Thu, Jul 3, 2025
[Archive]
Volume 20, Issue 12 (December 2022)
IJRM 2022, 20(12): 1007-1012 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Akhavansales Z, Mosadegh Mehrjardi A, Ashrafzadeh H R, Yavari S F, Tahoori M T, Bitaraf Sani M, et al . Evaluation of the FAS and FASL Gene changes in women with premature ovarian failure: A case-control study. IJRM 2022; 20 (12) :1007-1012
URL: http://ijrm.ir/article-1-2540-en.html
URL: http://ijrm.ir/article-1-2540-en.html
Zhima Akhavansales *1 

 , Alimohammad Mosadegh Mehrjardi2
, Alimohammad Mosadegh Mehrjardi2 

 , Hamid reza Ashrafzadeh3
, Hamid reza Ashrafzadeh3 

 , Shadnaz fakhteh Yavari4
, Shadnaz fakhteh Yavari4 

 , Mohammad Taher Tahoori5
, Mohammad Taher Tahoori5 

 , Morteza Bitaraf Sani6
, Morteza Bitaraf Sani6 

 , Mahnaz Mohammadi3
, Mahnaz Mohammadi3 

 , Fateme Montazeri3
, Fateme Montazeri3 

 , Nasrin Ghasemi3
, Nasrin Ghasemi3 




 , Alimohammad Mosadegh Mehrjardi2
, Alimohammad Mosadegh Mehrjardi2 

 , Hamid reza Ashrafzadeh3
, Hamid reza Ashrafzadeh3 

 , Shadnaz fakhteh Yavari4
, Shadnaz fakhteh Yavari4 

 , Mohammad Taher Tahoori5
, Mohammad Taher Tahoori5 

 , Morteza Bitaraf Sani6
, Morteza Bitaraf Sani6 

 , Mahnaz Mohammadi3
, Mahnaz Mohammadi3 

 , Fateme Montazeri3
, Fateme Montazeri3 

 , Nasrin Ghasemi3
, Nasrin Ghasemi3 


1- Department of Immunology, Faculty of Medicine, International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , dr.zhima@live.com
2- Department of Traditional Pharmacy, Faculty of Traditional Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Tissue Engineering and Applied Cell Science, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Science, Tehran, Iran.
5- Department of Immunology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
6- Animal Science Research Department, Yazd Agricultural and Natural Resources Research and Education Center, Agricultural Research, Education & Extension Organization (AREEO), Yazd, Iran.
2- Department of Traditional Pharmacy, Faculty of Traditional Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Tissue Engineering and Applied Cell Science, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Science, Tehran, Iran.
5- Department of Immunology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
6- Animal Science Research Department, Yazd Agricultural and Natural Resources Research and Education Center, Agricultural Research, Education & Extension Organization (AREEO), Yazd, Iran.
Full-Text [PDF 268 kb]
(1115 Downloads)
| Abstract (HTML) (1090 Views)
Full-Text: (193 Views)
1. Introduction
Infertility is a multifactorial disease that ranges from hormonal and genetic disorders to immunological changes. A gradual decrease in ovarian function is observed as menopause nears, leading to estrogen deficiency and a decrease in fertility. This process is associated with higher gonadotropin levels (1-3). Premature ovarian failure (POF), is a menopause that occurs before the age of 40, affecting 1-3% of women worldwide (4, 5). The development and maturation of ovulation depend on molecular signaling pathways responding to androgens (6, 7).
Estrogen is one of the 2 steroid sex hormones secreted by ovaries. Estrogen also have an important role in fetal development, the presence of secondary sexual features, the reproductive cycle, and the continuation of pregnancy. In addition, estrogen regulates the growth and differentiation of endometrial cells (8). In the reproductive cycle, implantation involves a series of events, including apoptosis in endometrial cells (9, 10). Evidence suggests that apoptosis helps maintain cellular homeostasis by removing senescent cells from the functional layer of the uterine endometrium (11).
FASL acts as a mediator of apoptosis between cell differentiation and embryonic development. It has been reported that polymorphisms in FAS and FASL have clinical worth in hormone-sensitive cancers such as ovarian and breast cancer. Estrogen, one of the most important sex hormones, increases the expression of the FASL protein (12). Genetic tests also show increased FASL mRNA expression by estradiol and progesterone. It was shown that increased FASL expression may mediate apoptosis in endometrial cells (9). Therefore, it can play an important role in trophoblast invasion and consequent implantation. FAS and FASL genes have many polymorphisms even in the gene promoter, which could be important in regulating the cell death signal (13-15).
The FASL gene has 4 exons. FASLINV2nt_124A/G rs5030722 is one of the most important polymorphisms reported in this gene, located within the intron. Considering the effect of estrogen on FAS and FASL regulation, we investigated the effect of standard FAS and FASL polymorphisms with POF.
2. Materials and Methods
2.1. Study subjects
Among 112 women who were admitted to the Recurrent Abortion Clinic of Yazd Reproductive Sciences Institute, Yazd, Iran 51 women met the diagnostic criteria of POF and 61 healthy women were considered as case control groups, respectively.
2.2. Genotype analysis
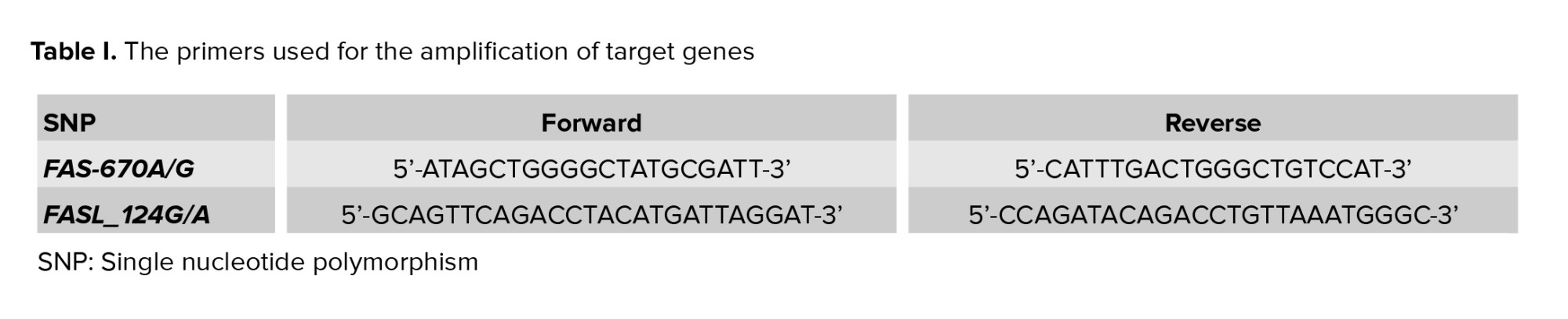
Genomic DNA was extracted from 5 ml of whole blood by the salting out method (16). DNA was amplified through polymerase chain reaction with designed primers of FAS and FASL genes (Table I). The solution for polymerase chain reaction reaction was made by 30 ng of genomic DNA, 10 p moles from each primer (Macrogen Inc., Korea), 10 μl of Master Mix RED (AMPLIQON, Denmark), plus distilled water up to 20 μl as the final volume. The reaction starts by denaturation for 5 min at 95°C, followes by 2 steps involving 10 cycles as the first step and 25 cycles as the second step. The first step contains 30 sec of denaturation at 95°C, 50 sec of annealing at 62°C, and 40 sec of extension at 72°C, and the second step contains 20 sec of denaturation at 95°C, 50 sec of annealing at 58°C, and 40 sec of extension at 72°C. The final extension was done within 3 min at 72°C. The products included 193 bp for FAS-670A/G and 230 bp for FASL_124G/A. They were restricted by MvaI (Fermentase, Germany) and FokI (BIOLAB, Germany) enzymes, respectively at 37°C overnight. The products stained by safe staining and separated on 2.5% agarose gel electrophoresis, resulting in 136 and 57 bp fragments in the -670G allele and 180 and 50 bp fragments in the FASLIVS2nt_124G allele (17, 18).
2.3. Ethical considerations
This study was approved by the ethics committee of Yazd Reproductive Science Institute, Yazd, Iran (Code: IR.SSU.RSI.REC.1396.27). The women were informed of the research goal and signed the consent form. Women with unplanned pregnancies and those who received assisted reproductive technologies were omitted from our study.
2.4. Statistical analysis
The frequency of the alleles, genotypes and haplotype in cases and controls were compared by Chi-square test (p-value < 0.05 was significant). Fisher’s exact test, Two-sided p-values, and odds ratio (OR) (with 95% confidence interval) were calculated by SPSS statistical software (Version 20). Genetic models (i.e., dominant, codominant, recessive, and overdominant) were analyzed using R software (the SNPassoc package) to assess the relationship between alleles. The PLINK software was employed for haplotype blocks.
3. Results
The genotype distribution of the 2 polymorphisms from both cases and the controls were in line with Hardy-Weinberg equilibrium (p > 0.05).
3.1. Allele frequencies
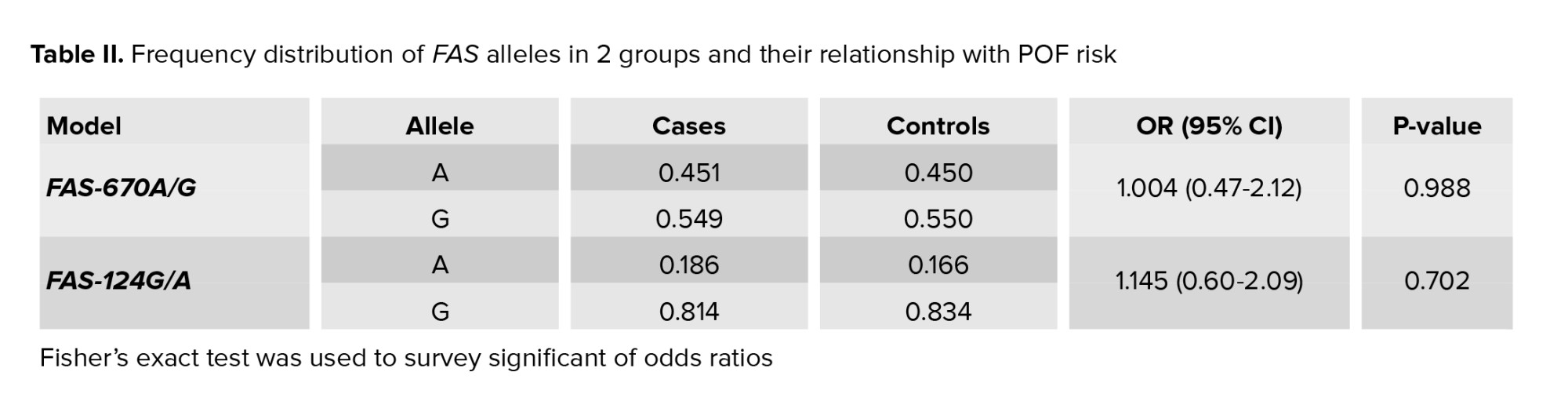
The allelic odds ratio of FAS-670A/G and FASL_124G/A were 1.004 and 1.145, respectively. No significant difference was observed between the allele frequencies of SNPs in this research (Table II). Allele frequencies within cases and controls are shown in table II. Also, odds ratio statistics with p-value for any allele were presented. Data were presented in percentages.
3.2. Genotype frequencies
Regarding the FASLIVS2nt_124, the frequency of AG genotype was higher in the controls (26.7%) than in cases (17.6%). However, the difference was not statistically significant (p = 0.23). Most POF women had AA genotype relative to FASLIVS2nt_124AG and GG genotypes (72.5%, 17.6% and 9.8%, respectively). Under the codominant model, the frequency of the FAS-670AG genotype was not significantly different between cases (47.1%) and controls (46.7%) (p = 0.99). Also, the AG genotype was the most common genotype in POF women (AG = 47.1%, GG = 31.4%, and AA = 21.6%) (Table III). No significant difference was observed in genotype frequencies of FASLIVS2nt_124 A/G and FAS-670 A/G variants between women with POF and healthy controls.
3.3. Haplotype frequency
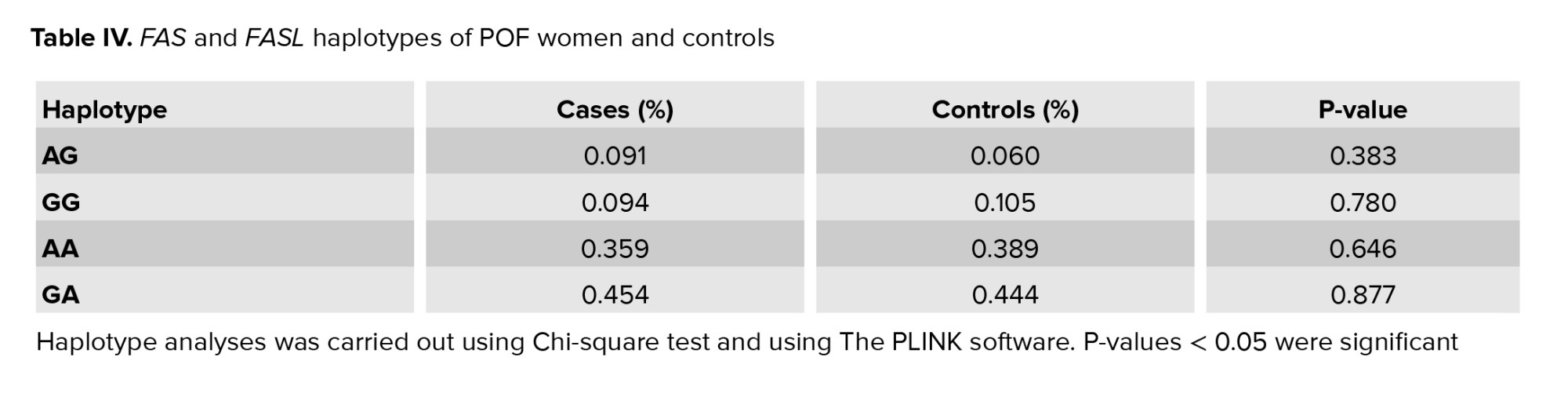
Haplotype analysis was done for these SNPs to investigate the association between a likely combination of FASLIVS2nt_124A/G and FAS-670A/G polymorphisms with POF. Most women with POF had GA and AA haplotypes (0.45% and 0.35%, respectively) (Table IV). There was no significant difference in the frequency of haplotypes between cases and controls.

4. Discussion
Apoptosis is vital for the formation of tissue structure during embryogenesis and cellular homeostasis, and it serves as a defense mechanism in facing pathogens (19, 20). Apoptosis is simultaneous with the implantation window (7). The expression of FASL gene is a important source of apoptosis, which changes in the ovary in female reproductive cycles. The levele of the FASL protein is corresponding to the estrogen-receptor beta-expression. Estrogen up-regulates the expression of FASL and higher mRNA finds in normal ovarian epithelial cells (21).
It has been suggested that regulation of FASL expression in human endometrium is dependent on steroid sex hormones. In immunohistochemistry tests, a gradual increase in the immune reaction of FASL has been observed in both stromal and glandular cells, and the strong expression of FASL has been detected in late reproductive and secretory phases )9(. In 2006, Slot and colleagues concluded in their experiments that the expression of FASL in normal ovaries was hormone-sensitive, and could play a key role in the physiology of normal ovarian tissue. They also found that estrogen is an important sex hormone, increasing the expression of FASL protein (21). Genetic tests have indicated an increase in the expression of FASL mRNA by estradiol and progesterone. It was revealed that higher FASL expression may mediate apoptosis in endometrial cells and can thus play an important role in trophoblast invasion and consequent implantation (9). It is believed that estrogen plays a vital role in oocyte maturation and fertilization (6). Disruption or polymorphism of apoptosis-related genes can impair the relevant immune responses (8).
Transcriptional activity of these genes change by Single-nucleotide polymorphisms in the promoter region. The -670A/G polymorphism of the FAS gene lies in the transcription binding site for the 2 main transcription factors of Sp1 and STAT1. Besides, FASLINV2nt_124A/G, rs5030772 in intron 2 of this gene have a important influence on the control of the expression of FASL (22). Considering the above statements, we evaluated the role of FAS and FASL genes polymorphisms in POF pathogenesis. Nevertheless, FASL expression in the normal ovary is sensitive to hormones and it could have a major role in the physiology of normal ovarian tissue (21). Based on our data, neither FAS nor FASL polymorphisms seem to be susceptibility factors for POF in our population.
5. Conclusion
This study showed that the gene variants under research were not involved in the pathogenesis of POF in the Iranian population. However, this does not completely exclude such genes as potential candidates for the occurrence of this disease. A comprehensive genetic analysis of the genes implicated in the intricate apoptosis regulation system could result in the identification of susceptibility factors for the disease and a better understanding of its etiology.
Acknowledgments
Authors like to thank the staffs of laboratory of the abortion research center for their help and effort.
Conflict of Interest
The authors declare that there is no conflict of interest.
Infertility is a multifactorial disease that ranges from hormonal and genetic disorders to immunological changes. A gradual decrease in ovarian function is observed as menopause nears, leading to estrogen deficiency and a decrease in fertility. This process is associated with higher gonadotropin levels (1-3). Premature ovarian failure (POF), is a menopause that occurs before the age of 40, affecting 1-3% of women worldwide (4, 5). The development and maturation of ovulation depend on molecular signaling pathways responding to androgens (6, 7).
Estrogen is one of the 2 steroid sex hormones secreted by ovaries. Estrogen also have an important role in fetal development, the presence of secondary sexual features, the reproductive cycle, and the continuation of pregnancy. In addition, estrogen regulates the growth and differentiation of endometrial cells (8). In the reproductive cycle, implantation involves a series of events, including apoptosis in endometrial cells (9, 10). Evidence suggests that apoptosis helps maintain cellular homeostasis by removing senescent cells from the functional layer of the uterine endometrium (11).
FASL acts as a mediator of apoptosis between cell differentiation and embryonic development. It has been reported that polymorphisms in FAS and FASL have clinical worth in hormone-sensitive cancers such as ovarian and breast cancer. Estrogen, one of the most important sex hormones, increases the expression of the FASL protein (12). Genetic tests also show increased FASL mRNA expression by estradiol and progesterone. It was shown that increased FASL expression may mediate apoptosis in endometrial cells (9). Therefore, it can play an important role in trophoblast invasion and consequent implantation. FAS and FASL genes have many polymorphisms even in the gene promoter, which could be important in regulating the cell death signal (13-15).
The FASL gene has 4 exons. FASLINV2nt_124A/G rs5030722 is one of the most important polymorphisms reported in this gene, located within the intron. Considering the effect of estrogen on FAS and FASL regulation, we investigated the effect of standard FAS and FASL polymorphisms with POF.
2. Materials and Methods
2.1. Study subjects
Among 112 women who were admitted to the Recurrent Abortion Clinic of Yazd Reproductive Sciences Institute, Yazd, Iran 51 women met the diagnostic criteria of POF and 61 healthy women were considered as case control groups, respectively.
2.2. Genotype analysis
Genomic DNA was extracted from 5 ml of whole blood by the salting out method (16). DNA was amplified through polymerase chain reaction with designed primers of FAS and FASL genes (Table I). The solution for polymerase chain reaction reaction was made by 30 ng of genomic DNA, 10 p moles from each primer (Macrogen Inc., Korea), 10 μl of Master Mix RED (AMPLIQON, Denmark), plus distilled water up to 20 μl as the final volume. The reaction starts by denaturation for 5 min at 95°C, followes by 2 steps involving 10 cycles as the first step and 25 cycles as the second step. The first step contains 30 sec of denaturation at 95°C, 50 sec of annealing at 62°C, and 40 sec of extension at 72°C, and the second step contains 20 sec of denaturation at 95°C, 50 sec of annealing at 58°C, and 40 sec of extension at 72°C. The final extension was done within 3 min at 72°C. The products included 193 bp for FAS-670A/G and 230 bp for FASL_124G/A. They were restricted by MvaI (Fermentase, Germany) and FokI (BIOLAB, Germany) enzymes, respectively at 37°C overnight. The products stained by safe staining and separated on 2.5% agarose gel electrophoresis, resulting in 136 and 57 bp fragments in the -670G allele and 180 and 50 bp fragments in the FASLIVS2nt_124G allele (17, 18).

2.3. Ethical considerations
This study was approved by the ethics committee of Yazd Reproductive Science Institute, Yazd, Iran (Code: IR.SSU.RSI.REC.1396.27). The women were informed of the research goal and signed the consent form. Women with unplanned pregnancies and those who received assisted reproductive technologies were omitted from our study.
2.4. Statistical analysis
The frequency of the alleles, genotypes and haplotype in cases and controls were compared by Chi-square test (p-value < 0.05 was significant). Fisher’s exact test, Two-sided p-values, and odds ratio (OR) (with 95% confidence interval) were calculated by SPSS statistical software (Version 20). Genetic models (i.e., dominant, codominant, recessive, and overdominant) were analyzed using R software (the SNPassoc package) to assess the relationship between alleles. The PLINK software was employed for haplotype blocks.
3. Results
The genotype distribution of the 2 polymorphisms from both cases and the controls were in line with Hardy-Weinberg equilibrium (p > 0.05).
3.1. Allele frequencies
The allelic odds ratio of FAS-670A/G and FASL_124G/A were 1.004 and 1.145, respectively. No significant difference was observed between the allele frequencies of SNPs in this research (Table II). Allele frequencies within cases and controls are shown in table II. Also, odds ratio statistics with p-value for any allele were presented. Data were presented in percentages.
3.2. Genotype frequencies
Regarding the FASLIVS2nt_124, the frequency of AG genotype was higher in the controls (26.7%) than in cases (17.6%). However, the difference was not statistically significant (p = 0.23). Most POF women had AA genotype relative to FASLIVS2nt_124AG and GG genotypes (72.5%, 17.6% and 9.8%, respectively). Under the codominant model, the frequency of the FAS-670AG genotype was not significantly different between cases (47.1%) and controls (46.7%) (p = 0.99). Also, the AG genotype was the most common genotype in POF women (AG = 47.1%, GG = 31.4%, and AA = 21.6%) (Table III). No significant difference was observed in genotype frequencies of FASLIVS2nt_124 A/G and FAS-670 A/G variants between women with POF and healthy controls.
3.3. Haplotype frequency
Haplotype analysis was done for these SNPs to investigate the association between a likely combination of FASLIVS2nt_124A/G and FAS-670A/G polymorphisms with POF. Most women with POF had GA and AA haplotypes (0.45% and 0.35%, respectively) (Table IV). There was no significant difference in the frequency of haplotypes between cases and controls.



4. Discussion
Apoptosis is vital for the formation of tissue structure during embryogenesis and cellular homeostasis, and it serves as a defense mechanism in facing pathogens (19, 20). Apoptosis is simultaneous with the implantation window (7). The expression of FASL gene is a important source of apoptosis, which changes in the ovary in female reproductive cycles. The levele of the FASL protein is corresponding to the estrogen-receptor beta-expression. Estrogen up-regulates the expression of FASL and higher mRNA finds in normal ovarian epithelial cells (21).
It has been suggested that regulation of FASL expression in human endometrium is dependent on steroid sex hormones. In immunohistochemistry tests, a gradual increase in the immune reaction of FASL has been observed in both stromal and glandular cells, and the strong expression of FASL has been detected in late reproductive and secretory phases )9(. In 2006, Slot and colleagues concluded in their experiments that the expression of FASL in normal ovaries was hormone-sensitive, and could play a key role in the physiology of normal ovarian tissue. They also found that estrogen is an important sex hormone, increasing the expression of FASL protein (21). Genetic tests have indicated an increase in the expression of FASL mRNA by estradiol and progesterone. It was revealed that higher FASL expression may mediate apoptosis in endometrial cells and can thus play an important role in trophoblast invasion and consequent implantation (9). It is believed that estrogen plays a vital role in oocyte maturation and fertilization (6). Disruption or polymorphism of apoptosis-related genes can impair the relevant immune responses (8).
Transcriptional activity of these genes change by Single-nucleotide polymorphisms in the promoter region. The -670A/G polymorphism of the FAS gene lies in the transcription binding site for the 2 main transcription factors of Sp1 and STAT1. Besides, FASLINV2nt_124A/G, rs5030772 in intron 2 of this gene have a important influence on the control of the expression of FASL (22). Considering the above statements, we evaluated the role of FAS and FASL genes polymorphisms in POF pathogenesis. Nevertheless, FASL expression in the normal ovary is sensitive to hormones and it could have a major role in the physiology of normal ovarian tissue (21). Based on our data, neither FAS nor FASL polymorphisms seem to be susceptibility factors for POF in our population.
5. Conclusion
This study showed that the gene variants under research were not involved in the pathogenesis of POF in the Iranian population. However, this does not completely exclude such genes as potential candidates for the occurrence of this disease. A comprehensive genetic analysis of the genes implicated in the intricate apoptosis regulation system could result in the identification of susceptibility factors for the disease and a better understanding of its etiology.
Acknowledgments
Authors like to thank the staffs of laboratory of the abortion research center for their help and effort.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
References
1. Christin-Maitre S, Givony M, Albarel F, Bachelot A, Bidet M, Blanc JV, et al. Position statement on the diagnosis and management of premature/primary ovarian insufficiency (except Turner Syndrome). Ann Endocrinol 2021; 82: 555-571. [DOI:10.1016/j.ando.2021.09.001] [PMID]
2. Direkvand-Moghadam A, Sayehmiri K, Delpisheh A, Direkvand-Moghadam A. The global trend of infertility: An original review and meta-analysis. International Journal of Epidemiologic Research 2014; 1: 35-43.
3. Arora P, Polson DW. Diagnosis and management of premature ovarian failure. The Obstetrician & Gynaecologist 2011; 13: 67-72. [DOI:10.1576/toag.13.2.67.27648]
4. Zhang Ch. The roles of different stem cells in premature ovarian failure. Curr Stem Cell Res Ther 2020; 15: 473-481. [DOI:10.2174/1574888X14666190314123006] [PMID]
5. Igboeli P, El Andaloussi A, Sheikh U, Takala H, ElSharoud A, McHugh A, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: Two case reports and a review of the literature. Journal of Medical Case Reports 2020; 14: 1-11. [DOI:10.1186/s13256-020-02426-5] [PMID] [PMCID]
6. Sari S. Analysis of androgen receptor gene mutations in female with infertility. Scientific Information Database 2017; 19: 1-7.
7. Pargianas M, Salta S, Apostolopoulou K, Lazaros L, Kyrgiou M, Tinelli A, et al. Pathways involved in premature ovarian failure: A systematic review of experimental studies. Curr Pharm Des 2020; 26: 2087-2095. [DOI:10.2174/1381612826666200316160145] [PMID]
8. Parsa E, Hoseini SM, Namayandeh SM, Akhavansales Zh, Sheikhha MH. Investigating the rate of different ovarian response in in vitro fertilization cycles based on estrogen receptor beta+ 1730 polymorphism: A cross-sectional study. Int J Reprod BioMed 2020; 18: 509-516. [DOI:10.18502/ijrm.v13i7.7368] [PMID] [PMCID]
9. Sbracia M, Valeri C, Antonini G, Biagiotti G, Pacchiarotti A, Pacchiarotti A. Fas and fas-ligand in eutopic and ectopic endometrium of women with endometriosis: The possible immune privilege of ectopic endometrium. Reprod Sci 2016; 23: 81-86. [DOI:10.1177/1933719115594019] [PMID]
10. Liu Z, Li F, Xue J, Wang M, Lai S, Bao H, et al. Esculentoside A rescues granulosa cell apoptosis and folliculogenesis in mice with premature ovarian failure. Aging (Albany NY) 2020; 12: 16951-16962. [DOI:10.18632/aging.103609] [PMID] [PMCID]
11. Harada T, Taniguchi F, Izawa M, Ohama Y, Takenaka Y, Tagashira Y, et al. Apoptosis and endometriosis. Frontiers in Bioscience 2007; 12: 3140-3151. [DOI:10.2741/2302] [PMID]
12. Lettau M, Paulsen M, Schmidt H, Janssen O. Insights into the molecular regulation of FasL (CD178) biology. Eur J Cell Biol 2011; 90: 456-466. [DOI:10.1016/j.ejcb.2010.10.006] [PMID]
13. Eslami MM, Rezaei R, Abdollahi S, Davari A, Ahmadvand M. FAS-670A > G gene polymorphism and the risk of allograft rejection after organ transplantation: A systematic review and meta-analysis. Blood Res 2021; 56: 17-25. [DOI:10.5045/br.2021.2020201] [PMID] [PMCID]
14. Sibley K, Rollinson S, Allan JM, Smith AG, Law GR, Roddam PL, et al. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res 2003; 63: 4327-4330.
15. Akhavan Sales Zh, Tahoori MT, Sheikhha MH, Seifati SM, Bitaraf Sani M. Identification of a FAS/FASL haplotype associated with endometriosis in Iranian patients. Gynecol Endocrinol 2020; 36: 261-264. [DOI:10.1080/09513590.2019.1655729] [PMID]
16. Jafari M, Nasiri MR, Sanaei R, Anoosheh S, Farnia P, Sepanjnia A, et al. The NRAMP1, VDR, TNF-α, ICAM1, TLR2 and TLR4 gene polymorphisms in Iranian patients with pulmonary tuberculosis: A case-control study. Infect Genet Evol 2016; 39: 92-98. [DOI:10.1016/j.meegid.2016.01.013] [PMID]
17. Mohammadzadeh A, Pourfathollah AA, Sahraian MA, Behmanesh M, Daneshmandi S, Moeinfar Z, et al. Evaluation of apoptosis-related genes: FAS (CD94), FASL (CD178) and TRAIL polymorphisms in Iranian multiple sclerosis patients. J Neurol Sci 2012; 312: 166-169. [DOI:10.1016/j.jns.2011.07.037] [PMID]
18. Zhang Z, Wang LE, Sturgis EM, El-Naggar AK, Hong WK, Amos CI, et al. Polymorphisms of FAS and FAS ligand genes involved in the death pathway and risk and progression of squamous cell carcinoma of the head and neck. Clin Cancer Res 2006; 12: 5596-5602. [DOI:10.1158/1078-0432.CCR-05-1739] [PMID]
19. Agic A, Djalali S, Diedrich K, Hornung D. Apoptosis in endometriosis. Gynecol Obstet Invest 2009; 68: 217-223. [DOI:10.1159/000235871] [PMID]
20. D'Arcy MS. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 2019; 43: 582-592. [DOI:10.1002/cbin.11137] [PMID]
21. Slot KA, Voorendt M, de Boer-Brouwer M, van Vugt HH, Teerds KJ. Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro-and active caspase-3 in the rat ovary. J Endocrinol 2006; 188: 179-192. [DOI:10.1677/joe.1.06165] [PMID]
22. Mohammadzadeh A, Pourfathollah AA, Tahoori MT, Daneshmandi S, Langroudi L, Akhlaghi M. Evaluation of apoptosis-related gene FAS (CD95) and FASL (CD178) polymorphisms in Iranian rheumatoid arthritis patients. Rheumatol Int 2012; 32: 2833-2836. [DOI:10.1007/s00296-011-2065-x] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





