Sat, Jan 31, 2026
[Archive]
Volume 21, Issue 8 (August 2023)
IJRM 2023, 21(8): 599-618 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zamani P, Hemati Z, Kelishadi R, Kolahdozan S, Dianatinasab M, Keikha M. Association between anogenital distance as a noninvasive index in the diagnosis and prognosis of reproductive disorder: A systematic review. IJRM 2023; 21 (8) :599-618
URL: http://ijrm.ir/article-1-2472-en.html
URL: http://ijrm.ir/article-1-2472-en.html
Parisa Zamani1 

 , Zeinab Hemati2
, Zeinab Hemati2 

 , Roya Kelishadi2
, Roya Kelishadi2 

 , Sakineh Kolahdozan3
, Sakineh Kolahdozan3 

 , Mostafa Dianatinasab4
, Mostafa Dianatinasab4 

 , Mojtaba Keikha *5
, Mojtaba Keikha *5 




 , Zeinab Hemati2
, Zeinab Hemati2 

 , Roya Kelishadi2
, Roya Kelishadi2 

 , Sakineh Kolahdozan3
, Sakineh Kolahdozan3 

 , Mostafa Dianatinasab4
, Mostafa Dianatinasab4 

 , Mojtaba Keikha *5
, Mojtaba Keikha *5 


1- Student Research Committee, School of Nursing and Midwifery, Shahroud University of Medical Sciences, Shahroud, Iran.
2- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Clinical Research Development Unit, Bahar Hospital, Shahroud University of Medical Sciences, Shahroud, Iran.
4- Department of Medical Sciences, School of Medical and Life Sciences, Sunway University, Malaysia. Department of Complex Genetics and Epidemiology, School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, The Netherlands.
5- Department of Biostatistics and Epidemiology, Faculty of Public Health, Kerman University of Medical Sciences, Kerman, Iran. ,mr.mojtabakeikha@gmail.com
2- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Clinical Research Development Unit, Bahar Hospital, Shahroud University of Medical Sciences, Shahroud, Iran.
4- Department of Medical Sciences, School of Medical and Life Sciences, Sunway University, Malaysia. Department of Complex Genetics and Epidemiology, School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, The Netherlands.
5- Department of Biostatistics and Epidemiology, Faculty of Public Health, Kerman University of Medical Sciences, Kerman, Iran. ,
Full-Text [PDF 365 kb]
(1012 Downloads)
| Abstract (HTML) (1357 Views)
2.3. Data management
For removing the duplicate references and managing retrieved evidences we used EndNote software.
2.4. Data extraction and abstraction
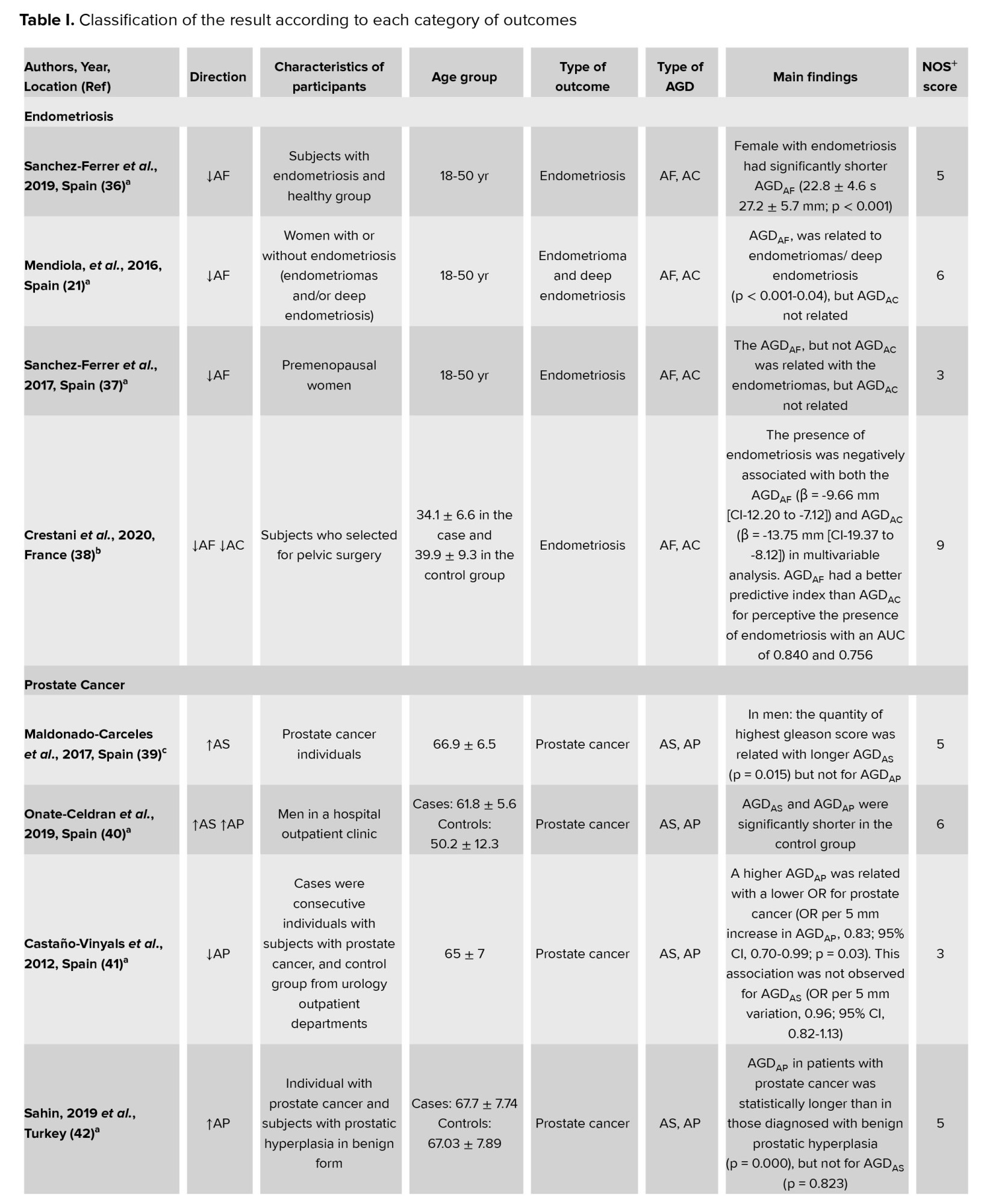
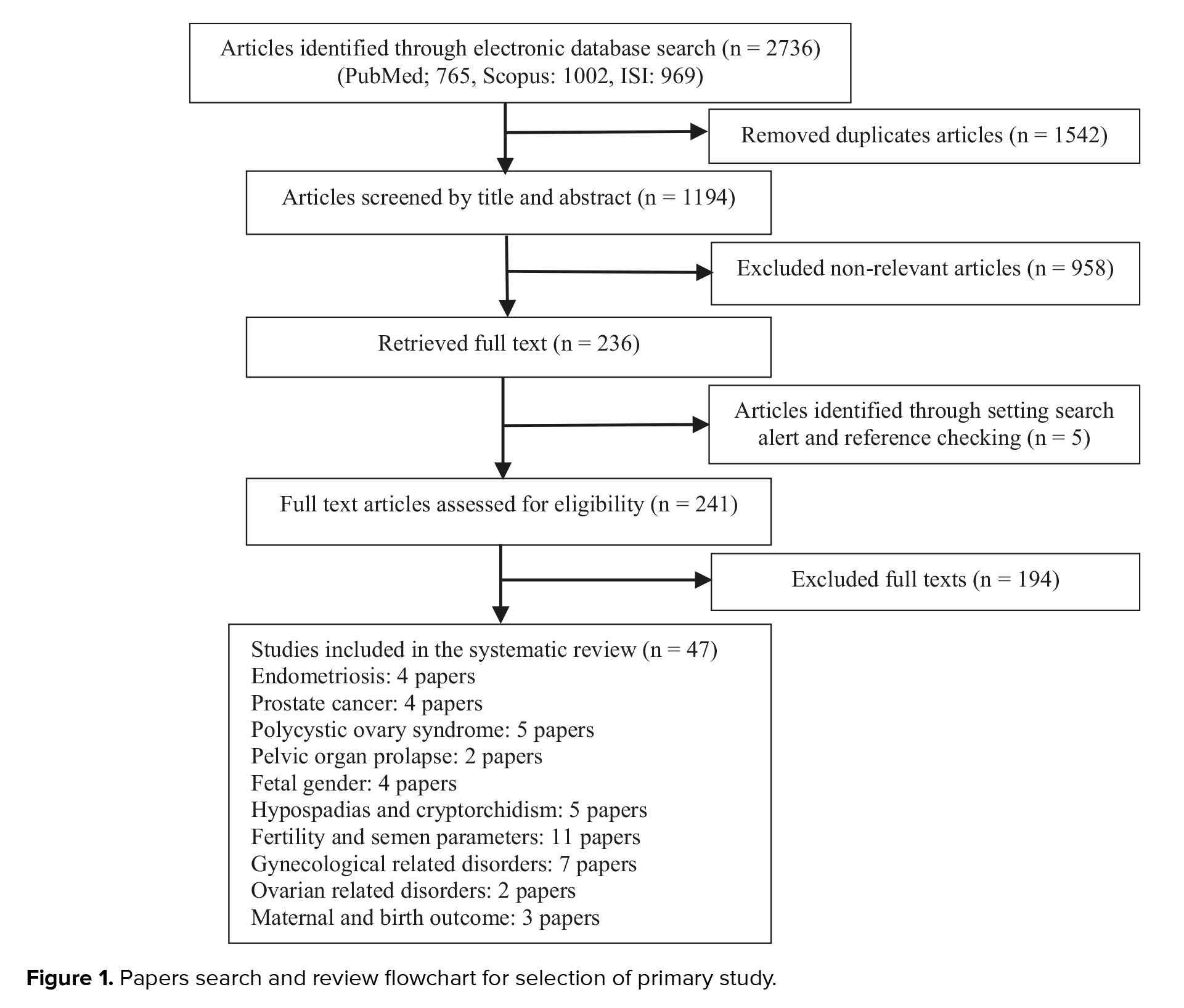
As presented in the PRISMA flowchart, we retrieved 1194 unique references after removing the duplicates. In total, 1542 articles were duplicated in basic search, found, and removed by the EndNote software. Another 958 were excluded after the title and abstract review. The full texts of the remaining 241 articles were retrieved and critically evaluated. This systematic review comprised 47 publications after the screening procedure (Figure 1).
2 independent reviewers (MK and PZ) reviewed the full text of publications identified by the literature search for their possible relevance or screened the titles and abstracts for inclusion in the review.
If there was a dispute, it was settled by consulting with a third reviewer (SK). The data was abstracted separately by 2 reviewers (ZH and MD). The following information was retrieved from all eligible papers: first author's name, year of publication, study location, age group, participant characteristics, study design, type of outcome, type of AGD, and key findings. According to high heterogeneity between included studies quantitative meta-analysis was not performed.
2.5. Quality assessment
The Newcastle-Ottawa Scale was used for assessing the quality of included studies (35). The Newcastle-Ottawa Scale score have 9 score and 3 main criteria. Each investigation was assessed based on 3 main criteria: 1) appropriate study population selection, 2) comparability of study groups, and 3) determination of the desired exposure (in cohort studies) or result (in case-control studies).
Each publication was evaluated independently by 2 reviewers. Disagreements were worked out via conversation until a consensus was reached. Studies with a score of 7 or more out of 9 were considered to be of high quality. Table I displays the results of each study's quality evaluation.


2.6. Ethical considerations
The present study was approved by Shahroud University of Medical Sciences Ethical Committee, Shahroud, Iran (Code: IR.SHMU.REC.1398.140).
3. Results
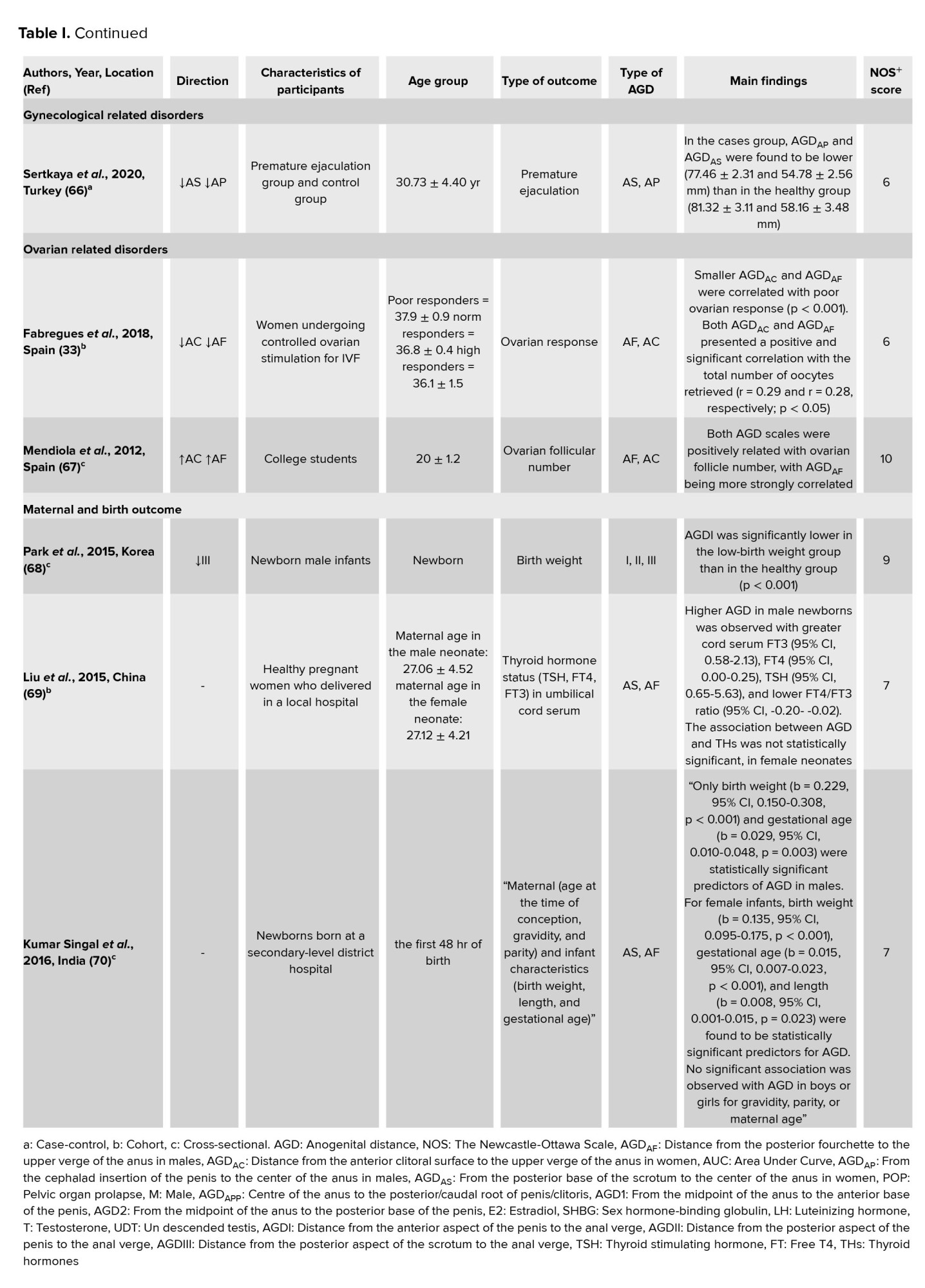
In this systematic review, we studied the association of AGD as a surrogate for diagnosing different diseases. Different outcomes have been studied, including endometriosis, prostate cancer, PCOS, POP, hypospadias, and cryptorchidism, fertility and semen parameters, maternal and birth outcomes, and ovarian and gynecological disorders related to pregnancy. The results of the included research are summarized in table I. We classified the results according to each outcome category.
Overall, a negative association was observed between AGD, endometriosis, and hypospadias, and a positive association between AGD and prostate cancer, PCOS, male fetal gender, and fertility parameters.
3.1. Quality assessments of studies
The methodological quality of the included articles according to NOS is provided in the supplementary file. Also, the overall NOS scores for the included studies are shown in table I. As shown, 22 high-quality and 12 medium-quality studies were included.
This is the first systematic review to assess the association of AGD as a non-invasive alternative to diagnostic and prognostic diseases requiring clinical intervention.
We did a comprehensive, systematic search of the literature to find studies that investigated the association of AGD as a non-invasive alternative to diagnostic and prognostic diseases. Important procedures, including searching, data extraction, and quality assessment, were also carried out independently by 2 experts.
3.2. Implications for clinical practice
Using quantitative indicators such as AGD to determine the prognosis and early diagnosis of diseases in the future may be of great help in therapeutic interventions and the treatment of individuals in the early stages of the disease.
3.3. AGD measurements and endometriosis
The current evidence showed that a shorter AGD was significantly associated with endometriosis. Few studies exist about AGD in women. Previous studies have shown that AGD was longer in women with a higher ovarian follicular number and higher testosterone levels (71), and disorder in the menstrual cycle before pregnancy (57). One study showed a strong relationship between endometriosis and shorter AGDAF (21). A case-control study of 114 women with endometriosis and 105 controls revealed that shorter AGD was seen in women with endometriosis (36). A prospective cohort study among 168 women over 18 yr old showed that the diagnosis of endometriosis was negatively associated with both the AGDAF and the AGDAC, and the AGDAF had a better predictive value than the AGDAC for discriminating the presence of endometriosis (38). Another study suggests that AGD biomarkers may be useful in diagnosing endometriosis in women (37). AGD is a bidirectional marker that is shorter in women with exposure to estrogens, for example, endometriosis, and in women with exposure to antiandrogens, such as phthalates (72). On the other hand, AGD is longer in a woman with relatively high levels of androgens, like PCOS. Therefore, AGD is a biomarker that may be useful in identifying the intrauterine environment from the prenatal period to adulthood (73).
3.4. AGD measurements and prostate cancer
Based on the included evidence, a correlation was observed between AGD and prostate cancer. One study demonstrated that AGDAP was higher in individuals with prostate cancer than in cases of prostatic hyperplasia (42). Another cross-sectional study among 120 prostate cancer patients showed that AGDAS was positively associated with the highest Gleason score and D'Amico nomogram (39). A previous study in 60 men with prostate cancer and 52 urological controls in 2 hospitals in Barcelona found that longer AGD in men with normal in-utero sexual development was related with a lower risk of prostate cancer (41). The results of 2 studies conducted on adult men showed that disconnection of androgen-mediated pathways in utero was related with the risk of prostate cancer (40). Therefore, having a longer AGDAS mention a higher chance of having higher testosterone in adult and, finally, a great risk of suffering a more strict form of prostate cancer (74).
3.5. AGD measurements and PCOS
Studies included in our systematic review showed that AGD measurements were longer in the PCOS group (31, 45). A case-control study of 156 PCOS cases and 180 reproductively healthy women showed that AGD was longer in individuals with PCOS than in the control group (31). A cohort study reported that AGD was more prevalent in newborn daughters of women with PCOS compared with a control group with no PCOS. This study suggested AGD may be a potential marker of the downstream risk of PCOS (44). The evidence confirm earlier study that identified during PCOS, women fetuses may expose higher T levels and suggest that AGD may supply postnatal ‘read-out’ of their prior intrauterine hormonal environment (75).
A cross-sectional study among healthy young women found that AGD was positively associated with the number of ovarian follicles. This relationship was confirmed in women with PCOS, suggesting that high prenatal testosterone levels and follicular growth in PCOS, may have common fetal origins (67). Indeed, androgen exposure in utero increased follicular recruitment in females (76).
Results from a descriptive study applying a retrospective list review of 128 patients aged 12-20, showed that androgen exposure in utero increased serum anti-Müllerian hormone, a marker for PCOS (77). Previous studies revealed that intrauterine androgen exposure increases the length of AGD (78, 79). Therefore, exposures to intrauterine and postnatal androgens are associated with PCOS and may also affect the length of AGD. Finally, AGD may have clinical utility when measuring human fetal androgen levels during pregnancy (43).
3.6. AGD measurements and POP
According to evidences included in the study, significant differences were observed between POP individuals and the controls for AGDAC and AGDAF. In that study, women with POP had longer AGDAC (46). A case-control study among 58 patients showed differences between the AGDAF (which is shorter in cases of prolapse), AGDAC distances, and length of genital hiatus (which is longer in cases) (29). As a result, AGD is presently utilized to quantify the volume of vaginal region hiatus in women with POP since it is less expensive and has a more accessible approach than other methods (80, 81). Hence, AGD may be a more accessible marker for clinical use in calculating the amount of the genital region hiatus in prolapses.
3.7. AGD measurements and fetal gender
Based on the study included in the current systematic review, AGD is a novel biomarker that may play a role in determining fetal sex. Few studies exist about fetal AGD and the differences between females and males. A previous cross-sectional study found that measuring AGD in the first trimester of pregnancy is a novel method for determining fetal sex. In that study, AGD was greater in male fetuses than in female fetuses (49). A previous study of 111 cases with a singleton pregnancy between 11 and 13 wk and 6 days found that when ultrasound detected AGD 4.8 mm, the likelihood of the pregnancy being female increased (11). Some evidence reported that AGD was significantly shorter in females than in males (48, 49). Previous study fetal gender was recognize by ultrasound in 310 singleton pregnancies at 11-14 wk of gestation, explain that a cut-off of 4.8 mm was determined to predict male (≥ 4.8 mm) or female (< 4.8 mm) fetuses (47). These results are in agreement with those of the Fowler study (48) although the cut-off value in this study (47), was 4.8 mm as opposed to 5 mm. This 4.8 mm cut-off demonstrated a high accuracy of AGD in specify male from female fetuses, resulting in sex determination in 87% of the males and 89% of the females. The results of a previous study in 87 term neonates (38 ≥ wk) showed that AGD was twice as common in male infants (average 22 mm) as in female infants (average 11 mm) (82).
3.8. AGD measurement, hypospadias, and cryptorchidism
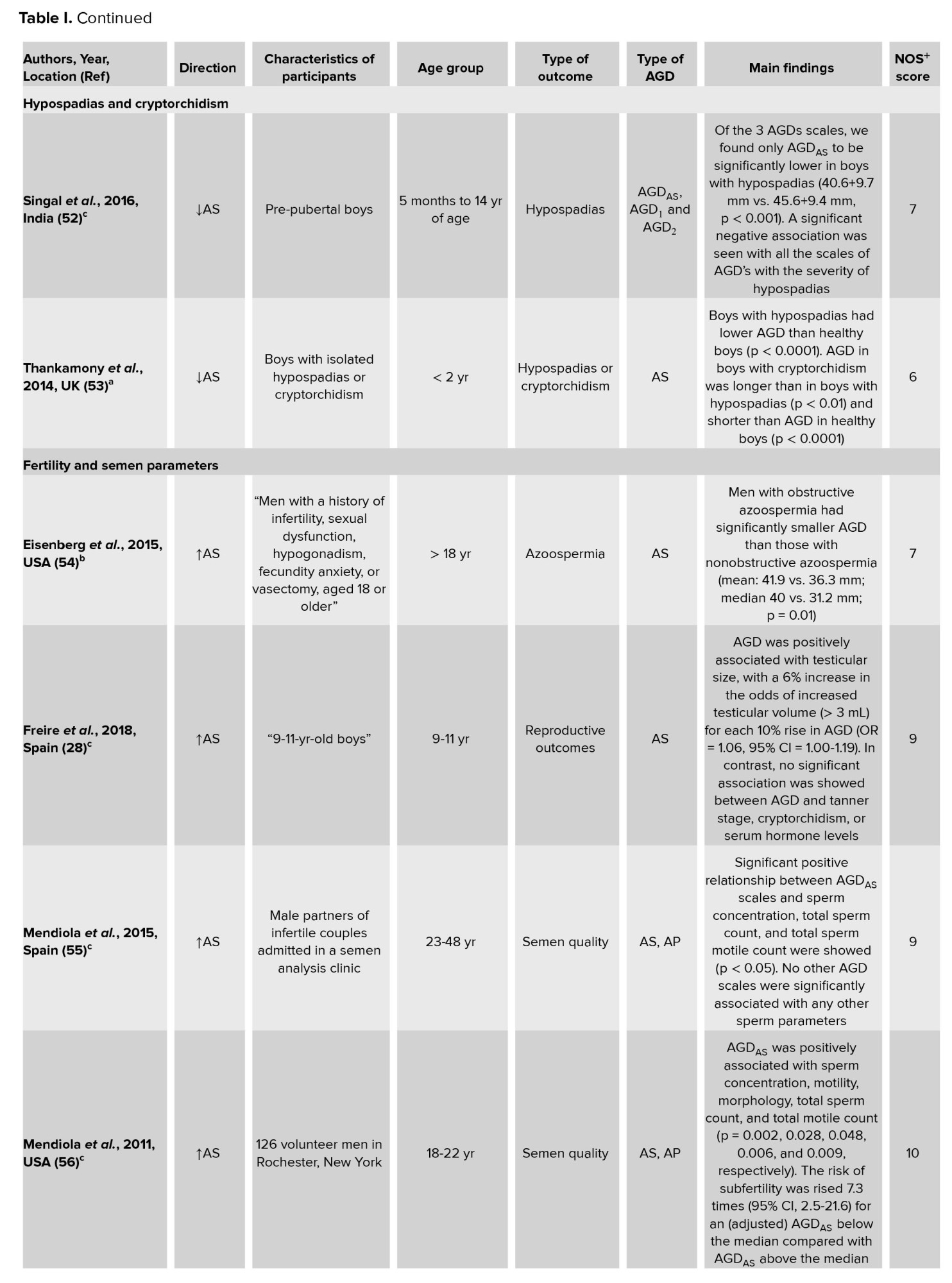
The results of the current systematic review reported a positive relationship between AGD, hypospadias, and cryptorchidism (50). The findings of the studies included in our study revealed that, when compared to the general population, shorter AGD were statistically significant in fetuses with hypospadias (30). Results of a systematic review and meta-analysis showed that AGD was shorter in boys with hypospadias and cryptorchidism (83). In a large cohort study of boys of pre-pubertal age, AGD was significantly shorter in boys with hypospadias compared to boys with normal genitalia in the healthy control group (52). Another study among boys < 2 yr of age diagnosed with cryptorchidism or isolated hypospadias and recruited from clinics at Cambridge University hospital showed that boys with hypospadias or cryptorchidism had significantly lower AGD and penile length than the healthy control group (53). 2 previous studies have shown the relationship between shortened AGD and cryptorchidism. Also, the latter study reported reduced AGD in boys with hypospadias (51, 84). One study among 52 fetuses suggested that, in the prenatal examination and counseling of male external genital abnormalities, AGD may be used as a supplemental objective sonographic measure (30).
3.9. AGD measurements, fertility and semen parameters
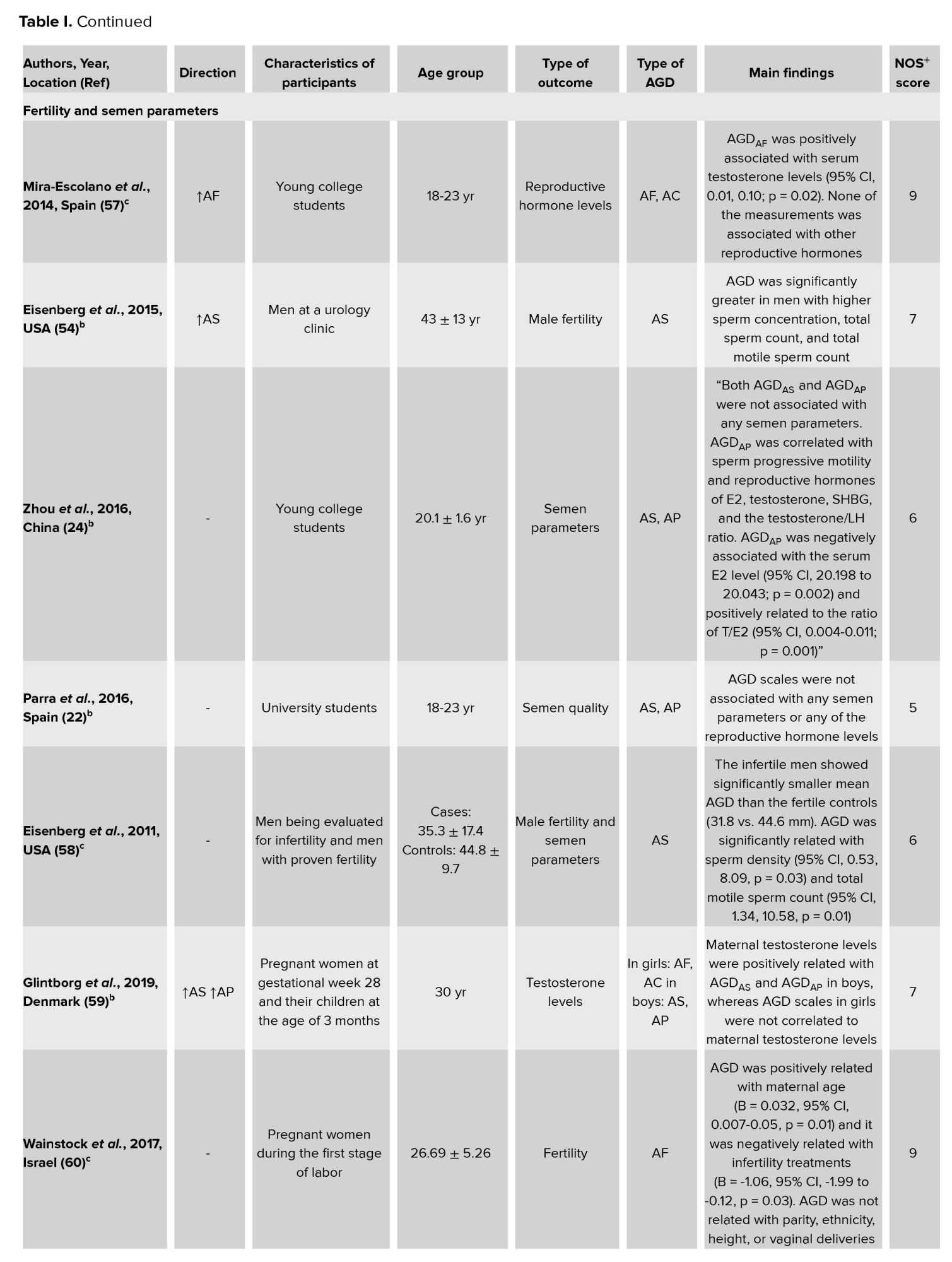
The present study shows that the majority of the studies demonstrated that AGD was related to semen parameters and fertility in men (60). A study among Spanish children aged between 9 and 11 yr, reported that longer AGD was positively associated with increased testicular volume (28). One study among 473 men showed that AGD was related to semen parameters. In that study, longer AGD was related with great sperm concentration, total sperm count, and total motile sperm count (54).
A cross-sectional study among infertile men aged between 25 and 38 yr detected a positive relationship between AGDAS and total sperm count, sperm concentration, and total sperm motile count (55). Similar results in another study conducted in the US on male students (56) and infertile men referred to andrology clinics (58) have been reported. However, study results among Caucasian young men from southern Spain reported that AGD was not related to the semen parameter (22), which is in opposite to the above results (56, 58, 59). However, the reasons for the controversial findings to date are not yet clear, but they could according to differences in studied age ranges, residual confounding, or even ethnic factors.
To our knowledge, the current reports represent the first presentation of the use of assessing AGD in clinical practice to assist patient care. AGD may prognosis normal male genital growth and sperm generation and could therefore provide a new tool to determine reproductive potential in men. Moreover, it may give the professionals additional prognostic information when counseling azoospermic men. Therefore, the results of this systematic review suggested that AGD can help diagnose reproductive function and infertility in men.
3.10. AGD measurements and gynecological related disorders
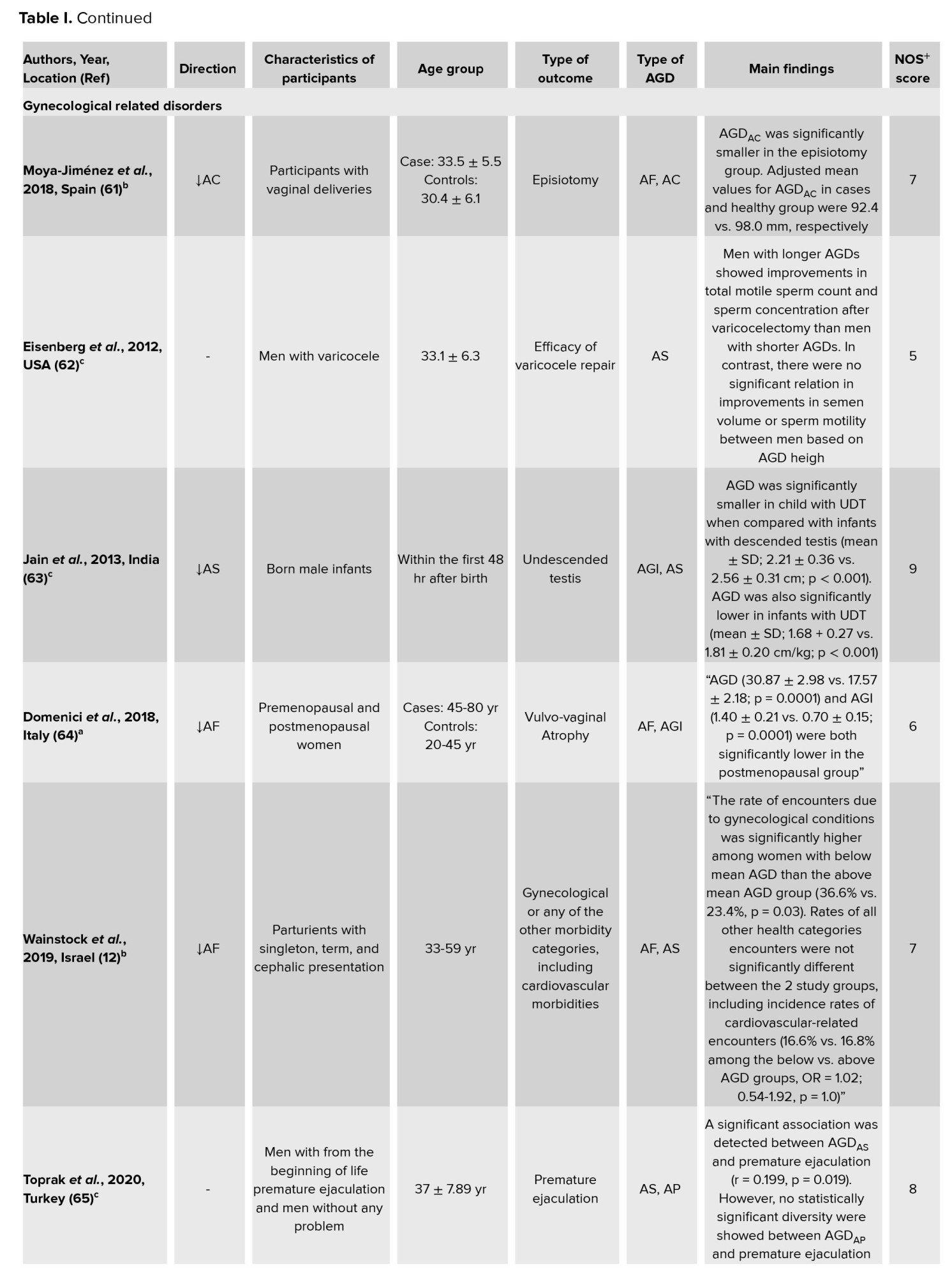
The findings of the studies in this systematic review demonstrated the relationship between AGD and gynecological conditions in women (64). A prospective cohort of 300 fertile women showed that, when comparing women with below-average AGD to those with above average AGD, the incidence of encounters owing to gynecological issues was much greater (12). Another cohort study among 119 women suggested measures of AGD as risk factors for episiotomy. That study introduced in the episiotomy group AGDAC was significantly shorter (61). Another study measured AGD in pregnant women (85). Better AGD measurements in women could thus be added to routine gynecological assessment and admission in the delivery room, providing critical data in women at risk for later gynecological morbidities (12). Another study among 1154 Indian infants reported that, compared with infants with descended testes, AGD was significantly shorter in infants with undescended testes (63). A prospective, observational study among 150 adult men aged 18-55 yr showed that in the premature ejaculation group, AGDAP and AGDAS were lower than in the control group (66). Therefore, AGD may be clinically useful as a measure of androgen action during pregnancy (65). Studies have shown that fertility and adult sperm production are related to AGD (56, 58). A cohort study suggested that total motile sperm count and sperm concentration after varicocelectomy were associated with longer AGD in men. However, no relationship was observed between improvements in semen volume or sperm motility and AGD length (62).
3.11. AGD measurements and ovarian follicular number
According to studies included in the present systematic review, poor ovarian response was associated with shorter AGDAC and AGDAF. In that study, AGDAC and AGDAF were positive and significant in relation to the total number of oocytes (33). A cross-sectional study reported that AGDAF was positively associated with ovarian follicle number (67). Some previous studies suggested that AGD length is associated with female reproductive function (57, 67, 71, 86). More studies reported that AGD was longer in women with PCOS (31), and daughters born of these women had longer AGD (78). So, our results may contribute to the claim for using AGD as a marker of the intrauterine hormonal milieu in epidemiological and clinical research.
3.12. AGD measurements, maternal and birth outcome
The studies included in our systematic review found links between AGD and maternal and infant characteristics. A cross-sectional study among 133 Korean infants reported that AGD1-3 in the low-birth-weight group were significantly lower than newborns in the control group with normal birth weight (68). A previous study showed that AGD in males was significantly associated with birth weight and gestational age. In that study, AGD in females was associated with birth weight, gestational age, and length (70). Another study demonstrated that AGD was longer in a male newborn with higher cord serum free triiodothyronine, free thyroxine, thyroid-stimulating hormone, and a lower thyroxine/triiodothyronine ratio. In that study, the association between AGD and thyroid-stimulating hormone was not statistically significant in the female neonate (69). A feasible practice is that T3 enhance skeletal muscle growth by adding the frequency and dimension of muscle fibers between the anus and the genital near the central perineal tendon (87). Another possibility is that the placenta influences fetal thyroid hormones and AGD (88).
4. Conclusion
Using quantitative indicators such as AGD to determine the prognosis and early diagnosis of diseases in the future may be of great help in therapeutic interventions and the treatment of cases in the early stages of the disease. Along the way, we should not neglect variables such as age, gender, and stressful life events that may confound the relationship pathway between AGD and disorders.
Author’s statement
We declare that this manuscript is original, has not been previously published or submitted elsewhere for publication, and is not under consideration by another journal.
Acknowledgments
The present study was supported by deputy of research of Shahroud University of Medical Sciences, Shahroud, Iran (grant no: 98120).
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (352 Views)
1. Introduction
Several lifestyle factors affect human reproductive performance (1). Exposure to endocrine-disrupting chemicals in fetal life can be concerning because the sexual organs are formed in this period, which is the foundation of reproductive health in adulthood (2, 3). Reproductive disorders and anogenital problems can be debilitating and negatively impact people's quality of life (4, 5). In the past decades, reproductive disorders and anogenital problems have increased dramatically (6-8).
Diagnostic procedures may generally be associated with health-related complications (9, 10). Some other methods result in high financial costs and time for the individuals and delay the “golden time” for diagnosis and treatment of the disease (11). Therefore, a noninvasive indicator that predicts and diagnoses the disease earlier seems important.
Hormonal dysfunction during fetal life can cause anogenital problems in adulthood (12-14). As a result, we can use anogenital distance (AGD) as a proxy for hormonal dysfunction in the past to determine whether or not a hormonally disruptive action occurred during fetal growth (15, 16). AGD is a noninvasive and easily measurable anthropometric measurement, the distance between the anus and the genital tubercle (17-20). It is considered the amount of distance between the anterior clitoral surface to the upper verge of the anus (AGDAC) and distance between posterior fourchette to the upper verge of the anus in women (AGDAF) (21). It is indicated the amount of distance between cephalad insertion of the penis to the center of the anus (AGDAP) and distance between posterior base of the scrotum to the center of the anus in male (AGDAS) (22).
AGD has been used as an indicator of fetal androgen dysfunction and as an adverse outcome in adulthood (23). AGD is a broad marker that retrospectively describes fetal androgen disruption and potentially adult reproductive disorders (24). Intrauterine hormonal changes can affect AGD (25, 26). Thus, AGD is a biomarker of prenatal hormonal exposure at birth and can also reflect reproductive health in adulthood (12, 27). In men, correlations have been found between the length of AGD and semen quality, testicular volume, hypospadias, and cryptorchidism (28-31). Also, in women, AGD is a potential determinant of some female gynecologic and reproductive disorders, such as polycystic ovary syndrome (PCOS), endometriosis, and pelvic organ prolapse (POP) (31-33). AGD can also determine the gender of the fetus in the first trimester of pregnancy (11).
There are large numbers of studies supporting the ability of AGD as a tool for disorder prediction. Thus, in this study, we have systematically summarized the available evidence related to assessing the association of AGD in the diagnosis and prediction of different health conditions.
2. Materials and Methods
2.1. Literature search
The current systematic review was performed according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (34). The electronic databases, including Medline via PubMed, Scopus, and ISI Web of Knowledge up to July 30, 2021 were systematically searched. The following keywords were used: "anogenital distance" OR "anogenital index" OR "ano genital distance" OR "ano genital index". Boolean Operators (AND, OR) were used in search strategy. Also, we review the reference of all included paper for any related articles. No any language limitation considered in the search strategy.
2.2. Selection criteria
The below criteria were used for selecting studies.
Inclusion criteria:
Several lifestyle factors affect human reproductive performance (1). Exposure to endocrine-disrupting chemicals in fetal life can be concerning because the sexual organs are formed in this period, which is the foundation of reproductive health in adulthood (2, 3). Reproductive disorders and anogenital problems can be debilitating and negatively impact people's quality of life (4, 5). In the past decades, reproductive disorders and anogenital problems have increased dramatically (6-8).
Diagnostic procedures may generally be associated with health-related complications (9, 10). Some other methods result in high financial costs and time for the individuals and delay the “golden time” for diagnosis and treatment of the disease (11). Therefore, a noninvasive indicator that predicts and diagnoses the disease earlier seems important.
Hormonal dysfunction during fetal life can cause anogenital problems in adulthood (12-14). As a result, we can use anogenital distance (AGD) as a proxy for hormonal dysfunction in the past to determine whether or not a hormonally disruptive action occurred during fetal growth (15, 16). AGD is a noninvasive and easily measurable anthropometric measurement, the distance between the anus and the genital tubercle (17-20). It is considered the amount of distance between the anterior clitoral surface to the upper verge of the anus (AGDAC) and distance between posterior fourchette to the upper verge of the anus in women (AGDAF) (21). It is indicated the amount of distance between cephalad insertion of the penis to the center of the anus (AGDAP) and distance between posterior base of the scrotum to the center of the anus in male (AGDAS) (22).
AGD has been used as an indicator of fetal androgen dysfunction and as an adverse outcome in adulthood (23). AGD is a broad marker that retrospectively describes fetal androgen disruption and potentially adult reproductive disorders (24). Intrauterine hormonal changes can affect AGD (25, 26). Thus, AGD is a biomarker of prenatal hormonal exposure at birth and can also reflect reproductive health in adulthood (12, 27). In men, correlations have been found between the length of AGD and semen quality, testicular volume, hypospadias, and cryptorchidism (28-31). Also, in women, AGD is a potential determinant of some female gynecologic and reproductive disorders, such as polycystic ovary syndrome (PCOS), endometriosis, and pelvic organ prolapse (POP) (31-33). AGD can also determine the gender of the fetus in the first trimester of pregnancy (11).
There are large numbers of studies supporting the ability of AGD as a tool for disorder prediction. Thus, in this study, we have systematically summarized the available evidence related to assessing the association of AGD in the diagnosis and prediction of different health conditions.
2. Materials and Methods
2.1. Literature search
The current systematic review was performed according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (34). The electronic databases, including Medline via PubMed, Scopus, and ISI Web of Knowledge up to July 30, 2021 were systematically searched. The following keywords were used: "anogenital distance" OR "anogenital index" OR "ano genital distance" OR "ano genital index". Boolean Operators (AND, OR) were used in search strategy. Also, we review the reference of all included paper for any related articles. No any language limitation considered in the search strategy.
2.2. Selection criteria
The below criteria were used for selecting studies.
Inclusion criteria:
- All quantitative research evaluated the association of AGD in diagnosing or prognosis of any disorder or disease.
- Studies that have a proper definition of AGD.
- All observational and comparative studies such as cross-sectional, case-control, and cohort studies.
Exclusion criteria:
- All studies that are not the original studies such as letter, editorial.
- Studies conducted on animals.
- Laboratory and in vivo studies.
2.3. Data management
For removing the duplicate references and managing retrieved evidences we used EndNote software.
2.4. Data extraction and abstraction
As presented in the PRISMA flowchart, we retrieved 1194 unique references after removing the duplicates. In total, 1542 articles were duplicated in basic search, found, and removed by the EndNote software. Another 958 were excluded after the title and abstract review. The full texts of the remaining 241 articles were retrieved and critically evaluated. This systematic review comprised 47 publications after the screening procedure (Figure 1).
2 independent reviewers (MK and PZ) reviewed the full text of publications identified by the literature search for their possible relevance or screened the titles and abstracts for inclusion in the review.
If there was a dispute, it was settled by consulting with a third reviewer (SK). The data was abstracted separately by 2 reviewers (ZH and MD). The following information was retrieved from all eligible papers: first author's name, year of publication, study location, age group, participant characteristics, study design, type of outcome, type of AGD, and key findings. According to high heterogeneity between included studies quantitative meta-analysis was not performed.
2.5. Quality assessment
The Newcastle-Ottawa Scale was used for assessing the quality of included studies (35). The Newcastle-Ottawa Scale score have 9 score and 3 main criteria. Each investigation was assessed based on 3 main criteria: 1) appropriate study population selection, 2) comparability of study groups, and 3) determination of the desired exposure (in cohort studies) or result (in case-control studies).
Each publication was evaluated independently by 2 reviewers. Disagreements were worked out via conversation until a consensus was reached. Studies with a score of 7 or more out of 9 were considered to be of high quality. Table I displays the results of each study's quality evaluation.








2.6. Ethical considerations
The present study was approved by Shahroud University of Medical Sciences Ethical Committee, Shahroud, Iran (Code: IR.SHMU.REC.1398.140).
3. Results
In this systematic review, we studied the association of AGD as a surrogate for diagnosing different diseases. Different outcomes have been studied, including endometriosis, prostate cancer, PCOS, POP, hypospadias, and cryptorchidism, fertility and semen parameters, maternal and birth outcomes, and ovarian and gynecological disorders related to pregnancy. The results of the included research are summarized in table I. We classified the results according to each outcome category.
Overall, a negative association was observed between AGD, endometriosis, and hypospadias, and a positive association between AGD and prostate cancer, PCOS, male fetal gender, and fertility parameters.
3.1. Quality assessments of studies
The methodological quality of the included articles according to NOS is provided in the supplementary file. Also, the overall NOS scores for the included studies are shown in table I. As shown, 22 high-quality and 12 medium-quality studies were included.
This is the first systematic review to assess the association of AGD as a non-invasive alternative to diagnostic and prognostic diseases requiring clinical intervention.
We did a comprehensive, systematic search of the literature to find studies that investigated the association of AGD as a non-invasive alternative to diagnostic and prognostic diseases. Important procedures, including searching, data extraction, and quality assessment, were also carried out independently by 2 experts.
3.2. Implications for clinical practice
Using quantitative indicators such as AGD to determine the prognosis and early diagnosis of diseases in the future may be of great help in therapeutic interventions and the treatment of individuals in the early stages of the disease.
3.3. AGD measurements and endometriosis
The current evidence showed that a shorter AGD was significantly associated with endometriosis. Few studies exist about AGD in women. Previous studies have shown that AGD was longer in women with a higher ovarian follicular number and higher testosterone levels (71), and disorder in the menstrual cycle before pregnancy (57). One study showed a strong relationship between endometriosis and shorter AGDAF (21). A case-control study of 114 women with endometriosis and 105 controls revealed that shorter AGD was seen in women with endometriosis (36). A prospective cohort study among 168 women over 18 yr old showed that the diagnosis of endometriosis was negatively associated with both the AGDAF and the AGDAC, and the AGDAF had a better predictive value than the AGDAC for discriminating the presence of endometriosis (38). Another study suggests that AGD biomarkers may be useful in diagnosing endometriosis in women (37). AGD is a bidirectional marker that is shorter in women with exposure to estrogens, for example, endometriosis, and in women with exposure to antiandrogens, such as phthalates (72). On the other hand, AGD is longer in a woman with relatively high levels of androgens, like PCOS. Therefore, AGD is a biomarker that may be useful in identifying the intrauterine environment from the prenatal period to adulthood (73).
3.4. AGD measurements and prostate cancer
Based on the included evidence, a correlation was observed between AGD and prostate cancer. One study demonstrated that AGDAP was higher in individuals with prostate cancer than in cases of prostatic hyperplasia (42). Another cross-sectional study among 120 prostate cancer patients showed that AGDAS was positively associated with the highest Gleason score and D'Amico nomogram (39). A previous study in 60 men with prostate cancer and 52 urological controls in 2 hospitals in Barcelona found that longer AGD in men with normal in-utero sexual development was related with a lower risk of prostate cancer (41). The results of 2 studies conducted on adult men showed that disconnection of androgen-mediated pathways in utero was related with the risk of prostate cancer (40). Therefore, having a longer AGDAS mention a higher chance of having higher testosterone in adult and, finally, a great risk of suffering a more strict form of prostate cancer (74).
3.5. AGD measurements and PCOS
Studies included in our systematic review showed that AGD measurements were longer in the PCOS group (31, 45). A case-control study of 156 PCOS cases and 180 reproductively healthy women showed that AGD was longer in individuals with PCOS than in the control group (31). A cohort study reported that AGD was more prevalent in newborn daughters of women with PCOS compared with a control group with no PCOS. This study suggested AGD may be a potential marker of the downstream risk of PCOS (44). The evidence confirm earlier study that identified during PCOS, women fetuses may expose higher T levels and suggest that AGD may supply postnatal ‘read-out’ of their prior intrauterine hormonal environment (75).
A cross-sectional study among healthy young women found that AGD was positively associated with the number of ovarian follicles. This relationship was confirmed in women with PCOS, suggesting that high prenatal testosterone levels and follicular growth in PCOS, may have common fetal origins (67). Indeed, androgen exposure in utero increased follicular recruitment in females (76).
Results from a descriptive study applying a retrospective list review of 128 patients aged 12-20, showed that androgen exposure in utero increased serum anti-Müllerian hormone, a marker for PCOS (77). Previous studies revealed that intrauterine androgen exposure increases the length of AGD (78, 79). Therefore, exposures to intrauterine and postnatal androgens are associated with PCOS and may also affect the length of AGD. Finally, AGD may have clinical utility when measuring human fetal androgen levels during pregnancy (43).
3.6. AGD measurements and POP
According to evidences included in the study, significant differences were observed between POP individuals and the controls for AGDAC and AGDAF. In that study, women with POP had longer AGDAC (46). A case-control study among 58 patients showed differences between the AGDAF (which is shorter in cases of prolapse), AGDAC distances, and length of genital hiatus (which is longer in cases) (29). As a result, AGD is presently utilized to quantify the volume of vaginal region hiatus in women with POP since it is less expensive and has a more accessible approach than other methods (80, 81). Hence, AGD may be a more accessible marker for clinical use in calculating the amount of the genital region hiatus in prolapses.
3.7. AGD measurements and fetal gender
Based on the study included in the current systematic review, AGD is a novel biomarker that may play a role in determining fetal sex. Few studies exist about fetal AGD and the differences between females and males. A previous cross-sectional study found that measuring AGD in the first trimester of pregnancy is a novel method for determining fetal sex. In that study, AGD was greater in male fetuses than in female fetuses (49). A previous study of 111 cases with a singleton pregnancy between 11 and 13 wk and 6 days found that when ultrasound detected AGD 4.8 mm, the likelihood of the pregnancy being female increased (11). Some evidence reported that AGD was significantly shorter in females than in males (48, 49). Previous study fetal gender was recognize by ultrasound in 310 singleton pregnancies at 11-14 wk of gestation, explain that a cut-off of 4.8 mm was determined to predict male (≥ 4.8 mm) or female (< 4.8 mm) fetuses (47). These results are in agreement with those of the Fowler study (48) although the cut-off value in this study (47), was 4.8 mm as opposed to 5 mm. This 4.8 mm cut-off demonstrated a high accuracy of AGD in specify male from female fetuses, resulting in sex determination in 87% of the males and 89% of the females. The results of a previous study in 87 term neonates (38 ≥ wk) showed that AGD was twice as common in male infants (average 22 mm) as in female infants (average 11 mm) (82).
3.8. AGD measurement, hypospadias, and cryptorchidism
The results of the current systematic review reported a positive relationship between AGD, hypospadias, and cryptorchidism (50). The findings of the studies included in our study revealed that, when compared to the general population, shorter AGD were statistically significant in fetuses with hypospadias (30). Results of a systematic review and meta-analysis showed that AGD was shorter in boys with hypospadias and cryptorchidism (83). In a large cohort study of boys of pre-pubertal age, AGD was significantly shorter in boys with hypospadias compared to boys with normal genitalia in the healthy control group (52). Another study among boys < 2 yr of age diagnosed with cryptorchidism or isolated hypospadias and recruited from clinics at Cambridge University hospital showed that boys with hypospadias or cryptorchidism had significantly lower AGD and penile length than the healthy control group (53). 2 previous studies have shown the relationship between shortened AGD and cryptorchidism. Also, the latter study reported reduced AGD in boys with hypospadias (51, 84). One study among 52 fetuses suggested that, in the prenatal examination and counseling of male external genital abnormalities, AGD may be used as a supplemental objective sonographic measure (30).
3.9. AGD measurements, fertility and semen parameters
The present study shows that the majority of the studies demonstrated that AGD was related to semen parameters and fertility in men (60). A study among Spanish children aged between 9 and 11 yr, reported that longer AGD was positively associated with increased testicular volume (28). One study among 473 men showed that AGD was related to semen parameters. In that study, longer AGD was related with great sperm concentration, total sperm count, and total motile sperm count (54).
A cross-sectional study among infertile men aged between 25 and 38 yr detected a positive relationship between AGDAS and total sperm count, sperm concentration, and total sperm motile count (55). Similar results in another study conducted in the US on male students (56) and infertile men referred to andrology clinics (58) have been reported. However, study results among Caucasian young men from southern Spain reported that AGD was not related to the semen parameter (22), which is in opposite to the above results (56, 58, 59). However, the reasons for the controversial findings to date are not yet clear, but they could according to differences in studied age ranges, residual confounding, or even ethnic factors.
To our knowledge, the current reports represent the first presentation of the use of assessing AGD in clinical practice to assist patient care. AGD may prognosis normal male genital growth and sperm generation and could therefore provide a new tool to determine reproductive potential in men. Moreover, it may give the professionals additional prognostic information when counseling azoospermic men. Therefore, the results of this systematic review suggested that AGD can help diagnose reproductive function and infertility in men.
3.10. AGD measurements and gynecological related disorders
The findings of the studies in this systematic review demonstrated the relationship between AGD and gynecological conditions in women (64). A prospective cohort of 300 fertile women showed that, when comparing women with below-average AGD to those with above average AGD, the incidence of encounters owing to gynecological issues was much greater (12). Another cohort study among 119 women suggested measures of AGD as risk factors for episiotomy. That study introduced in the episiotomy group AGDAC was significantly shorter (61). Another study measured AGD in pregnant women (85). Better AGD measurements in women could thus be added to routine gynecological assessment and admission in the delivery room, providing critical data in women at risk for later gynecological morbidities (12). Another study among 1154 Indian infants reported that, compared with infants with descended testes, AGD was significantly shorter in infants with undescended testes (63). A prospective, observational study among 150 adult men aged 18-55 yr showed that in the premature ejaculation group, AGDAP and AGDAS were lower than in the control group (66). Therefore, AGD may be clinically useful as a measure of androgen action during pregnancy (65). Studies have shown that fertility and adult sperm production are related to AGD (56, 58). A cohort study suggested that total motile sperm count and sperm concentration after varicocelectomy were associated with longer AGD in men. However, no relationship was observed between improvements in semen volume or sperm motility and AGD length (62).
3.11. AGD measurements and ovarian follicular number
According to studies included in the present systematic review, poor ovarian response was associated with shorter AGDAC and AGDAF. In that study, AGDAC and AGDAF were positive and significant in relation to the total number of oocytes (33). A cross-sectional study reported that AGDAF was positively associated with ovarian follicle number (67). Some previous studies suggested that AGD length is associated with female reproductive function (57, 67, 71, 86). More studies reported that AGD was longer in women with PCOS (31), and daughters born of these women had longer AGD (78). So, our results may contribute to the claim for using AGD as a marker of the intrauterine hormonal milieu in epidemiological and clinical research.
3.12. AGD measurements, maternal and birth outcome
The studies included in our systematic review found links between AGD and maternal and infant characteristics. A cross-sectional study among 133 Korean infants reported that AGD1-3 in the low-birth-weight group were significantly lower than newborns in the control group with normal birth weight (68). A previous study showed that AGD in males was significantly associated with birth weight and gestational age. In that study, AGD in females was associated with birth weight, gestational age, and length (70). Another study demonstrated that AGD was longer in a male newborn with higher cord serum free triiodothyronine, free thyroxine, thyroid-stimulating hormone, and a lower thyroxine/triiodothyronine ratio. In that study, the association between AGD and thyroid-stimulating hormone was not statistically significant in the female neonate (69). A feasible practice is that T3 enhance skeletal muscle growth by adding the frequency and dimension of muscle fibers between the anus and the genital near the central perineal tendon (87). Another possibility is that the placenta influences fetal thyroid hormones and AGD (88).
4. Conclusion
Using quantitative indicators such as AGD to determine the prognosis and early diagnosis of diseases in the future may be of great help in therapeutic interventions and the treatment of cases in the early stages of the disease. Along the way, we should not neglect variables such as age, gender, and stressful life events that may confound the relationship pathway between AGD and disorders.
Author’s statement
We declare that this manuscript is original, has not been previously published or submitted elsewhere for publication, and is not under consideration by another journal.
Acknowledgments
The present study was supported by deputy of research of Shahroud University of Medical Sciences, Shahroud, Iran (grant no: 98120).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Epidemiology
References
1. Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: A review. Hum Reprod Update 2007; 13: 209-223. [DOI:10.1093/humupd/dml056] [PMID]
2. Johansson HKL, Svingen T, Fowler PA, Vinggaard AM, Boberg J. Environmental influences on ovarian dysgenesis - developmental windows sensitive to chemical exposures. Nat Rev Endocrinol 2017; 13: 400-414. [DOI:10.1038/nrendo.2017.36] [PMID]
3. Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol Rev 2016; 96: 55-97. [DOI:10.1152/physrev.00017.2015] [PMID] [PMCID]
4. Della Corte L, Di Filippo C, Gabrielli O, Reppuccia S, La Rosa VL, Ragusa R, et al. The burden of endometriosis on women's lifespan: A narrative overview on quality of life and psychosocial wellbeing. Int J Environ Res Public Health 2020; 17: 4683. [DOI:10.3390/ijerph17134683] [PMID] [PMCID]
5. Sánchez-Ferrer ML, Adoamnei E, Prieto-Sánchez MT, Mendiola J, Corbalán-Biyang Sh, Moñino-García M, et al. Health-related quality of life in women with polycystic ovary syndrome attending to a tertiary hospital in Southeastern Spain: A case-control study. Health Qual Life Outcomes 2020; 18: 232. [DOI:10.1186/s12955-020-01484-z] [PMID] [PMCID]
6. Main KM, Skakkebaek NE, Virtanen HE, Toppari J. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab 2010; 24: 279-289. [DOI:10.1016/j.beem.2009.10.003] [PMID]
7. Misseri R. Association between male genital anomalies and adult male reproductive disorders: A population-based data linkage study spanning more than 40 years. J Pediatr Urol 2020; 16: 504-505. [DOI:10.1016/j.jpurol.2020.07.010] [PMID]
8. Manríquez D, Velez J, Pinedo PJ. Incidence and risk factors for reproductive disorders in organic certified dairies. J Dairy Sci 2020; 103: 10797-10808. [DOI:10.3168/jds.2020-18592] [PMID]
9. Yang Sh, Shih Y-ChT, Huo J, Mehta HJ, Wu Y, Salloum RG, et al. Procedural complications associated with invasive diagnostic procedures after lung cancer screening with low-dose computed tomography. Lung Cancer 2022; 165: 141-144. [DOI:10.1016/j.lungcan.2021.12.020] [PMID] [PMCID]
10. Halliday SJ, Aboudara MC, Maldonado F. Complication rates in a study of invasive diagnostic procedures for lung abnormalities. JAMA Inter Med 2019; 179: 846-847. [DOI:10.1001/jamainternmed.2019.0960] [PMID]
11. Sipahi M, Tokgöz VY, Alanya Tosun Ş. An appropriate way to predict fetal gender at first trimester: Anogenital distance. J Matern Fetal Neonatal Med 2019; 32: 2012-2016. [DOI:10.1080/14767058.2018.1424131] [PMID]
12. Wainstock T, Yoles I, Sergienko R, Walfisch A. The association between anogenital distance, reproductive and general health in adult females- a prospective cohort of 17 years. Reprod Toxicol 2019; 90: 77-81. [DOI:10.1016/j.reprotox.2019.08.007] [PMID]
13. Di Nisio A, Sabovic I, Valente U, Tescari S, Rocca MS, Guidolin D, et al. Endocrine disruption of androgenic activity by perfluoroalkyl substances: Clinical and experimental evidence. J Clin Endocrinol Metab 2019; 104: 1259-1271. [DOI:10.1210/jc.2018-01855] [PMID]
14. Lymperi S, Giwercman A. Endocrine disruptors and testicular function. Metabolism 2018; 86: 79-90. [DOI:10.1016/j.metabol.2018.03.022] [PMID]
15. Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl 2012; 35: 236-244. [DOI:10.1111/j.1365-2605.2011.01190.x] [PMID]
16. Liu C, Xu X, Huo X. Anogenital distance and its application in environmental health research. Environ Sci Pollut Res Int 2014; 21: 5457-5464. [DOI:10.1007/s11356-014-2570-z] [PMID]
17. Kurzrock EA, Jegatheesan P, Cunha GR, Baskin LS. Urethral development in the fetal rabbit and induction of hypospadias: A model for human development. J Urol 2000; 164: 1786-1792.
https://doi.org/10.1016/S0022-5347(05)67107-8 [DOI:10.1097/00005392-200011000-00102] [PMID]
18. Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: Relationship to male reproductive development and its disorders. J Clin Endocrinol Metab 2013; 98: 2230-2238. [DOI:10.1210/jc.2012-4057] [PMID]
19. Adibi JJ, Lee MK, Naimi AI, Barrett E, Nguyen RH, Sathyanarayana S, et al. Human chorionic gonadotropin partially mediates phthalate association with male and female anogenital distance. J Clin Endocrinol Metab 2015; 100: E1216-E1224. [DOI:10.1210/jc.2015-2370] [PMID] [PMCID]
20. Aydin E, Holt R, Chaplin D, Hawkes R, Allison C, Hackett G, et al. Fetal anogenital distance using ultrasound. Prenat Diagn 2019; 39: 527-535. [DOI:10.1002/pd.5459] [PMID] [PMCID]
21. Mendiola J, Sánchez-Ferrer ML, Jiménez-Velázquez R, Cánovas-López L, Hernández-Peñalver AI, Corbalán-Biyang S, et al. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum Reprod 2016; 31: 2377-2383. [DOI:10.1093/humrep/dew163] [PMID] [PMCID]
22. Parra MD, Mendiola J, Jørgensen N, Swan SH, Torres-Cantero AM. Anogenital distance and reproductive parameters in young men. Andrologia 2016; 48: 3-10. [DOI:10.1111/and.12403] [PMID]
23. Zarean M, Keikha M, Feizi A, Kazemitabaee M, Kelishadi R. The role of exposure to phthalates in variations of anogenital distance: A systematic review and meta-analysis. Environ Pollut 2019; 247: 172-179. [DOI:10.1016/j.envpol.2019.01.026] [PMID]
24. Zhou N, Sun L, Yang H, Chen Q, Wang X, Yang H, et al. Anogenital distance is associated with serum reproductive hormones, but not with semen quality in young men. Hum Reprod 2016; 31: 958-967. [DOI:10.1093/humrep/dew052] [PMID]
25. Thankamony A, Pasterski V, Ong KK, Acerini CL, Hughes IA. Anogenital distance as a marker of androgen exposure in humans. Andrology 2016; 4: 616-625. [DOI:10.1111/andr.12156] [PMID] [PMCID]
26. Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U, Svingen T. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxicol 2019; 93: 253-272. [DOI:10.1007/s00204-018-2350-5] [PMID]
27. Jain VG, Goyal V, Chowdhary V, Swarup N, Singh RJ, Singal A, et al. Anogenital distance is determined during early gestation in humans. Hum Reprod 2018; 33: 1619-1627. [DOI:10.1093/humrep/dey265] [PMID]
28. Freire C, Ocón-Hernández O, Dávila-Arias C, Pérez-Lobato R, Calvente I, Ramos R, et al. Anogenital distance and reproductive outcomes in 9-to 11-year-old boys: the INMA-Granada cohort study. Andrology 2018; 6: 874-881. [DOI:10.1111/andr.12544] [PMID]
29. Sánchez-Ferrer ML, Moya-Jiménez LC, Mendiola J. Comparison of the anogenital distance and anthropometry of the perineum in patients with and without pelvic organ prolapse. Actas Urol Esp 2016; 40: 628-634. [DOI:10.1016/j.acuro.2016.04.004] [PMID]
30. Gilboa Y, Perlman S, Kivilevitch Z, Messing B, Achiron R. Prenatal anogenital distance is shorter in fetuses with hypospadias. J Ultrasound Med 2017; 36: 175-182. [DOI:10.7863/ultra.16.01006] [PMID]
31. Wu Y, Zhong G, Chen Sh, Zheng Ch, Liao D, Xie M. Polycystic ovary syndrome is associated with anogenital distance, a marker of prenatal androgen exposure. Hum Reprod 2017; 32: 937-943. [DOI:10.1093/humrep/dex042] [PMID]
32. Sánchez-Ferrer ML, Mendiola J, Hernández-Peñalver AI, Corbalán-Biyang S, Carmona-Barnosi A, Prieto-Sánchez MT, et al. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum Reprod 2017; 32: 2315-2323. [DOI:10.1093/humrep/dex274] [PMID]
33. Fabregues F, González-Foruria I, Peñarrubia J, Carmona F. Ovarian response is associated with anogenital distance in patients undergoing controlled ovarian stimulation for IVF. Hum Reprod 2018; 33: 1696-1704. [DOI:10.1093/humrep/dey244] [PMID]
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI:10.1371/journal.pmed.1000097] [PMID] [PMCID]
35. Wells GA, O'Connell D, Peterson J, Welch V, Losos M, Fogwell P. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
36. Sánchez-Ferrer ML, Jiménez-Velázquez R, Mendiola J, Prieto-Sánchez MT, Cánovas-López L, Carmona-Barnosi A, et al. Accuracy of anogenital distance and anti-müllerian hormone in the diagnosis of endometriosis without surgery. Int J Gynaecol Obstet 2019; 144: 90-96. [DOI:10.1002/ijgo.12691] [PMID]
37. Sánchez-Ferrer Ml, Mendiola J, Jiménez-Velázquez R, Cánovas-López L, Corbalán-Biyang S, Hernández-Peñalver AI, et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod Biomed Online 2017; 34: 375-382. [DOI:10.1016/j.rbmo.2017.01.002] [PMID]
38. Crestani A, Arfi A, Ploteau S, Breban M, Boudy A-S, Bendifallah S, et al. Anogenital distance in adult women is a strong marker of endometriosis: Results of a prospective study with laparoscopic and histological findings. Hum Reprod Open 2020; 3: 1-9. [DOI:10.1093/hropen/hoaa023] [PMID] [PMCID]
39. Maldonado-Cárceles AB, Sánchez-Rodríguez C, Vera-Porras EM, Árense-Gonzalo JJ, Oñate-Celdrán J, Samper-Mateo P, et al. Anogenital distance, a biomarker of prenatal androgen exposure is associated with prostate cancer severity. Prostate 2017; 77: 406-411. [DOI:10.1002/pros.23279] [PMID]
40. Oñate-Celdrán J, Arense-Gonzalo JJ, Mendiola J, Samper-Mateo P, Sánchez-Rodríguez C, García-Escudero D, et al. [Prostate cancer is associated with the anogenital distance, a biomarker of prenatal androgen milieu]. Arch Esp Urol 2019; 72: 9-15. (in Spanish)
41. Castaño-Vinyals G, Carrasco E, Lorente JA, Sabaté Y, Cirac-Claveras J, Pollán M, et al. Anogenital distance and the risk of prostate cancer. BJU Int 2012; 110: E707-E710. [DOI:10.1111/j.1464-410X.2012.11516.x] [PMID]
42. Sahin A, Kutluhan MA, Toprak T, Vural Y, Ürkmez A, Akan S, et al. Assesment of anogenital distance as a marker in diagnosis of prostate cancer. Arch Ital Urol Androl 2019; 91: 163-165. [DOI:10.4081/aiua.2019.3.163] [PMID]
43. Hernández-Peñalver AI, Sánchez-Ferrer ML, Mendiola J, Adoamnei E, Prieto-Sánchez MT, Corbalán-Biyang S, et al. Assessment of anogenital distance as a diagnostic tool in polycystic ovary syndrome. Reprod Biomed Online 2018; 37: 741-749. [DOI:10.1016/j.rbmo.2018.08.020] [PMID]
44. Barrett E, Hoeger K, Sathyanarayana S, Redmon JB, Nguyen R, Swan SH. Anogenital distance, a biomarker of prenatal androgen exposure, is longer among newborn daughters of women with polycystic ovary syndrome (PCOS). Endocr Rev 2016; 37 (Suppl.).
45. Simsir C, Pekcan MK, Aksoy RT, Ecemis T, Coskun B, Kilic SH, et al. The ratio of anterior anogenital distance to posterior anogenital distance: A novel-biomarker for polycystic ovary syndrome. J Chin Med Assoc 2019; 82: 782-786. [DOI:10.1097/JCMA.0000000000000150] [PMID]
46. Sánchez-Ferrer ML, Prieto-Sánchez MT, Moya-Jiménez C, Mendiola J, García-Hernández CM, Carmona-Barnosi A, et al. Anogenital distance and perineal measurements of the pelvic organ prolapse (POP) quantification system. J Vis Exp 2018; 139: 57912.
https://doi.org/10.3791/57912 [DOI:10.3791/57912-v] [PMID] [PMCID]
47. Arfi A, Cohen J, Canlorbe G, Bendifallah S, Thomassin-Naggara I, Darai E, et al. First-trimester determination of fetal gender by ultrasound: Measurement of the ano-genital distance. Eur J Obstet Gynecol Reprod Biol 2016; 203: 177-181. [DOI:10.1016/j.ejogrb.2016.06.001] [PMID]
48. Fowler PA, Filis P, Bhattacharya S, Bizec Bl, Antignac J-P, Morvan M-L, et al. Human anogenital distance: An update on fetal smoke-exposure and integration of the perinatal literature on sex differences. Hum Reprod 2016; 31: 463-472. [DOI:10.1093/humrep/dev323] [PMID] [PMCID]
49. Najdi N, Safi F, Hashemi-Dizaji S, Sahraian G, Jand Y. First trimester determination of fetal gender by ultrasonographic measurement of anogenital distance: A cross-sectional study. Int J Reprod BioMed 2019; 17: 51-56. [DOI:10.18502/ijrm.v17i1.3820] [PMID] [PMCID]
50. Cox K, Kyriakou A, Amjad B, O'Toole S, Flett ME, Welsh M, et al. Shorter anogenital and anoscrotal distances correlate with the severity of hypospadias: A prospective study. J Pediatr Urol 2017; 13: 57. [DOI:10.1016/j.jpurol.2016.08.006] [PMID]
51. Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr Urol Rep 2008; 9: 137-142. [DOI:10.1007/s11934-008-0025-0] [PMID]
52. Singal AK, Jain VG, Gazali Z, Shekhawat P. Shorter anogenital distance correlates with the severity of hypospadias in pre-pubertal boys. Hum Reprod 2016; 31: 1406-1410. [DOI:10.1093/humrep/dew115] [PMID]
53. Thankamony A, Lek N, Carroll D, Williams M, Dunger DB, Acerini CL, et al. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: Comparison with normative data. Environ Health Perspect 2014; 122: 207-211. [DOI:10.1289/ehp.1307178] [PMID] [PMCID]
54. Eisenberg ML, Lipshultz LI. Anogenital distance as a measure of human male fertility. J Assist Reprod Genet 2015; 32: 479-484. [DOI:10.1007/s10815-014-0410-1] [PMID] [PMCID]
55. Mendiola J, Melgarejo M, Moñino-García M, Cutillas-Tolín A, Noguera-Velasco JA, Torres-Cantero AM. Is anogenital distance associated with semen quality in male partners of subfertile couples? Andrology 2015; 3: 672-676. [DOI:10.1111/andr.12059] [PMID]
56. Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect 2011; 119: 958-963. [DOI:10.1289/ehp.1103421] [PMID] [PMCID]
57. Mira-Escolano MP, Mendiola J, Mı'nguez-Alarco'n L, Melgarejo M, Cutillas-Tolı'n A, Roca M, et al. Longer anogenital distance is associated with higher testosterone levels in women: A cross-sectional study. BJOG 2014; 121: 1359-1364. [DOI:10.1111/1471-0528.12627] [PMID]
58. Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One 2011; 6: e18973. [DOI:10.1371/journal.pone.0018973] [PMID] [PMCID]
59. Glintborg D, Jensen RC, Schmedes AV, Brandslund I, Kyhl HB, Jensen TK, et al. Anogenital distance in children born of mothers with polycystic ovary syndrome: the Odense child cohort. Hum Reprod 2019; 34: 2061-2070. [DOI:10.1093/humrep/dez122] [PMID]
60. Wainstock T, Shoham-Vardi I, Sheiner E, Walfisch A. Fertility and anogenital distance in women. Reprod Toxicol 2017; 73: 345-349. [DOI:10.1016/j.reprotox.2017.07.009] [PMID]
61. Moya-Jiménez LC, Sánchez-Ferrer ML, Adoamnei E, Mendiola J. New approach to the evaluation of perineal measurements to predict the likelihood of the need for an episiotomy. Int Urogynecol J 2018; 30: 815-821. [DOI:10.1007/s00192-018-3745-9] [PMID]
62. Eisenberg ML, Shy M, Herder D, Walters RC, Lipshultz LI. The relationship between anogenital distance and the efficacy of varicocele repair. BJU Int 2012; 110: E927-E930. [DOI:10.1111/j.1464-410X.2012.11154.x]
63. Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: A detailed genital anthropometric analysis in human newborns. Hum Reprod 2013; 28: 2343-2349. [DOI:10.1093/humrep/det286] [PMID]
64. Domenici L, Musella A, Bracchi C, Lecce F, Schiavi MC, Colagiovanni V, et al. Comparison of anogenital distance and correlation with vulvo-vaginal atrophy: A pilot study on premenopausal and postmenopausal women. J Menopausal Med 2018; 24: 108-112. [DOI:10.6118/jmm.2018.24.2.108] [PMID] [PMCID]
65. Toprak T, Şahin A, Akgul K, Kutluhan MA, Ramazanoglu MA, Yilmaz M, et al. The relationship between anogenital distance and lifelong premature ejaculation. Andrology 2020; 8: 353-357. [DOI:10.1111/andr.12709] [PMID]
66. Sertkaya Z, Ertaş K, Tokuç E. The relationship between premature ejaculation and anogenital distance. Andrologia 2020; 52: e13571. [DOI:10.1111/and.13571]
67. Mendiola J, Roca M, Mínguez-Alarcón L, Mira-Escolano MP, López-Espín JJ, Barrett ES, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: A cross-sectional study. Environ Health 2012; 11: 90. [DOI:10.1186/1476-069X-11-90] [PMID] [PMCID]
68. Park JY, Lim G, Oh KW, Ryu DS, Park S, Jeon JC, et al. Penile length, digit length, and anogenital distance according to birth weight in newborn male infants. Korean J Urol 2015; 56: 248-253. [DOI:10.4111/kju.2015.56.3.248] [PMID] [PMCID]
69. Liu R, Xu X, Zhang Y, Zheng X, Kim SS, Dietrich KN, et al. Thyroid hormone status in umbilical cord serum is positively associated with male anogenital distance. J Clin Endocrinol Metab 2015; 10: 3378-3385. [DOI:10.1210/jc.2015-3872] [PMID] [PMCID]
70. Singal AK, Jain VG. Maternal and infant characteristics influencing the anogenital distance and penile length in newborns. Andrologia 2016; 48: 708-713. [DOI:10.1111/and.12507] [PMID]
71. Mira-Escolano MP, Mendiola J, Mı'nguez-Alarco'n L, Roca M, Cutillas-Tolı'n A, pez-Espı'n JJL, et al. Anogenital distance of women in relation to their mother's gynaecological characteristics before or during pregnancy. Reprod Biomed Online 2014; 28: 209-215. [DOI:10.1016/j.rbmo.2013.09.026] [PMID]
72. Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 2015; 30: 963-972. [DOI:10.1093/humrep/deu363] [PMID] [PMCID]
73. Gilboa Y, Kivilevitch Z, Oren M, Cohen YP, Katorza E, Achiron R. Anogenital distance in male and female fetuses at 20 to 35 weeks of gestation: Centile charts and reference ranges. Prenat Diagn 2014; 34: 946-951. [DOI:10.1002/pd.4399] [PMID]
74. Boyle P, Koechlin A, Bota M, Onofrio A, Zaridze DG, Perrin P, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: A meta-analysis. BJU Int 2016; 118: 731-741. [DOI:10.1111/bju.13417] [PMID]
75. Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JEAK, David AL, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol 2010; 30: 444-446. [DOI:10.3109/01443615.2010.485254] [PMID]
76. Wu X-Y, Li Zh-L, Wu Ch-Y, Liu Y-M, Lin H, Wang Sh-H, et al. Endocrine traits of polycystic ovary syndrome in prenatally androgenized female Sprague-Dawley rats. Endocr J 2010; 57: 201-209. [DOI:10.1507/endocrj.K09E-205] [PMID]
77. Hart R, Doherty DA, Norman RJ, Franks S, Dickinson JE, Hickey M, et al. Serum anti-Mullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS). Fertil Steril 2010; 94: 1118-1121. [DOI:10.1016/j.fertnstert.2009.11.002] [PMID]
78. Barrett ES, Hoeger KM, Sathyanarayana S, Abbott DH, Redmon JB, Nguyen RHN. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis 2018; 9: 307-314. [DOI:10.1017/S2040174417001118] [PMID] [PMCID]
79. Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 2008; 108: 177-184. [DOI:10.1016/j.envres.2008.08.007] [PMID] [PMCID]
80. Khunda A, Shek KL, Dietz HP. Can ballooning of the levator hiatus be determined clinically? Am J Obstet Gynecol 2012; 206: e1-e4. [DOI:10.1016/j.ajog.2011.10.876] [PMID]
81. Dietz HP. Ultrasound imaging of the pelvic floor. Part II: Three-dimensional or volume imaging. Ultrasound Obstet Gynecol 2004; 23: 615-625. [DOI:10.1002/uog.1072] [PMID]
82. Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: A descriptive, cross-sectional study. Environ Health Perspect 2004; 3: 8. [DOI:10.1186/1476-069X-3-8] [PMID] [PMCID]
83. Hua X-G, Hu R, Hu Ch-Y, Li FL, Jiang W, Zhang XJ. Associations between hypospadias, cryptorchidism and anogenital distance: Systematic review and meta‐analysis. Andrologia 2018; 50: e13152. [DOI:10.1111/and.13152] [PMID]
84. Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005; 113: 1056-1061. [DOI:10.1289/ehp.8100] [PMID] [PMCID]
85. Eid SM. Is perineal body length measurement reinforcing the decision about performance or avoidance of episiotomy? Asian Acad Manage J 2011; 3: 209-226.
86. Barrett ES, Parlett LE, Swan SH. Stability of proposed biomarkers of prenatal androgen exposure over the menstrual cycle. J Dev Orig Health Dis 2015; 6: 149-157. [DOI:10.1017/S2040174414000646] [PMID] [PMCID]
87. Lee JW, Kim NH, Milanesi A. Thyroid hormone signaling in muscle development, repair and metabolism. J Endocrinol Diabetes Obes 2014; 2: 1046.
88. Loubière LS, Vasilopoulou E, Glazier JD, Taylor PM, Franklyn JA, Kilby MD, et al. Expression and function of thyroid hormone transporters in the microvillous plasma membrane of human term placental syncytiotrophoblast. Endocrinology 2012; 153: 6126-6135. [DOI:10.1210/en.2012-1753] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




