Tue, Feb 24, 2026
[Archive]
Volume 20, Issue 11 (November 2022)
IJRM 2022, 20(11): 941-954 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shafie A, Kianian F, Ashabi G, Kadkhodaee M, Ranjbaran M, Hajiaqaei M, et al . Beneficial effects of combination therapy with testosterone and hydrogen sulfide by reducing oxidative stress and apoptosis: Rat experimental varicocele model. IJRM 2022; 20 (11) :941-954
URL: http://ijrm.ir/article-1-2423-en.html
URL: http://ijrm.ir/article-1-2423-en.html
Anahid Shafie1 

 , Farzaneh Kianian1
, Farzaneh Kianian1 

 , Ghorbangol Ashabi1
, Ghorbangol Ashabi1 

 , Mehri Kadkhodaee1
, Mehri Kadkhodaee1 

 , Mina Ranjbaran1
, Mina Ranjbaran1 

 , Mahdi Hajiaqaei1
, Mahdi Hajiaqaei1 

 , Keivan Lorian2
, Keivan Lorian2 

 , Arash Abdi1
, Arash Abdi1 

 , Behjat Seifi *3
, Behjat Seifi *3 




 , Farzaneh Kianian1
, Farzaneh Kianian1 

 , Ghorbangol Ashabi1
, Ghorbangol Ashabi1 

 , Mehri Kadkhodaee1
, Mehri Kadkhodaee1 

 , Mina Ranjbaran1
, Mina Ranjbaran1 

 , Mahdi Hajiaqaei1
, Mahdi Hajiaqaei1 

 , Keivan Lorian2
, Keivan Lorian2 

 , Arash Abdi1
, Arash Abdi1 

 , Behjat Seifi *3
, Behjat Seifi *3 


1- Department of Physiology, Faculty of Medicine, Tehran University of Medical Science, Tehran, Iran.
2- 2. Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Physiology, Faculty of Medicine, Tehran University of Medical Science, Tehran, Iran ,b-seifi@tums.ac.ir
2- 2. Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Physiology, Faculty of Medicine, Tehran University of Medical Science, Tehran, Iran ,
Keywords: Apoptosis genes, Hydrogen sulfide, Oxidative stress, Sperm count, Testosterone, Varicocele.
Full-Text [PDF 2534 kb]
(1339 Downloads)
| Abstract (HTML) (1904 Views)
Full-Text: (318 Views)
1. Introduction
Male fertility requires the continuous production of an adequate number of motile and morphologically normal spermatozoa (1). This process strongly depends on testosterone produced by the Leydig cells in the testes; thus, testosterone deficiency leads to impaired male fertility (2). In this regard, many studies have reported testosterone deficiency in patients with varicocele. The abnormal dilatation of the pampiniform plexus, based on the world health organization, is the leading cause of infertility in men (3-5). Several studies have shown that varicocele is associated with decreased sperm count, motility, morphology, and semen volume (6, 7). To date, surgical varicocelectomy is considered as the gold standard treatment for varicocele. Nevertheless, besides having several side effects, the association between varicocelectomy and improved testosterone levels remains controversial (8).
Testosterone therapy has long been the main approach for solving the testosterone deficiency problem. Administration of testosterone in appropriate doses stimulates spermatogenesis, increases sperm concentration and motility, and ameliorates inflammation, oxidative stress, and apoptosis by suppressing testicular damage (9). However, despite its effectiveness and simplicity, the clinical use of testosterone therapy has been bounded by reason of dose-dependent numerous side effects such as prostate cancer and cardiovascular risks (10). In addition, long-term exogenous administration of testosterone suppresses the hypothalamic-pituitary-gonadal axis, which may partially or completely stop spermatogenesis by reducing follicle-stimulating hormone and luteinizing hormone (11). Therefore, it is essential to investigate how to take advantage of testosterone therapy while at the same time reducing its side effects. An interesting result of research on the action mechanism of testosterone is that the beneficial effects of this hormone are mediated, at least in part, by hydrogen sulfide (H2S), being toxic in high concentrations but acts as an endogenous gaseous signaling molecule in low concentrations (12, 13). On the other hand, it has been shown that H2S can increase testosterone levels (14). These findings drew our attention to H2S to see if a low (subeffective) dose of testosterone combined with a subeffective dose of H2S can be effective in treating varicocele through a possible additive effect. Furthermore, given the involvement of different pathological mechanisms such as oxidative stress and apoptosis in the pathophysiology of varicocele, the fact that H2S, similar to testosterone, has various beneficial biological properties, including antioxidant and antiapoptotic activities prompted us further to choose H2S for this combination therapy (1, 15).
To our knowledge, the therapeutic effects of a subeffective dose of testosterone in combination with a subeffective dose of the H2S donor sodium hydrosulfide (NaHS) on infertility disorders have not been studied so far, the present study aims to investigate the beneficial effects of the combined administration of subeffective doses of testosterone and NaHS on a rat model of varicocele along with the underlying mechanisms.
2. Materials and Methods
2.1. Animals
For this experimental study, 8-10 wk-old, outbred albino Wistar rats weighing 200-250 gr were obtained from the Department of Physiology of Tehran University of Medical Sciences, Tehran, Iran. Animals were maintained in an air-conditioned room at 22 ± 2°C under a 12 hr light/dark cycle with access to food and water ad libitum. 3 rats were kept within each cage. Deep sedation was induced to prevent pain, and antibiotic tetracycline was used at the surgical site to reduce infection (16).
2.2. Varicocele induction
The varicocele model was induced by partial ligation of the left renal vein (17, 18). Briefly, rats were intraperitoneally anesthetized with 100 mg/kg ketamine (Rotexmedica, Germany) and 10 mg/kg xylazine (Sigma, USA). A 4-0 silk thread was placed around the left renal vein over a 0.7 mm needle and tied snugly; then the needle was removed. Sham rats were subjected to the same procedures, except that the left renal vein was not tied.
2.3. Dose-response
To select a subeffective dose of testosterone (Padgin Teb Co, Iran), 5 wk after the induction of varicocele, rats were subcutaneously injected with testosterone at doses of 100 (19), 200, and 400 µg/kg (20), 5 times per wk for 4 consecutive weeks, and then sperm parameters such as count, motility, viability, and morphology were assessed. However, the treatment duration was chosen according to the spermatogenesis period in rats. Our results showed significant improvements in all these parameters in rats that received 400 µg/kg testosterone, but not in 100 and 200 µg/kg testosterone (data not shown). On the other hand, to select a subeffective dose of NaHS (Merck, Germany), 5 wk after the induction of varicocele, other rats received 15 and 30 μmol/L of NaHS (21) in drinking water daily for 4 consecutive weeks, and then mentioned sperm parameters were evaluated. Our findings revealed marked improvements in all these parameters in rats that received 30 μmol/L NaHS but not 15 μmol/L NaHS. Therefore, 200 µg/kg testosterone and 15 μmol/L NaHS as half of the effective dose were selected as the subeffective doses for further experiments.
2.4. Experimental design
Thirty rats were divided randomly into 5 groups (n = 6) as in previous work in our laboratory: (1) sham, (2) varicocele, (3) testosterone (200 µg/kg, 5 times per wk for 4 consecutive week), (4) NaHS (15 μmol/L, daily for 4 wk), and (5) testosterone + NaHS (200 µg/kg, 5 times per wk + 15 μmol/L, daily, both for 4 wk). 5 wk after varicocele induction, animals in all treatment groups were treated. 8 wk after varicocele induction, serum samples were collected to measure testosterone levels. Then, the left caudal epididymis was separated, placed in a petri dish containing 1 ml of 37°C sperm media (Ham's F-10, Thermo Fisher Scientific, USA), and cut into pieces to remove the spermatozoa. Then, the sperm samples were placed in the incubator at 37°C. After 15 min, sperm motility, viability, count, and morphology were assessed. Testicular tissues were removed and washed in cold normal saline. The left testes were kept at -80°C to evaluate malondialdehyde (MDA), H2S levels, Bcl-2-associated X protein (Bax), and B-cell lymphoma 2 (Bcl-2) protein expression. A part of the right testes was stored at -80°C to determine superoxide dismutase (SOD) activity, and another part of these tissues was fixed in 10% formalin for histopathological study.
2.5. Measurement of serum testosterone levels
Serum testosterone levels were measured by enzyme-linked immunosorbent assay. Before starting the experiment, all reagents, standard diluents, control, and test samples were placed in the laboratory to reach room temperature. Then all procedures were carried out based on the manufacturer’s instructions (Padgin Teb Co, Iran). In this test, the quantitative sandwich enzyme immunoassay technique was used. The reactions were quantified by reading the optical density at 450 nm by an enzyme-linked immunosorbent assay reader.
2.6. Assessment of testicular histopathology
After fixation in 10% formalin (Merck, Germany), testicular tissues were embedded in paraffin, sectioned, and stained with hematoxylin-eosin solution (Inocolon, Iran). The tissue sections were then evaluated for pathological changes in the seminiferous tubules, basement membranes, and surrounding interstitial tissues using a light microscope at ×400 magnification.
2.7. Assessment of sperm count, motility, viability, and morphology
For sperm count, 500 µL of sperm suspension was mixed with 1,200 µL of 20% formalin, and the mixture (10 µL) was then placed on a hemocytometer. In 5 microscopic fields, sperm heads were counted using a light microscope at ×400 magnification (22). For sperm motility, sperm suspension (10 µL) was placed on a 37°C glass slide, and then the motility of 200 spermatozoa was evaluated using a light microscope at ×400 magnification. The evaluation of progressive motile spermatozoa, non-progressive motile spermatozoa and non-motile spermatozoa were performed according to the World Health Organization criteria (23). For sperm viability, 1% eosin and 10% nigrosin (Inocolon, Iran) were separately prepared. 1% eosin (2 volumes) was added to sperm suspension (one volume). After 30 sec, an equal volume of 10% nigrosin was added to the mixture, and then a thin smear was made on a 37°C glass slide. The viability of 100 spermatozoa was evaluated using a light microscope at 1,000× magnification. Viable spermatozoa remained colorless, whereas dead spermatozoa stained pink. For sperm morphology, the eosin-nigrosin staining was used. Eosin and nigrosin (10 µL) were added to sperm suspension (50 µL) on a glass slide, and the smear was incubated for 60 min at room temperature. The morphology of 100 spermatozoa was evaluated using a light microscope at 1,000× magnification (24, 25). The hook-shaped head was considered normal, while the round head, pinhead, double head, amorphous head, bent neck, asymmetrical neck, excess residual cytoplasm more than one-third head, double tail, bent tail, coiled tail, and short tail were considered abnormal (23).
2.8. Morphometric assessment of the seminiferous tubules
In the testicular sections stained with hematoxylin-eosin stain, the tubular diameter, area, and thickness of the seminiferous epithelium of the seminiferous tubules with rounded contours in 10 sections per animal were examined using Motic Image Plus 2.0 ML software. The diameter was defined as the average of the 2 parallel tangent lines on the outer edge of the tubule. The area was determined with the following formula: π (D/2)2. The thickness was calculated as the average of the 4 quadrants of the tubule (90°, 180°, 270°, and 360°) (24).
2.9. Measurement of testicular H2S levels
50 mg of testicular tissues were homogenized in 500 µl of phosphate-buffered saline. After incubating the homogenates with L-cysteine (Sigma, USA), pyridoxal phosphate (Sigma, USA), and normal saline for thirty min at 37°C, trichloroacetic acid (Sigma, USA) and zinc acetate (Merck, Germany) were added. 15 min after adding N, N-dimethyl-p-phenylenediamine sulfate (Sigma, USA) and ferric chloride (Sigma, USA), the absorbance of aliquots was measured at 660 nm by a microplate reader (26).
2.10. Assessment of the number of the Sertoli cells and spermatogonia
In hematoxylin-eosin-stained testicular sections, the Sertoli cells and spermatogonia in 50 seminiferous tubules per animal were counted using a light microscope at ×400 magnification.
2.11. Measurement of testicular MDA levels
MDA levels of testicular tissues were measured using the Esterbauer and Cheeseman method (27). The testicular tissue was mixed with 10% trichloroacetic acid (Sigma, USA) based on this method. After centrifugation of the mixture at 3000 g for 15 min at 4°C, the supernatant was separated and reacted with thiobarbituric acid (Sigma, USA) in 100°C water for 15 min. The reaction of MDA with thiobarbituric acid produces a pink pigment that has a maximum absorption at 532 nm (28).
2.12. Measurement of testicular SOD activity
SOD activity of testicular tissues was evaluated using the Nasdox™ assay kit according to the manufacturer’s protocol (Navand Salamat, Iran). 50 μL of the samples were blended with reagent 1 (200 μL), and reagent 2 (50 μL) was poured into a microplate. After 5 min of incubation, the optical density was determined at 405 nm by a spectrophotometer (29).
2.13. Testicular protein extraction and western blotting
Testicular tissues were homogenized in lysis buffer and centrifuged at 13,000 g for 15 min at 4°C. Then, protein concentrations in cell lysates were measured according to the Bradford method (30). Proteins (20 mg each) were electrophoresed in 12% SDS-PAGE and blotted on a polyvinylidene difluoride membrane. Anti-Bax and anti-Bcl-2 antibodies (Cell Signaling Technology, MA, USA) were used for membrane incubation and probed with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, MA, USA) in the next step. The reactive bands were distinguished by a chemiluminescence detection system, and Image J software was used for quantification of the blots. For normalization, the same polyvinylidene difluoride membrane was re-blotted with an anti-β-actin antibody (Cell Signaling Technology, MA, USA).
2.14. Ethical considerations
All experiments, including working with laboratory animals, were approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.MEDICINE.REC.1398.760).
2.15. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). A comparison among groups was performed by one-way analysis of variance (ANOVA) and Tukey’s test. P-values < 0.05 were considered significant. All statistical analyses were performed using SPSS software (SPSS, version 22, Chicago, IL, USA).
3. Results
3.1. Effects of subeffective doses of testosterone and NaHS alone or in combination on serum testosterone and testicular H2S levels
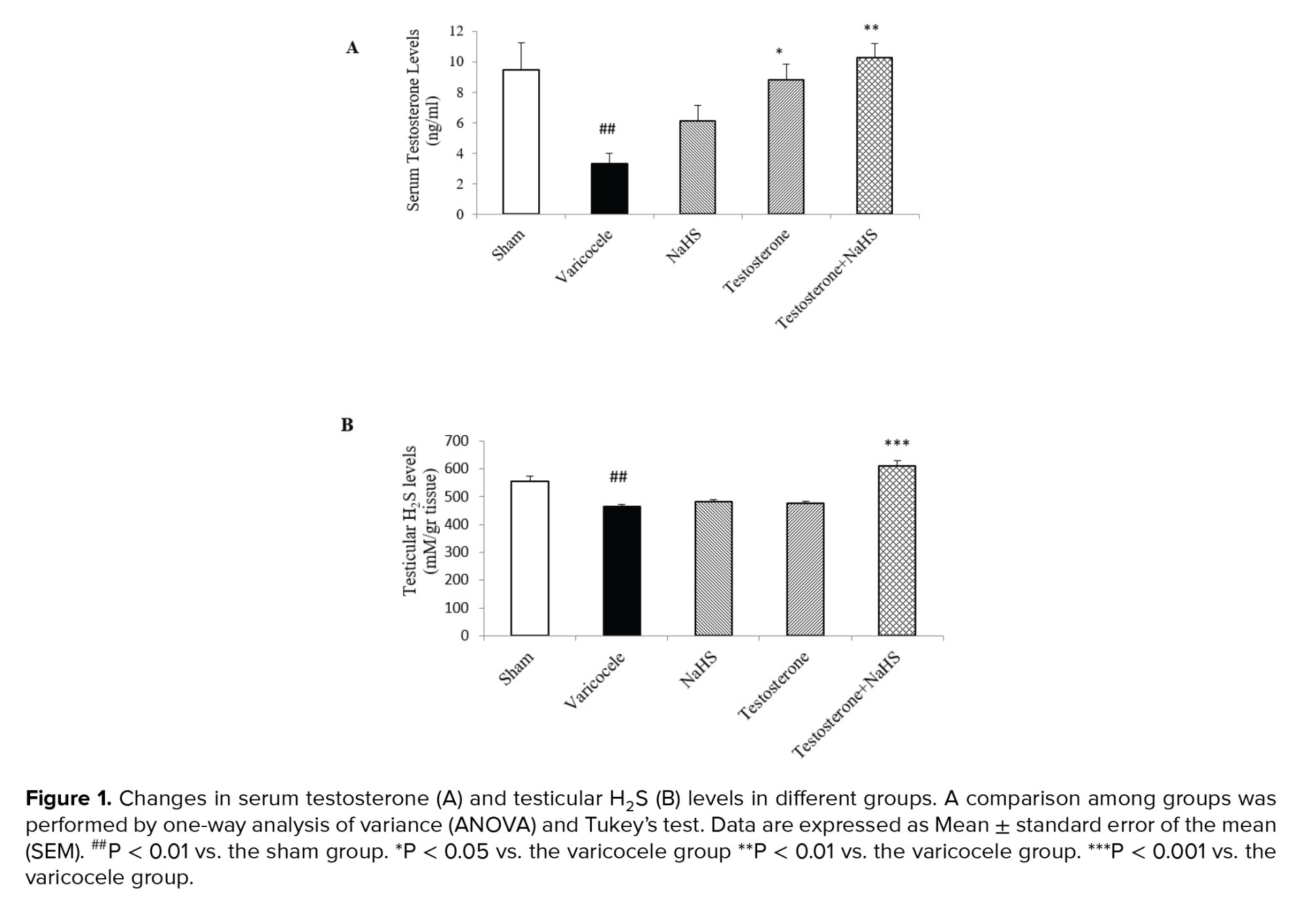
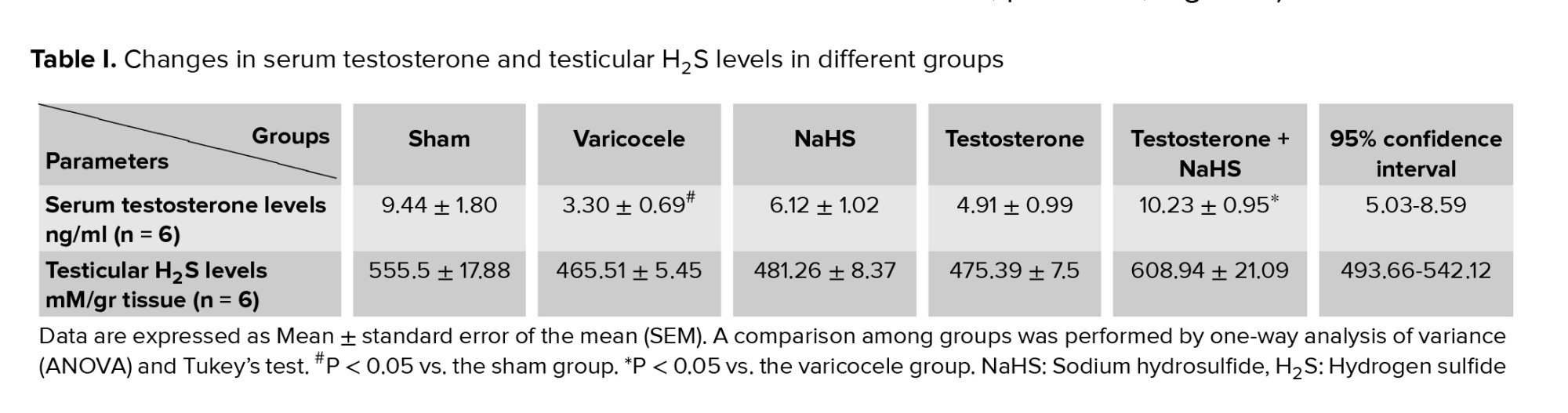
The varicocele induction in rats significantly decreased serum testosterone levels compared with the sham group (p = 0.02, Figure 1A, Table I). There were no considerable differences in serum testosterone levels in the groups of testosterones and NaHS alone in comparison with the varicocele group (Figure 1A, Table I). However, the combined administration of testosterone and NaHS significantly increased serum testosterone levels compared to the varicocele group (p = 0.01, Figure 1A, Table I). The varicocele induction in rats markedly decreased testicular H2S levels compared with the sham group (p < 0.001 Figure 1B, Table I). There were no considerable differences in testicular H2S levels in the groups of testosterones and NaHS alone in comparison with the varicocele group (Figure 1B, Table I). However, the combined administration of testosterone and NaHS significantly increased testicular H2S levels compared with the varicocele group (p < 0.001, Figure 1B, Table I).
3.2. Effects of subeffective doses of testosterone and NaHS alone or in combination on testicular histopathology
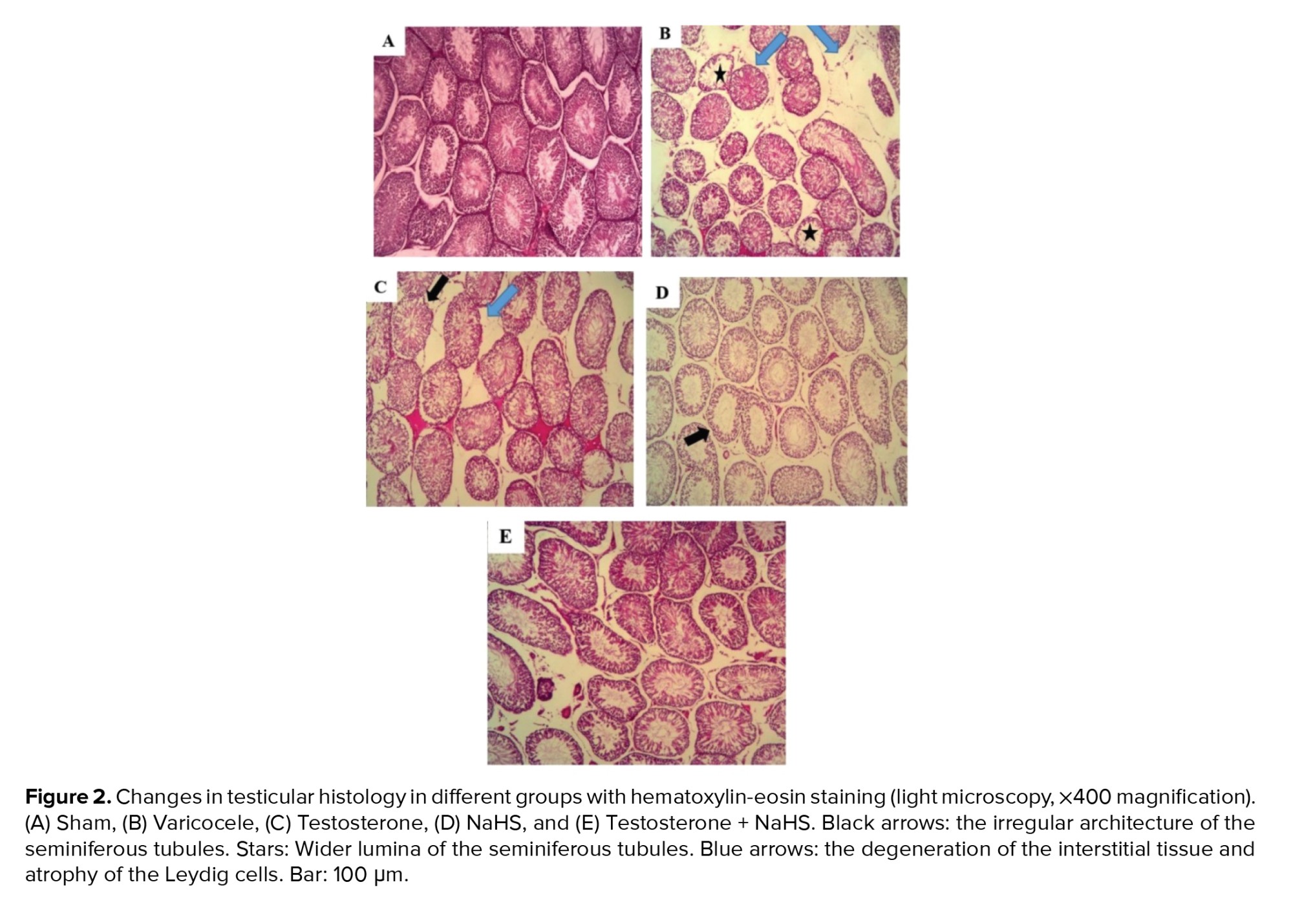
There was no detectable damage to the testicular tissues of the sham group (Figure 2A). The testicular tissues in the varicocele group showed severe changes in comparison with the sham group (Figure 2B). These changes include the irregular architecture and wider lumina of the seminiferous tubules and widening of the spaces between these tubules. The disorganized epithelium with changes in the basement membrane thickness, degeneration of the interstitial tissue, and atrophy of the Leydig cells were also seen. The combined but not individual administration of testosterone and NaHS considerably reduced the amount of histopathological damage (Figures 2C-E).
3.3. Effects of subeffective doses of testosterone and NaHS alone or in combination on sperm count, motility, viability, and morphology
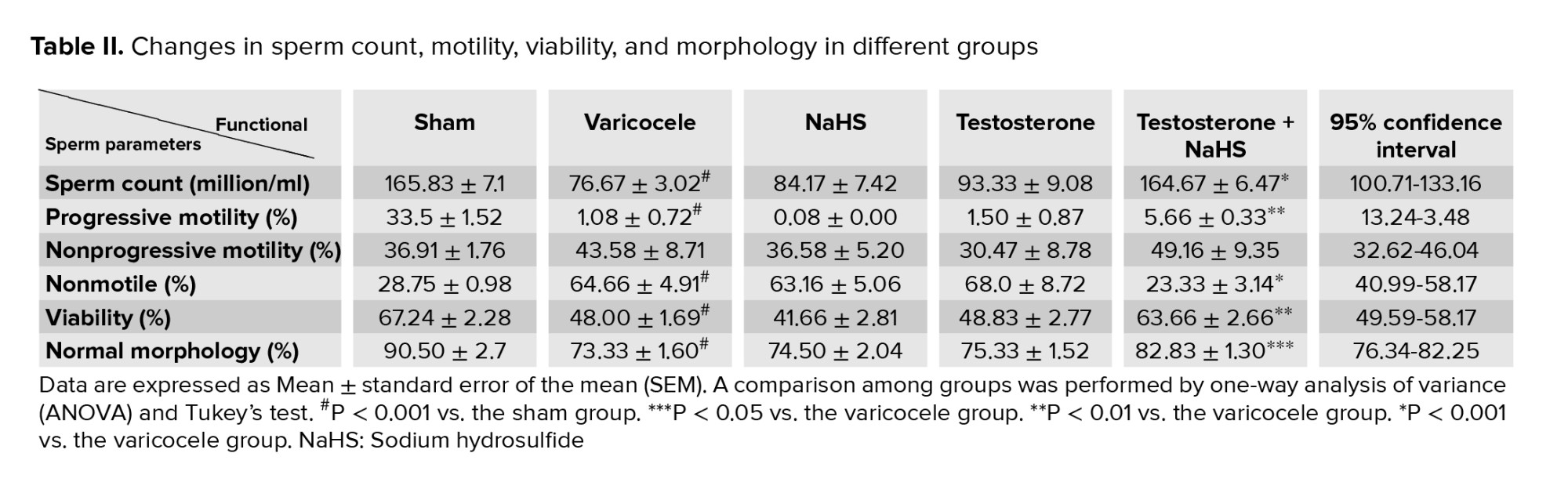
The varicocele induction in rats significantly decreased the number of spermatozoa compared to the sham group (p < 0.001, Table II). There were no considerable differences in the number of spermatozoa in the testosterone groups and NaHS alone compared with the varicocele group (Table II). However, the combined administration of testosterone and NaHS significantly increased the number of spermatozoa compared to the varicocele group (p < 0.001, Table II). The induction of varicocele in rats significantly decreased the percentage of progressively motile spermatozoa and increased the percentage of non-motile spermatozoa (both p < 0.001, Table II), but did not change the percentage of non-progressive motile spermatozoa (Table II) in comparison with the sham group. There were no considerable differences in these parameters in the groups of testosterones and NaHS alone compared to the varicocele group (Table II). However, the combined administration of testosterone and NaHS significantly increased the percentage of progressively motile spermatozoa and decreased the percentage of non-motile spermatozoa (p < 0.001 and p < 0.001, respectively), but did not change the percentage of non-progressive motile spermatozoa (Table II) compared to the varicocele group.
The varicocele induction in rats significantly decreased the percentage of viable spermatozoa compared to the sham group (p < 0.001, Table II). There were no considerable differences in the percentage of viable spermatozoa in the groups of testosterones and NaHS alone compared to the varicocele group (Table II). However, the combined administration of testosterone and NaHS remarkably enhanced the percentage of viable spermatozoa in comparison with the varicocele group (p < 0.001, Table II). The varicocele induction in rats significantly decreased the percentage of morphologically normal spermatozoa compared to the sham group (p < 0.001, Table II). There were no considerable differences in the percentage of morphologically normal spermatozoa in the groups of testosterones and NaHS alone compared to the varicocele group (Table II). However, the combined administration of testosterone and NaHS markedly enhanced the percentage of morphologically normal spermatozoa in comparison with the varicocele group (p = 0.01, Table II).
3.4. Effects of subeffective doses of testosterone and NaHS alone or in combination on morphometric features of the seminiferous tubules and number of the Sertoli cells and spermatogonia
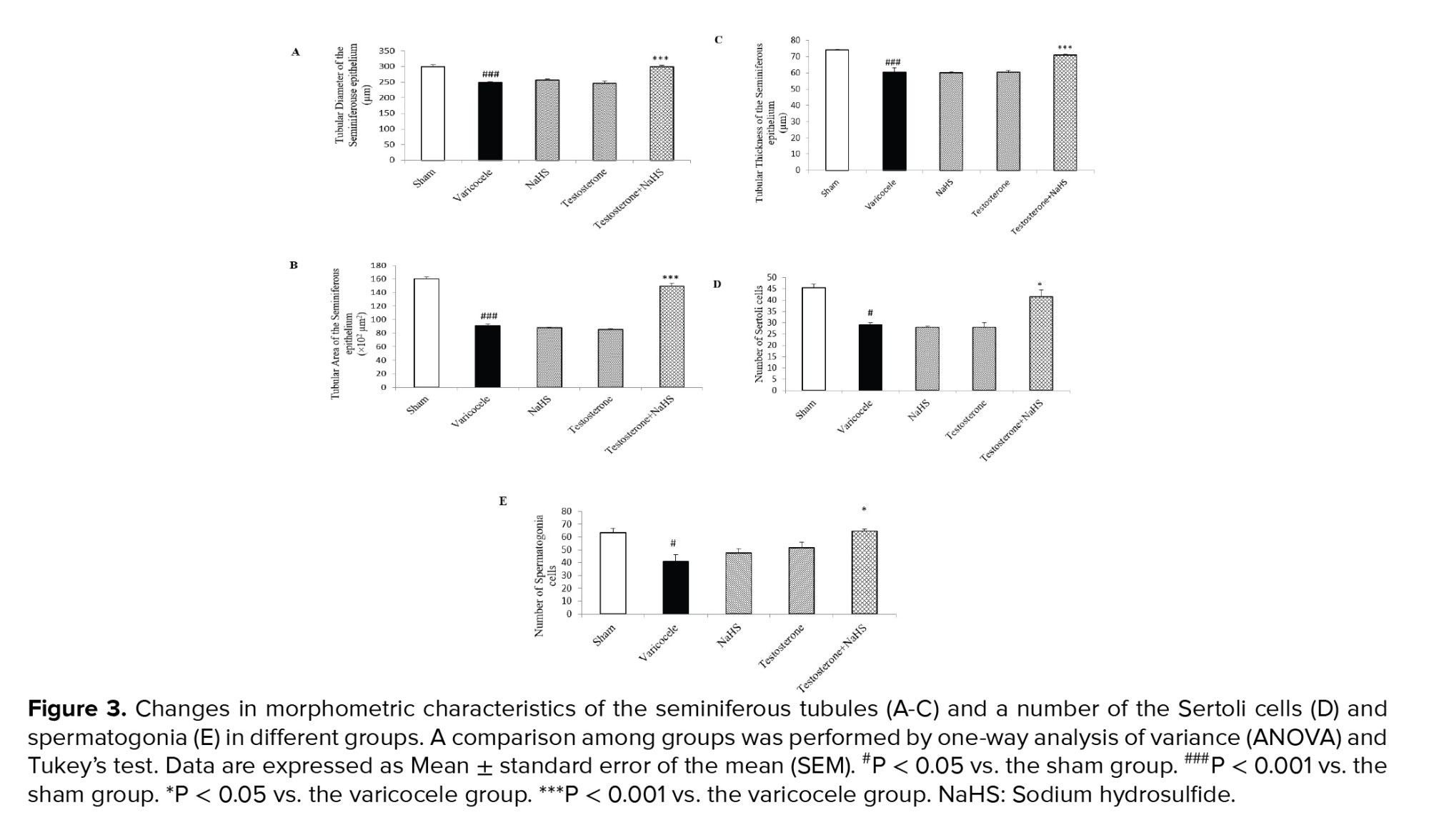
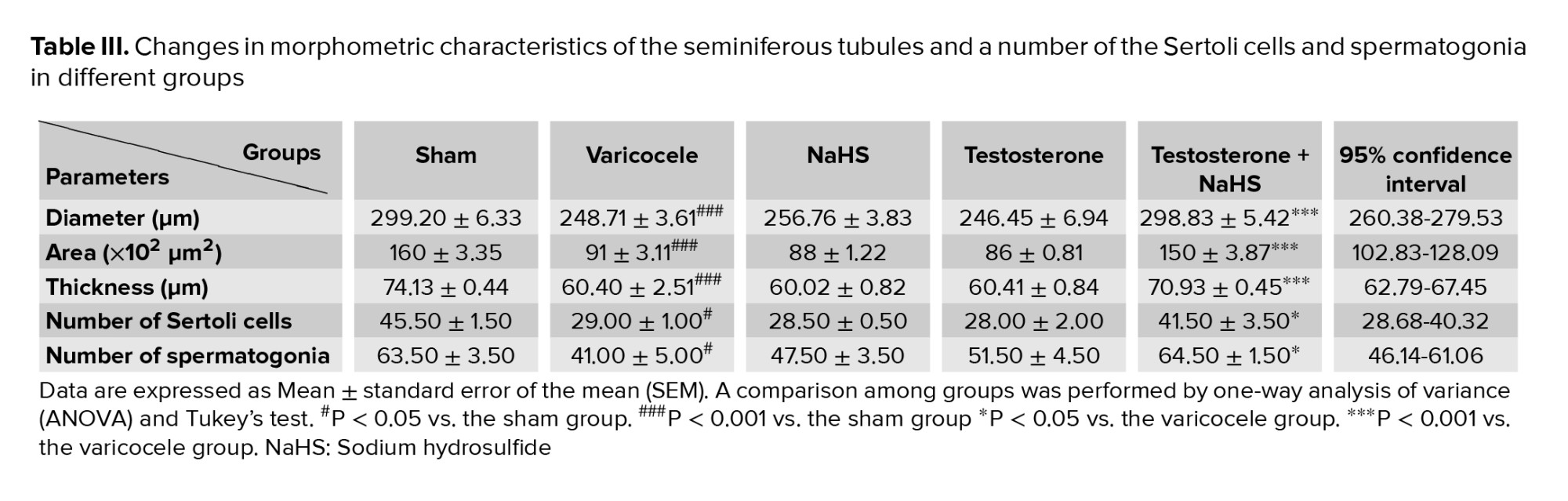
The induction of varicocele in rats significantly decreased the tubular diameter and area, and thickness of the seminiferous epithelium of the seminiferous tubules in comparison with the sham group (all p < 0.001, Figures 3A-C, respectively, Table III). There were no considerable differences in these parameters in the groups of testosterones and NaHS alone compared to the varicocele group (Figures 3A-C, Table III). However, the combined administration of testosterone and NaHS significantly increased the tubular diameter and area, and thickness of the seminiferous epithelium of the seminiferous tubules in comparison with the varicocele group (all p < 0.001, Figures 3A-C, respectively, Table III). The varicocele induction in rats remarkably reduced the Sertoli cells and spermatogonia number compared to the sham group (p = 0.01 and p = 0.04, Figures 3D and E, respectively, Table III). There were no considerable differences in the number of the Sertoli cells and spermatogonia in the groups of testosterone and NaHS alone in comparison with the varicocele group (Figures 3D and E, respectively, Table III). However, the combined administration of testosterone and NaHS markedly enhanced the number of the Sertoli cells and spermatogonia in comparison with the varicocele group (both p = 0.03, Figures 3D and E, respectively, Table III).
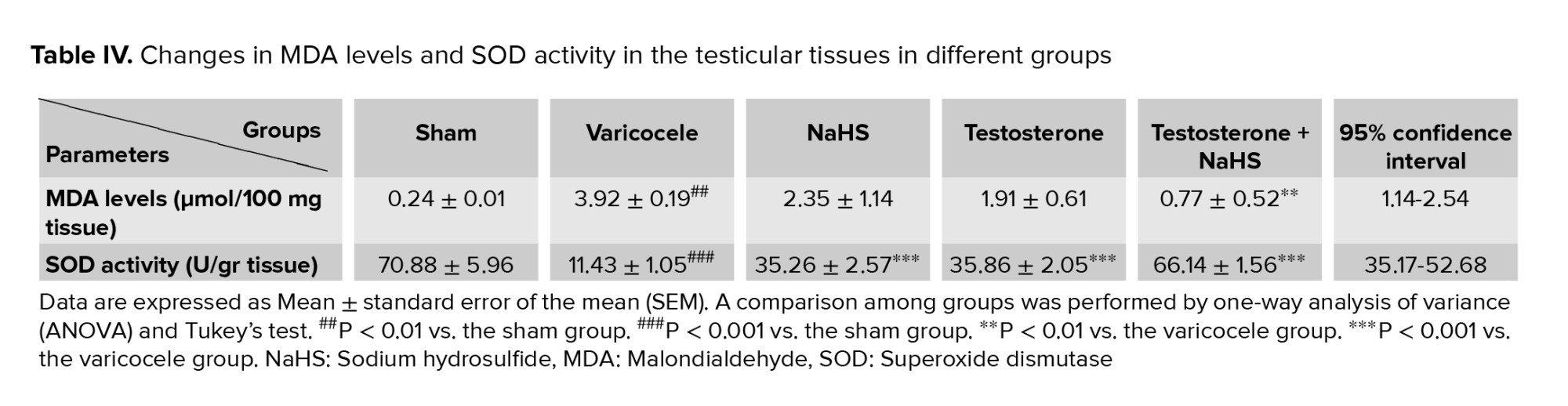
3.5. Effects of subeffective doses of testosterone and NaHS alone or in combination on MDA levels and SOD activity in the testicular tissues
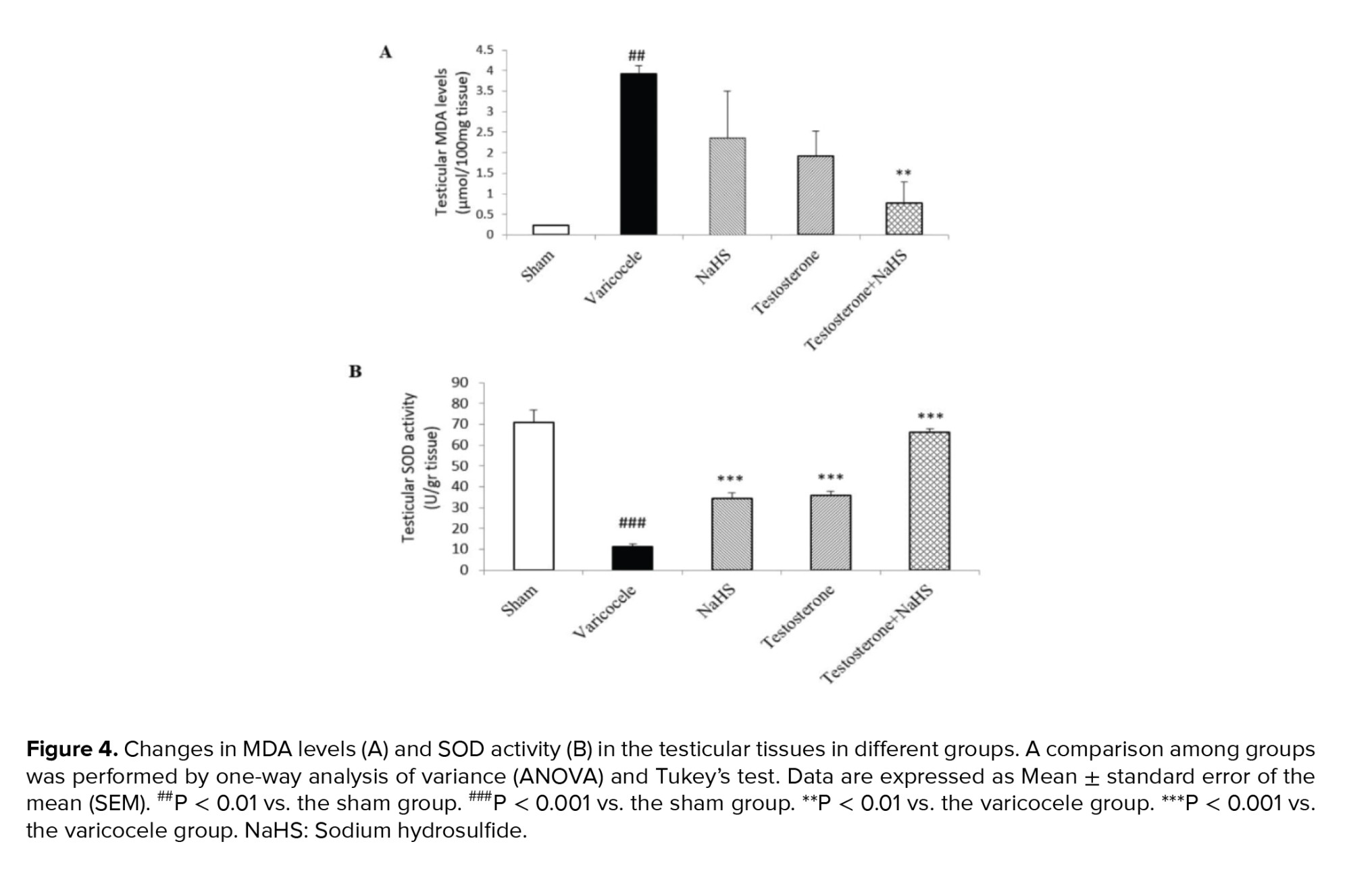
The varicocele induction in rats significantly increased testicular MDA levels compared to the sham group (p < 0.001, Figure 4A, Table IV). There were no considerable differences in testicular MDA levels in the groups of testosterone and NaHS alone than the varicocele group (Figure 4A, Table IV). However, the combined administration of testosterone and NaHS significantly decreased testicular MDA levels compared with the varicocele group (p < 0.001, Figure 4A, Table IV). The varicocele induction in rats significantly decreased testicular SOD activity compared to the sham group (p < 0.001, Figure 4B, Table IV). However, both the individual and combined administration of testosterone and NaHS significantly enhanced testicular SOD activity in comparison with the varicocele group (all p < 0.001, Figure 4B, Table IV).
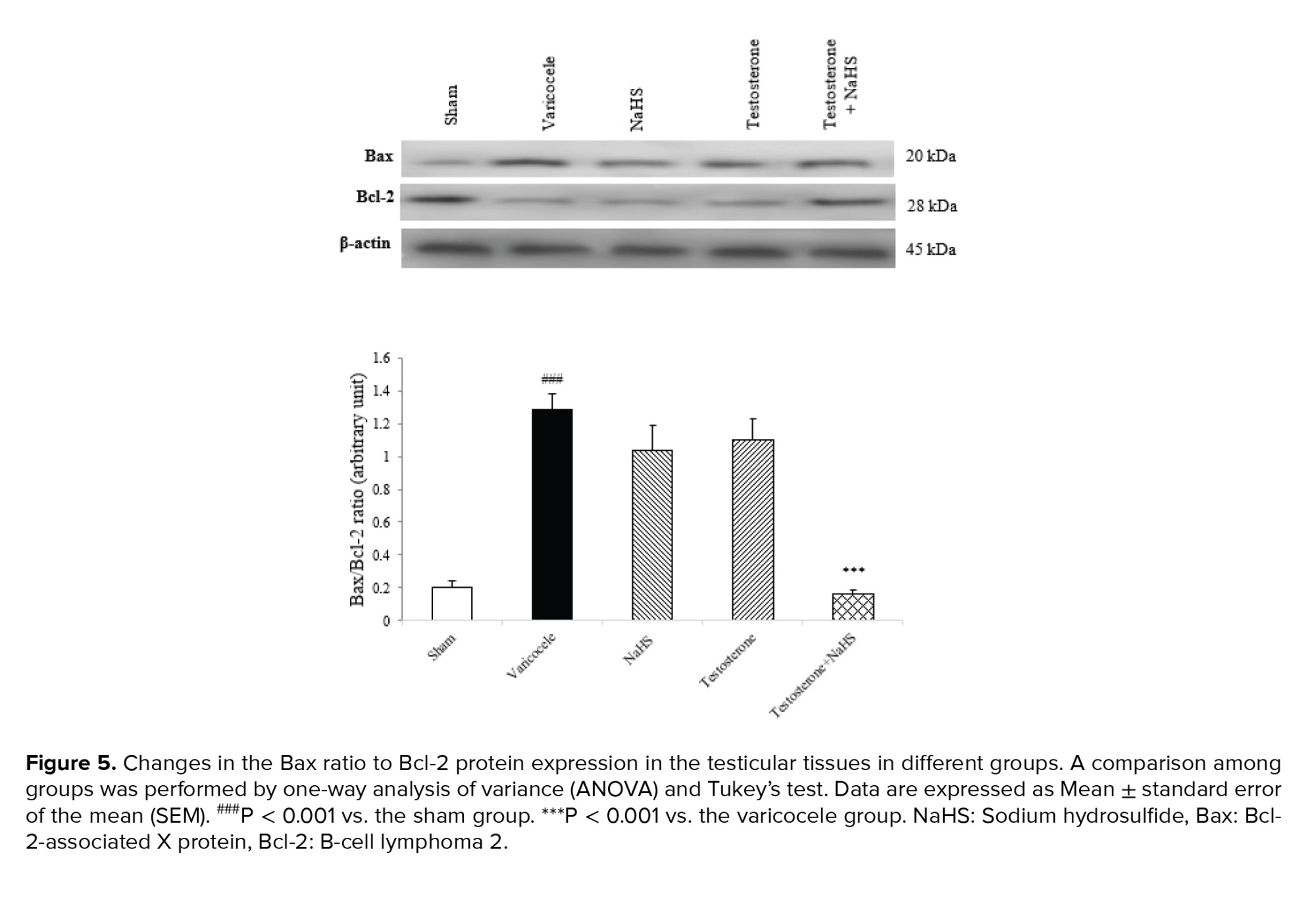
3.6. Effects of subeffective doses of testosterone and NaHS alone or in combination on the ratio of Bax to Bcl-2 protein expression in the testicular tissues
The varicocele induction in rats considerably enhanced the ratio of Bax to Bcl-2 protein expression in the testicular tissues compared to the sham group (1.28 ± 0.08 vs. 0.20 ± 0.03, p < 0.001, Figure 5). There were no considerable differences in the ratio of Bax to Bcl-2 protein expression in the testicular tissues in the groups of testosterone (1.03 ± 0.13) and NaHS (1.19 ± 0.10) alone in comparison with the varicocele group (Figure 5). However, the combined administration of testosterone and NaHS significantly decreased the Bax to Bcl-2 protein expression ratio in the testicular tissues compared to the varicocele group (0.16 ± 0.01, 95% CI: 0.53-0.95, p < 0.001, Figure 5).
4. Discussion
In the current study, to the best of our knowledge for the first time, it was examined whether a subeffective dose of testosterone combined with a subeffective dose of H2S can improve varicocele-induced damages through a possible additive effect. As a starting point, we assessed the protective effects of the treatment regimen on the functional status of testicular tissues in varicocele rats since it is well known that varicocele causes testicular dysfunction (31). The first main function of the testes, steroidogenesis, is a multistep process by which cholesterol is converted to steroids such as testosterone (32). In this regard, preclinical and clinical studies have shown varicocele-induced impairment of steroidogenesis by a reduction in serum testosterone levels (33, 34). Similarly, this study observed a significant decrease in serum testosterone levels in rats subjected to varicocele. In addition, the subsequent histopathological examination of the testicular tissues confirmed this finding as it showed the Leydig cell atrophy in varicocele rats, indicating a reduction in testosterone-producing cells with varicocele induction. The second main function of the testes, spermatogenesis, is a process of multiple germ cell divisions to enhance their number and then differentiate into spermatozoa in the seminiferous tubules (35). Importantly, this process is highly dependent on testosterone, as it has been shown many times in the medical literature that hypospermatogenesis is associated with testosterone deficiency. Moreover, testosterone deficiency is reported to have detrimental effects on other sperm characteristics, including motility, viability, and morphology as well as morphometric features of the seminiferous tubules such as tubular diameter and thickness of the seminiferous epithelium (36). Accordingly, we found the deleterious effects of varicocele on these parameters along with decreased serum testosterone levels. However, from the therapeutic point of view, the combined administration of subeffective doses of testosterone and NaHS to varicocele rats reversed all the above pathological changes to the levels measured before varicocele induction, suggesting the improvement of testicular dysfunction.
In the next step, we evaluated the beneficial effects of the treatment regimen on testicular H2S levels in rats subjected to varicocele because it is thought that decreased H2S levels in testes contribute to varicocele-induced infertility (37). Our result showed a significant reduction in testicular H2S levels in varicocele, which was interestingly reversed by the combined administration of subeffective doses of testosterone and NaHS. Along with this, our histopathological examination of the testicular tissues revealed another interesting finding. In addition to the Leydig cell atrophy mentioned earlier, there was a considerable reduction in the number of Sertoli cells and spermatogonia in the testes of varicocele rats, which was markedly enhanced by the combined administration of subeffective doses of testosterone and NaHS. Importantly, in the male reproductive tract, H2S is mainly produced by cystathionine-β-synthase in the Leydig cells and cystathionine γ-lyase in the Sertoli cells and spermatogonia (13). Thus, our findings indicated the potential of this treatment regimen for protecting H2S-producing cells in testes and thereby maintaining normal testicular H2S levels.
After achieving such promising results from the treatment regimen, it was decided to elucidate some of the underlying molecular mechanisms. Varicocele-induced oxidative stress is well known to play a crucial role in damaging the male reproductive tract, including the testes (37). Oxidative stress is an imbalance between reactive oxygen species formation and their removal by antioxidant systems, leading to lipid peroxidation. Substantial evidence suggests that increased scrotal temperature due to retrograde blood flow to the pampiniform plexus is the most likely cause of oxidative stress induced by varicocele (38). In our study, the presence of oxidative stress, a significant increase in MDA levels is a major lipid peroxidation byproduct, and a decrease of SOD activity as an important antioxidant enzyme in the testicular tissues of varicocele rats were reported. From the therapeutic point of view, although the individual administration of subeffective doses of testosterone or NaHS to varicocele rats considerably enhanced testicular SOD activity, their co-administration provided better protection on lipid peroxidation and thus reduced oxidative stress in the testicular tissues.
In addition to its antioxidant effects, we evaluated the anti-apoptotic effects of the treatment regimen because it is indicated that varicocele-induced oxidative stress and apoptosis in the male reproductive tract are closely related processes (38). Although the mechanism is unknown, reactive oxygen species overproduction has been suggested to cause the efflux of cytochrome C from the mitochondria, which triggers signaling pathways for apoptosis, a type of cell death (39). In this terms, pro-apoptotic Bcl-2 family members (e.g., Bax), suppress the activity of anti-apoptotic Bcl-2 family members (e.g., Bcl-2) which is existing in the outer mitochondrial membrane, declaring that the ratio of pro- and anti-apoptotic Bcl-2 family members can be a good indicator to assess the occurrence of apoptosis (40). In this study, our findings showed a marked increase in the ratio of Bax to Bcl-2 protein expression levels in the testicular tissues of varicocele rats, which was remarkably reduced by the co-administration of subeffective doses of testosterone and NaHS.
The limitation of the present study was not evaluating the side effects of combined administration of subeffective doses of testosterone and NaHS in varicocele. Therefore, evaluating these side effects are suggested.
5. Conclusion
In the present study, the experimental varicocele caused decrease in the levels of serum testosterone and testicular H2S, as well as detrimental effects on sperm parameters and testicular histology. In contrast, the co-administration of subeffective doses of testosterone and the H2S donor NaHS, by reducing testicular oxidative stress and apoptosis through a possible additive effect, reversed all the above indicators to the levels measured before varicocele induction. Therefore, this study interestingly introduces a novel approach to benefit from the beneficial effects of testosterone therapy and decrease its side effects at the same time. Nevertheless, further preclinical investigations are needed to confirm the effectiveness of this therapeutic approach for clinical use.
Acknowledgments
This research was supported by Tehran University of Medical Sciences, Tehran, Iran (grant no = 45380). The authors are grateful to the Tehran University of Medical Sciences for supporting the present research.
Conflict of interest
The authors declare no conflict of interest.
Male fertility requires the continuous production of an adequate number of motile and morphologically normal spermatozoa (1). This process strongly depends on testosterone produced by the Leydig cells in the testes; thus, testosterone deficiency leads to impaired male fertility (2). In this regard, many studies have reported testosterone deficiency in patients with varicocele. The abnormal dilatation of the pampiniform plexus, based on the world health organization, is the leading cause of infertility in men (3-5). Several studies have shown that varicocele is associated with decreased sperm count, motility, morphology, and semen volume (6, 7). To date, surgical varicocelectomy is considered as the gold standard treatment for varicocele. Nevertheless, besides having several side effects, the association between varicocelectomy and improved testosterone levels remains controversial (8).
Testosterone therapy has long been the main approach for solving the testosterone deficiency problem. Administration of testosterone in appropriate doses stimulates spermatogenesis, increases sperm concentration and motility, and ameliorates inflammation, oxidative stress, and apoptosis by suppressing testicular damage (9). However, despite its effectiveness and simplicity, the clinical use of testosterone therapy has been bounded by reason of dose-dependent numerous side effects such as prostate cancer and cardiovascular risks (10). In addition, long-term exogenous administration of testosterone suppresses the hypothalamic-pituitary-gonadal axis, which may partially or completely stop spermatogenesis by reducing follicle-stimulating hormone and luteinizing hormone (11). Therefore, it is essential to investigate how to take advantage of testosterone therapy while at the same time reducing its side effects. An interesting result of research on the action mechanism of testosterone is that the beneficial effects of this hormone are mediated, at least in part, by hydrogen sulfide (H2S), being toxic in high concentrations but acts as an endogenous gaseous signaling molecule in low concentrations (12, 13). On the other hand, it has been shown that H2S can increase testosterone levels (14). These findings drew our attention to H2S to see if a low (subeffective) dose of testosterone combined with a subeffective dose of H2S can be effective in treating varicocele through a possible additive effect. Furthermore, given the involvement of different pathological mechanisms such as oxidative stress and apoptosis in the pathophysiology of varicocele, the fact that H2S, similar to testosterone, has various beneficial biological properties, including antioxidant and antiapoptotic activities prompted us further to choose H2S for this combination therapy (1, 15).
To our knowledge, the therapeutic effects of a subeffective dose of testosterone in combination with a subeffective dose of the H2S donor sodium hydrosulfide (NaHS) on infertility disorders have not been studied so far, the present study aims to investigate the beneficial effects of the combined administration of subeffective doses of testosterone and NaHS on a rat model of varicocele along with the underlying mechanisms.
2. Materials and Methods
2.1. Animals
For this experimental study, 8-10 wk-old, outbred albino Wistar rats weighing 200-250 gr were obtained from the Department of Physiology of Tehran University of Medical Sciences, Tehran, Iran. Animals were maintained in an air-conditioned room at 22 ± 2°C under a 12 hr light/dark cycle with access to food and water ad libitum. 3 rats were kept within each cage. Deep sedation was induced to prevent pain, and antibiotic tetracycline was used at the surgical site to reduce infection (16).
2.2. Varicocele induction
The varicocele model was induced by partial ligation of the left renal vein (17, 18). Briefly, rats were intraperitoneally anesthetized with 100 mg/kg ketamine (Rotexmedica, Germany) and 10 mg/kg xylazine (Sigma, USA). A 4-0 silk thread was placed around the left renal vein over a 0.7 mm needle and tied snugly; then the needle was removed. Sham rats were subjected to the same procedures, except that the left renal vein was not tied.
2.3. Dose-response
To select a subeffective dose of testosterone (Padgin Teb Co, Iran), 5 wk after the induction of varicocele, rats were subcutaneously injected with testosterone at doses of 100 (19), 200, and 400 µg/kg (20), 5 times per wk for 4 consecutive weeks, and then sperm parameters such as count, motility, viability, and morphology were assessed. However, the treatment duration was chosen according to the spermatogenesis period in rats. Our results showed significant improvements in all these parameters in rats that received 400 µg/kg testosterone, but not in 100 and 200 µg/kg testosterone (data not shown). On the other hand, to select a subeffective dose of NaHS (Merck, Germany), 5 wk after the induction of varicocele, other rats received 15 and 30 μmol/L of NaHS (21) in drinking water daily for 4 consecutive weeks, and then mentioned sperm parameters were evaluated. Our findings revealed marked improvements in all these parameters in rats that received 30 μmol/L NaHS but not 15 μmol/L NaHS. Therefore, 200 µg/kg testosterone and 15 μmol/L NaHS as half of the effective dose were selected as the subeffective doses for further experiments.
2.4. Experimental design
Thirty rats were divided randomly into 5 groups (n = 6) as in previous work in our laboratory: (1) sham, (2) varicocele, (3) testosterone (200 µg/kg, 5 times per wk for 4 consecutive week), (4) NaHS (15 μmol/L, daily for 4 wk), and (5) testosterone + NaHS (200 µg/kg, 5 times per wk + 15 μmol/L, daily, both for 4 wk). 5 wk after varicocele induction, animals in all treatment groups were treated. 8 wk after varicocele induction, serum samples were collected to measure testosterone levels. Then, the left caudal epididymis was separated, placed in a petri dish containing 1 ml of 37°C sperm media (Ham's F-10, Thermo Fisher Scientific, USA), and cut into pieces to remove the spermatozoa. Then, the sperm samples were placed in the incubator at 37°C. After 15 min, sperm motility, viability, count, and morphology were assessed. Testicular tissues were removed and washed in cold normal saline. The left testes were kept at -80°C to evaluate malondialdehyde (MDA), H2S levels, Bcl-2-associated X protein (Bax), and B-cell lymphoma 2 (Bcl-2) protein expression. A part of the right testes was stored at -80°C to determine superoxide dismutase (SOD) activity, and another part of these tissues was fixed in 10% formalin for histopathological study.
2.5. Measurement of serum testosterone levels
Serum testosterone levels were measured by enzyme-linked immunosorbent assay. Before starting the experiment, all reagents, standard diluents, control, and test samples were placed in the laboratory to reach room temperature. Then all procedures were carried out based on the manufacturer’s instructions (Padgin Teb Co, Iran). In this test, the quantitative sandwich enzyme immunoassay technique was used. The reactions were quantified by reading the optical density at 450 nm by an enzyme-linked immunosorbent assay reader.
2.6. Assessment of testicular histopathology
After fixation in 10% formalin (Merck, Germany), testicular tissues were embedded in paraffin, sectioned, and stained with hematoxylin-eosin solution (Inocolon, Iran). The tissue sections were then evaluated for pathological changes in the seminiferous tubules, basement membranes, and surrounding interstitial tissues using a light microscope at ×400 magnification.
2.7. Assessment of sperm count, motility, viability, and morphology
For sperm count, 500 µL of sperm suspension was mixed with 1,200 µL of 20% formalin, and the mixture (10 µL) was then placed on a hemocytometer. In 5 microscopic fields, sperm heads were counted using a light microscope at ×400 magnification (22). For sperm motility, sperm suspension (10 µL) was placed on a 37°C glass slide, and then the motility of 200 spermatozoa was evaluated using a light microscope at ×400 magnification. The evaluation of progressive motile spermatozoa, non-progressive motile spermatozoa and non-motile spermatozoa were performed according to the World Health Organization criteria (23). For sperm viability, 1% eosin and 10% nigrosin (Inocolon, Iran) were separately prepared. 1% eosin (2 volumes) was added to sperm suspension (one volume). After 30 sec, an equal volume of 10% nigrosin was added to the mixture, and then a thin smear was made on a 37°C glass slide. The viability of 100 spermatozoa was evaluated using a light microscope at 1,000× magnification. Viable spermatozoa remained colorless, whereas dead spermatozoa stained pink. For sperm morphology, the eosin-nigrosin staining was used. Eosin and nigrosin (10 µL) were added to sperm suspension (50 µL) on a glass slide, and the smear was incubated for 60 min at room temperature. The morphology of 100 spermatozoa was evaluated using a light microscope at 1,000× magnification (24, 25). The hook-shaped head was considered normal, while the round head, pinhead, double head, amorphous head, bent neck, asymmetrical neck, excess residual cytoplasm more than one-third head, double tail, bent tail, coiled tail, and short tail were considered abnormal (23).
2.8. Morphometric assessment of the seminiferous tubules
In the testicular sections stained with hematoxylin-eosin stain, the tubular diameter, area, and thickness of the seminiferous epithelium of the seminiferous tubules with rounded contours in 10 sections per animal were examined using Motic Image Plus 2.0 ML software. The diameter was defined as the average of the 2 parallel tangent lines on the outer edge of the tubule. The area was determined with the following formula: π (D/2)2. The thickness was calculated as the average of the 4 quadrants of the tubule (90°, 180°, 270°, and 360°) (24).
2.9. Measurement of testicular H2S levels
50 mg of testicular tissues were homogenized in 500 µl of phosphate-buffered saline. After incubating the homogenates with L-cysteine (Sigma, USA), pyridoxal phosphate (Sigma, USA), and normal saline for thirty min at 37°C, trichloroacetic acid (Sigma, USA) and zinc acetate (Merck, Germany) were added. 15 min after adding N, N-dimethyl-p-phenylenediamine sulfate (Sigma, USA) and ferric chloride (Sigma, USA), the absorbance of aliquots was measured at 660 nm by a microplate reader (26).
2.10. Assessment of the number of the Sertoli cells and spermatogonia
In hematoxylin-eosin-stained testicular sections, the Sertoli cells and spermatogonia in 50 seminiferous tubules per animal were counted using a light microscope at ×400 magnification.
2.11. Measurement of testicular MDA levels
MDA levels of testicular tissues were measured using the Esterbauer and Cheeseman method (27). The testicular tissue was mixed with 10% trichloroacetic acid (Sigma, USA) based on this method. After centrifugation of the mixture at 3000 g for 15 min at 4°C, the supernatant was separated and reacted with thiobarbituric acid (Sigma, USA) in 100°C water for 15 min. The reaction of MDA with thiobarbituric acid produces a pink pigment that has a maximum absorption at 532 nm (28).
2.12. Measurement of testicular SOD activity
SOD activity of testicular tissues was evaluated using the Nasdox™ assay kit according to the manufacturer’s protocol (Navand Salamat, Iran). 50 μL of the samples were blended with reagent 1 (200 μL), and reagent 2 (50 μL) was poured into a microplate. After 5 min of incubation, the optical density was determined at 405 nm by a spectrophotometer (29).
2.13. Testicular protein extraction and western blotting
Testicular tissues were homogenized in lysis buffer and centrifuged at 13,000 g for 15 min at 4°C. Then, protein concentrations in cell lysates were measured according to the Bradford method (30). Proteins (20 mg each) were electrophoresed in 12% SDS-PAGE and blotted on a polyvinylidene difluoride membrane. Anti-Bax and anti-Bcl-2 antibodies (Cell Signaling Technology, MA, USA) were used for membrane incubation and probed with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, MA, USA) in the next step. The reactive bands were distinguished by a chemiluminescence detection system, and Image J software was used for quantification of the blots. For normalization, the same polyvinylidene difluoride membrane was re-blotted with an anti-β-actin antibody (Cell Signaling Technology, MA, USA).
2.14. Ethical considerations
All experiments, including working with laboratory animals, were approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.MEDICINE.REC.1398.760).
2.15. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). A comparison among groups was performed by one-way analysis of variance (ANOVA) and Tukey’s test. P-values < 0.05 were considered significant. All statistical analyses were performed using SPSS software (SPSS, version 22, Chicago, IL, USA).
3. Results
3.1. Effects of subeffective doses of testosterone and NaHS alone or in combination on serum testosterone and testicular H2S levels
The varicocele induction in rats significantly decreased serum testosterone levels compared with the sham group (p = 0.02, Figure 1A, Table I). There were no considerable differences in serum testosterone levels in the groups of testosterones and NaHS alone in comparison with the varicocele group (Figure 1A, Table I). However, the combined administration of testosterone and NaHS significantly increased serum testosterone levels compared to the varicocele group (p = 0.01, Figure 1A, Table I). The varicocele induction in rats markedly decreased testicular H2S levels compared with the sham group (p < 0.001 Figure 1B, Table I). There were no considerable differences in testicular H2S levels in the groups of testosterones and NaHS alone in comparison with the varicocele group (Figure 1B, Table I). However, the combined administration of testosterone and NaHS significantly increased testicular H2S levels compared with the varicocele group (p < 0.001, Figure 1B, Table I).
3.2. Effects of subeffective doses of testosterone and NaHS alone or in combination on testicular histopathology
There was no detectable damage to the testicular tissues of the sham group (Figure 2A). The testicular tissues in the varicocele group showed severe changes in comparison with the sham group (Figure 2B). These changes include the irregular architecture and wider lumina of the seminiferous tubules and widening of the spaces between these tubules. The disorganized epithelium with changes in the basement membrane thickness, degeneration of the interstitial tissue, and atrophy of the Leydig cells were also seen. The combined but not individual administration of testosterone and NaHS considerably reduced the amount of histopathological damage (Figures 2C-E).
3.3. Effects of subeffective doses of testosterone and NaHS alone or in combination on sperm count, motility, viability, and morphology
The varicocele induction in rats significantly decreased the number of spermatozoa compared to the sham group (p < 0.001, Table II). There were no considerable differences in the number of spermatozoa in the testosterone groups and NaHS alone compared with the varicocele group (Table II). However, the combined administration of testosterone and NaHS significantly increased the number of spermatozoa compared to the varicocele group (p < 0.001, Table II). The induction of varicocele in rats significantly decreased the percentage of progressively motile spermatozoa and increased the percentage of non-motile spermatozoa (both p < 0.001, Table II), but did not change the percentage of non-progressive motile spermatozoa (Table II) in comparison with the sham group. There were no considerable differences in these parameters in the groups of testosterones and NaHS alone compared to the varicocele group (Table II). However, the combined administration of testosterone and NaHS significantly increased the percentage of progressively motile spermatozoa and decreased the percentage of non-motile spermatozoa (p < 0.001 and p < 0.001, respectively), but did not change the percentage of non-progressive motile spermatozoa (Table II) compared to the varicocele group.
The varicocele induction in rats significantly decreased the percentage of viable spermatozoa compared to the sham group (p < 0.001, Table II). There were no considerable differences in the percentage of viable spermatozoa in the groups of testosterones and NaHS alone compared to the varicocele group (Table II). However, the combined administration of testosterone and NaHS remarkably enhanced the percentage of viable spermatozoa in comparison with the varicocele group (p < 0.001, Table II). The varicocele induction in rats significantly decreased the percentage of morphologically normal spermatozoa compared to the sham group (p < 0.001, Table II). There were no considerable differences in the percentage of morphologically normal spermatozoa in the groups of testosterones and NaHS alone compared to the varicocele group (Table II). However, the combined administration of testosterone and NaHS markedly enhanced the percentage of morphologically normal spermatozoa in comparison with the varicocele group (p = 0.01, Table II).
3.4. Effects of subeffective doses of testosterone and NaHS alone or in combination on morphometric features of the seminiferous tubules and number of the Sertoli cells and spermatogonia
The induction of varicocele in rats significantly decreased the tubular diameter and area, and thickness of the seminiferous epithelium of the seminiferous tubules in comparison with the sham group (all p < 0.001, Figures 3A-C, respectively, Table III). There were no considerable differences in these parameters in the groups of testosterones and NaHS alone compared to the varicocele group (Figures 3A-C, Table III). However, the combined administration of testosterone and NaHS significantly increased the tubular diameter and area, and thickness of the seminiferous epithelium of the seminiferous tubules in comparison with the varicocele group (all p < 0.001, Figures 3A-C, respectively, Table III). The varicocele induction in rats remarkably reduced the Sertoli cells and spermatogonia number compared to the sham group (p = 0.01 and p = 0.04, Figures 3D and E, respectively, Table III). There were no considerable differences in the number of the Sertoli cells and spermatogonia in the groups of testosterone and NaHS alone in comparison with the varicocele group (Figures 3D and E, respectively, Table III). However, the combined administration of testosterone and NaHS markedly enhanced the number of the Sertoli cells and spermatogonia in comparison with the varicocele group (both p = 0.03, Figures 3D and E, respectively, Table III).
3.5. Effects of subeffective doses of testosterone and NaHS alone or in combination on MDA levels and SOD activity in the testicular tissues
The varicocele induction in rats significantly increased testicular MDA levels compared to the sham group (p < 0.001, Figure 4A, Table IV). There were no considerable differences in testicular MDA levels in the groups of testosterone and NaHS alone than the varicocele group (Figure 4A, Table IV). However, the combined administration of testosterone and NaHS significantly decreased testicular MDA levels compared with the varicocele group (p < 0.001, Figure 4A, Table IV). The varicocele induction in rats significantly decreased testicular SOD activity compared to the sham group (p < 0.001, Figure 4B, Table IV). However, both the individual and combined administration of testosterone and NaHS significantly enhanced testicular SOD activity in comparison with the varicocele group (all p < 0.001, Figure 4B, Table IV).
3.6. Effects of subeffective doses of testosterone and NaHS alone or in combination on the ratio of Bax to Bcl-2 protein expression in the testicular tissues
The varicocele induction in rats considerably enhanced the ratio of Bax to Bcl-2 protein expression in the testicular tissues compared to the sham group (1.28 ± 0.08 vs. 0.20 ± 0.03, p < 0.001, Figure 5). There were no considerable differences in the ratio of Bax to Bcl-2 protein expression in the testicular tissues in the groups of testosterone (1.03 ± 0.13) and NaHS (1.19 ± 0.10) alone in comparison with the varicocele group (Figure 5). However, the combined administration of testosterone and NaHS significantly decreased the Bax to Bcl-2 protein expression ratio in the testicular tissues compared to the varicocele group (0.16 ± 0.01, 95% CI: 0.53-0.95, p < 0.001, Figure 5).









4. Discussion
In the current study, to the best of our knowledge for the first time, it was examined whether a subeffective dose of testosterone combined with a subeffective dose of H2S can improve varicocele-induced damages through a possible additive effect. As a starting point, we assessed the protective effects of the treatment regimen on the functional status of testicular tissues in varicocele rats since it is well known that varicocele causes testicular dysfunction (31). The first main function of the testes, steroidogenesis, is a multistep process by which cholesterol is converted to steroids such as testosterone (32). In this regard, preclinical and clinical studies have shown varicocele-induced impairment of steroidogenesis by a reduction in serum testosterone levels (33, 34). Similarly, this study observed a significant decrease in serum testosterone levels in rats subjected to varicocele. In addition, the subsequent histopathological examination of the testicular tissues confirmed this finding as it showed the Leydig cell atrophy in varicocele rats, indicating a reduction in testosterone-producing cells with varicocele induction. The second main function of the testes, spermatogenesis, is a process of multiple germ cell divisions to enhance their number and then differentiate into spermatozoa in the seminiferous tubules (35). Importantly, this process is highly dependent on testosterone, as it has been shown many times in the medical literature that hypospermatogenesis is associated with testosterone deficiency. Moreover, testosterone deficiency is reported to have detrimental effects on other sperm characteristics, including motility, viability, and morphology as well as morphometric features of the seminiferous tubules such as tubular diameter and thickness of the seminiferous epithelium (36). Accordingly, we found the deleterious effects of varicocele on these parameters along with decreased serum testosterone levels. However, from the therapeutic point of view, the combined administration of subeffective doses of testosterone and NaHS to varicocele rats reversed all the above pathological changes to the levels measured before varicocele induction, suggesting the improvement of testicular dysfunction.
In the next step, we evaluated the beneficial effects of the treatment regimen on testicular H2S levels in rats subjected to varicocele because it is thought that decreased H2S levels in testes contribute to varicocele-induced infertility (37). Our result showed a significant reduction in testicular H2S levels in varicocele, which was interestingly reversed by the combined administration of subeffective doses of testosterone and NaHS. Along with this, our histopathological examination of the testicular tissues revealed another interesting finding. In addition to the Leydig cell atrophy mentioned earlier, there was a considerable reduction in the number of Sertoli cells and spermatogonia in the testes of varicocele rats, which was markedly enhanced by the combined administration of subeffective doses of testosterone and NaHS. Importantly, in the male reproductive tract, H2S is mainly produced by cystathionine-β-synthase in the Leydig cells and cystathionine γ-lyase in the Sertoli cells and spermatogonia (13). Thus, our findings indicated the potential of this treatment regimen for protecting H2S-producing cells in testes and thereby maintaining normal testicular H2S levels.
After achieving such promising results from the treatment regimen, it was decided to elucidate some of the underlying molecular mechanisms. Varicocele-induced oxidative stress is well known to play a crucial role in damaging the male reproductive tract, including the testes (37). Oxidative stress is an imbalance between reactive oxygen species formation and their removal by antioxidant systems, leading to lipid peroxidation. Substantial evidence suggests that increased scrotal temperature due to retrograde blood flow to the pampiniform plexus is the most likely cause of oxidative stress induced by varicocele (38). In our study, the presence of oxidative stress, a significant increase in MDA levels is a major lipid peroxidation byproduct, and a decrease of SOD activity as an important antioxidant enzyme in the testicular tissues of varicocele rats were reported. From the therapeutic point of view, although the individual administration of subeffective doses of testosterone or NaHS to varicocele rats considerably enhanced testicular SOD activity, their co-administration provided better protection on lipid peroxidation and thus reduced oxidative stress in the testicular tissues.
In addition to its antioxidant effects, we evaluated the anti-apoptotic effects of the treatment regimen because it is indicated that varicocele-induced oxidative stress and apoptosis in the male reproductive tract are closely related processes (38). Although the mechanism is unknown, reactive oxygen species overproduction has been suggested to cause the efflux of cytochrome C from the mitochondria, which triggers signaling pathways for apoptosis, a type of cell death (39). In this terms, pro-apoptotic Bcl-2 family members (e.g., Bax), suppress the activity of anti-apoptotic Bcl-2 family members (e.g., Bcl-2) which is existing in the outer mitochondrial membrane, declaring that the ratio of pro- and anti-apoptotic Bcl-2 family members can be a good indicator to assess the occurrence of apoptosis (40). In this study, our findings showed a marked increase in the ratio of Bax to Bcl-2 protein expression levels in the testicular tissues of varicocele rats, which was remarkably reduced by the co-administration of subeffective doses of testosterone and NaHS.
The limitation of the present study was not evaluating the side effects of combined administration of subeffective doses of testosterone and NaHS in varicocele. Therefore, evaluating these side effects are suggested.
5. Conclusion
In the present study, the experimental varicocele caused decrease in the levels of serum testosterone and testicular H2S, as well as detrimental effects on sperm parameters and testicular histology. In contrast, the co-administration of subeffective doses of testosterone and the H2S donor NaHS, by reducing testicular oxidative stress and apoptosis through a possible additive effect, reversed all the above indicators to the levels measured before varicocele induction. Therefore, this study interestingly introduces a novel approach to benefit from the beneficial effects of testosterone therapy and decrease its side effects at the same time. Nevertheless, further preclinical investigations are needed to confirm the effectiveness of this therapeutic approach for clinical use.
Acknowledgments
This research was supported by Tehran University of Medical Sciences, Tehran, Iran (grant no = 45380). The authors are grateful to the Tehran University of Medical Sciences for supporting the present research.
Conflict of interest
The authors declare no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Physiology
References
1. 1. Hassanin AM, Ahmed HH, Kaddah AN. A global view of the pathophysiology of varicocele. Andrology 2018; 6: 654-661.
2. 2. Ohlander SJ, Lindgren MC, Lipshultz LI. Testosterone and male infertility. Urol Clin North AM 2016; 43: 195-202.
3. 3. Çayan S, Akbay E, Saylam B, Kadıoğlu A. Effect of varicocele and its treatment on testosterone in hypogonadal men with varicocele: Review of the literature. Balkan Med J 2020; 37: 121-124.
4. 4. Panach-Navarrete J, Morales-Giraldo A, Ferrandis-Cortes C, Garcia-Morata F, Pastor-Lence JC, Martínez-Jabaloyas JM. Is there a relationship between varicocele and testosterone levels? The Aging Male 2020; 23: 592-598.
5. 5. Bernie HL, Goldstein M. Varicocele repair versus testosterone therapy for older hypogonadal men with clinical varicocele and low testosterone. Eur Urol Focus 2018; 4: 314-316.
6. 6. Agarwal A, Sharma R, Harlev A, Esteves SC. Effect of varicocele on semen characteristics according to the new 2010 world health organization criteria: A systematic review and meta-analysis. Asian J Androl 2016; 18: 163-170.
7. 7. Birowo P, Rahendra Wijaya J, Atmoko W, Rasyid N. The effects of varicocelectomy on the DNA fragmentation index and other sperm parameters: A meta-analysis. Basic Clin Androl 2020; 30: 1-9.
8. 8. Jangkhah M, Farrahi F, Gilani MAS, Hosseini SJ, Dadkhah F, Salmanyazdi R, et al. Effects of varicocelectomy on serum testosterone levels among infertile men with varicocele. Int J Fertil Steril 2018; 12: 169-172.
9. 9. An Q, Zhang K, Fu L, Guo Y, Zhang C, Ge Z, et al. The impact of exogenous testosterone supplementation on spermatogenesis in a rat model of oligoasthenospermia. Int J Clin Exp Pathol 2020; 13: 1287-1299.
10. 10. Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: A review. Sex Med Rev 2018; 6: 106-113.
11. 11. Fusco F, Verze P, Capece M, Napolitano L. Suppression of spermatogenesis by exogenous testosterone. Curr Pharm Des 2021; 27: 2750-2753.
12. 12. Aroca A, Gotor C, Bassham DC, Romero LC. Hydrogen sulfide: From a toxic molecule to a key molecule of cell life. Antioxidants 2020; 9: 621.
13. 13. Wang J, Wang J, Shen T, Hong R, Tang S, Zhao X. H2S catalysed by CBS regulates testosterone synthesis through affecting the sulfhydrylation of PDE. J Cell Mol Med 2021; 25: 3460-3468.
14. 14. Azarbarz N, Shafiei Seifabadi Z, Moaiedi MZ, Mansouri E. Assessment of the effect of sodium hydrogen sulfide (hydrogen sulfide donor) on cisplatin-induced testicular toxicity in rats. Environ Sci Pollut Res Int 2020; 27: 8119-8128.
15. 15. Kang J, Jia Zh, Ping Y, Liu Z, Yan X, Xing G, et al. Testosterone alleviates mitochondrial ROS accumulation and mitochondria-mediated apoptosis in the gastric mucosa of orchiectomized rats. Arch Biochem Biophys 2018; 649: 53-59.
16. 16. Kianian F, Seifi B, Kadkhodaee M, Sajedizadeh A, Ahghari P. Protective effects of celecoxib on ischemia reperfusion-induced acute kidney injury: Comparing between male and female rats. Iran J Basic Med Sci 2019; 22: 43-48.
17. 17. Siregar S, Noegroho BS, Adriansjah R, Mustafa A, Bonar A. The effect of intratesticular injection of human adipose-derived mesenchymal cell on testicular oxidative stress and spermatogenesis process in the varicocele rat model. Res Rep Urol 2021; 13: 759-765.
18. 18. Turner TT. The study of varicocele through the use of animal models. Hum Reprod Update 2001; 7: 78-84.
19. 19. Fu L, Liu Y, Wang J, Sun Y, Zhang L, Wu T, et al. Cardioprotection by low-dose of estrogen and testosterone at the physiological ratio on ovariectomized rats during ischemia/reperfusion injury. J Cardiovas Pharmacol 2017; 70: 87-93.
20. 20. Khosravanian N, Razi M, Farokhi F, Khosravanian H. Testosterone and vitamin E administration up-regulated varicocele-reduced Hsp70-2 protein expression and ameliorated biochemical alterations. J Assist Reprod Genet 2014; 31: 341-354.
21. 21. Lorian K, Kadkhodaee M, Kianian F, Abdi A, Seifi B. Administration of sodium hydrosulfide reduces remote organ injury by an anti-oxidant mechanism in a rat model of varicocele. Iran J Basic Med Sci 2020; 23: 236-243.
22. 22. Hedayati Emami N, Mahmoudi Lafout F, Mohammadghasemi F. Administration of melatonin protects against acetylsalicylic acid-induced impairment of male reproductive function in mice. Iran J Basic Med Sci 2018; 21: 124-129.
23. 23. Boitrelle F, Shah R, Saleh R, Henkel R, Kandil H, Chung E, et al. The sixth edition of the WHO manual for human semen analysis: A critical review and SWOT analysis. Life (Basel) 2021; 11: 1368.
24. 24. Lorian K, Kadkhodaee M, Kianian F, Abdi A, Ranjbaran M, Ashabi G, et al. Long‐term NaHS administration reduces oxidative stress and apoptosis in a rat model of left‐side varicocele. Andrologia 2020; 52: e13496.
25. 25. Cecere JT. Eosin‐Nigrosin staining in the evaluation of sperm. In: Dascanio J, McCue P. Equine reproductive procedures. 2nd Ed. USA: John Wiley & Sons Inc; 2014: 373-376.
26. 26. Seifi B, Sajedizadeh A, Kadkhodaee M, Ranjbaran M. Long-term exercise restores hydrogen sulfide in the kidney and contributes to exercise benefits in 5/6 nephrectomized rats. Clin Exp Hypertens 2019; 41: 87-91.
27. 27. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol 1990; 186: 407-421.
28. 28. Kianian F, Seifi B, Kadkhodaee M, Sadeghipour HR, Ranjbaran M. Nephroprotection through modifying the apoptotic tnf-α/erk1/2/bax signaling pathway and oxidative stress by long-term sodium hydrosulfide administration in ovalbumin-induced chronic asthma. Immunol Invest 2022; 51: 602-618.
29. 29. Pourmirzaei F, Ranjbaran M, Kadkhodaee M, Kianian F, Lorian K, Abdi A, et al. Sperm and testicular dysfunction during cecal ligation and puncture-induced sepsis in male rats and effects of tannic acid through reducing testicular oxidative stress and inflammation. Iran J Basic Med Sci 2021; 24: 1554-1560.
30. 30. Talebi H, Farahpour MR, Hamishehkar H. The effectiveness of rutin for prevention of surgical induced endometriosis development in a rat model. Sci Rep 2021; 11: 7180.
31. 31. Zhang J, Jin PP, Gong M, Yi QT, Zhu RJ. Role of leptin and the leptin receptor in the pathogenesis of varicocele-induced testicular dysfunction. Mol Med Rep 2018; 17: 7065-7072.
32. 32. Miller WL. Steroidogenesis: Unanswered questions. Trends Endocrinol Metab 2017; 28: 771-793.
33. 33. Abdel-Meguid TA, Farsi HM, Al-Sayyad A, Tayib A, Mosli HA, Halawani AH. Effects of varicocele on serum testosterone and changes of testosterone after varicocelectomy: A prospective controlled study. Urology 2014; 84: 1081-1087.
34. 34. Wang K, Gao Y, Wang Ch, Liang M, Liao Y, Hu K. Role of oxidative stress in varicocele. Front Genet 2022; 13: 850114.
35. 35. Nishimura H, L’Hernault SW. Spermatogenesis. Curr Biol 2017; 27: R988-R994.
36. 36. Hayden RP, Tanrikut C. Testosterone and varicocele. Urol Clin North Am 2016; 43: 223-232.
37. 37. Lorian K, Kadkhodaee M, Kianian F, Abdi A, Sadeghipour H, Seifi B. Oxidative stress, nitric oxide and inflammation in the pathophysiology of varicocele and the effect of hydrogen sulfide as a potential treatment. Physiol Pharmacol 2019; 23: 249-260.
38. 38. Shiraishi K, Takihara H, Matsuyama H. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J Urol 2010; 28: 359-364.
39. 39. Gur FM, Timurkaan S, Taskin E, Guven C, Gur HE, Senturk M, et al. Thymoquinone improves testicular damage and sperm quality in experimentally varicocele‐induced adolescent rats. Andrologia 2021; 53: e14033.
40. 40. Flores‐Romero H, Hohorst L, John M, Albert MC, King LE, Beckmann L, et al. BCL‐2‐family protein tBID can act as a BAX‐like effector of apoptosis. EMBO J 2022; 41: e108690.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





