BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijrm.ir/article-1-25-en.html

 , Mohammad Taghizadieh
, Mohammad Taghizadieh 
 , Mehrzad Mahzad-Sadaghiani
, Mehrzad Mahzad-Sadaghiani 
 , Farahnaz Noroozinia
, Farahnaz Noroozinia 
 , Bahman Jahandideh
, Bahman Jahandideh 

Introduction
Leydig cell tumor constitutes the single most common type of testicular sex cord-stromal tumor (1,2). They may occur at any ages, but are most commonly seen between the second and the sixth decades of life. Approximately 20% of these tumors are detected in the prepubertal age group (1,3). The etiology of Leydig cell tumors is unknown. In contrast to germ cell tumors, there appears to be no association with cryptorchidism and Leydig cell tumor (4). Malignant clinical behavior occurs in approximately 10% and metastasis is the major criterion of malignancy (5). On gross examination, Leydig cell tumors are most commonly 2 to 5 cm and when the size is greater than 5 cm, suggest more possibility of malignancy (1, 4). Testicular swelling, decreased libido (20%) and gynecomastia (15%) are common symptoms in adults. Pseudoprecocity is usually seen in children (3, 6).
Case report
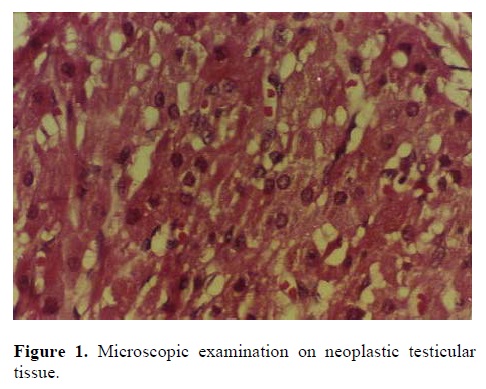
A 24 year old man comes with chief complaint of infertility since a year after marriage. Spermatogram revealed azoospermia and bilateral testicular tumor was shown by ultrasound examination. Hormonal assay showed increase of plasma FSH and LH levels and low normal serum testosterone. Frozen section during surgery from bilateral testes was suggestive for Leydig cell tumor which was confirmed by following histopathology. The patient underwent right inguinal radical orchiectomy. The gross examination showed a well circumscribed, brown homogeneous solid mass with soft consistency measuring 1.5 cm in diameter in the middle part of the testis. The microscopic examination revealed, diffuse sheet-like pattern of large, polygonal cells with round nuclei and eosinophilic cytoplasm. Mitoses and pleomorphism was scarce. Necrosis and hemorrhage was not seen (Figure 1). In non-neoplastic testicular tissue, the seminiferous tubules showed only sertoli cells with complete spermatogenetic arrest (Figures 2).

The patient didn’t accept left side orchiectomy or tumoral excision due to consequences of castration.
Discussion
Leydig cell tumor is the most common sex cord tumors. This tumor is always benign in children and approximately 90% are benign in adults (7). Clinical presentations are as follow: testicular enlargement, gynecomastia, sexual activity disturbances such as decreased libido, infertility and azoospermia (8). In prepubertal cases, presenting manifestations are usually those of isosexual precocity. Asymptomatic tumors may be detected incidentally in testicular sonography (8).
Hormonal activity detect in 20% of the tumors, including increased estradiol and testosterone serum level, leading to adult feminization and children musculinizaion respectively (9,10). The endocrinologic manifestation may precede the palpable testis mass (3).
Semen analysis may show oligozoospermia, cryptozoospermia or azoospermia (11). Clinical and hormonal manifestations subside in 90% of patients following orchiectomy (4).
Change in hormonal assay can be used as a marker of tumor recurrence in patients follow up.
Leydig cell tumor metastasis is very uncommon. Cellular atypia, necrosis, lymphatic invasion, high mitotic activity, atypical mitotic forms, infiltrative margins, extratesticular invasion and DNA aneuploidy show metastatic and invasive potential (1-3, 5).
Immunohistochemistry for oncogenic markers of Ki -67, p53 and bcl-2 could be of diagnostic value in the identification of malignant and borderline cases (positive) of Leydig cell tumor (12).
Adrenal cortex and testis have mesodermal origin. Gotoh et al. (1995) reported a case of bilateral Leydig cell tumor with adrenal cortex adenoma, they suggest adrenal gland evaluation in patients with Leydig cell tumor (13).
Radical inguinal orchiectomy is the initial treatment of choice. Retroperitoneal lymph node dissection, chemotherapy and radiotherapy (relatively radioresistance) has been recommended in patients whose Leydig cell tumors appear histologically or biochemically malignant (4).
The prognosis for benign Leydig cell tumors is excellent (8). The persistence of virilizing and feminizing features after orchiectomy is not necessarily an indication of malignancy and these features may be to some extent irreversible (4).
The average survival time in malignant cases is approximately 3 years after surgery.
Conclusion
We recommend the following approach in patients with infertility and azoospermia:
- Bilateral testicular physical exam and sonography (11).
- Due to coincidence of Leydig cell tumor and azoospermia with klinefelter’s syndrome, karyotyping of patients is necessary (14).
- Adrenal cortex evaluation due to coincidence of adrenal and testicular lesions (13).
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


