Sat, Feb 21, 2026
[Archive]
Volume 21, Issue 5 (May 2023)
IJRM 2023, 21(5): 387-394 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soltani S, Aghaee A, Zakavi S R, Mottaghi M, Emadzadeh M, Rahmani S et al . Effects of radioiodine therapy on fertility indicators among men with differentiated thyroid cancer: A cohort study. IJRM 2023; 21 (5) :387-394
URL: http://ijrm.ir/article-1-2543-en.html
URL: http://ijrm.ir/article-1-2543-en.html
Salman Soltani1 

 , Atena Aghaee2
, Atena Aghaee2 

 , Seyed Rasoul Zakavi2
, Seyed Rasoul Zakavi2 

 , Mahdi Mottaghi1
, Mahdi Mottaghi1 

 , Maryam Emadzadeh3
, Maryam Emadzadeh3 

 , Sahar Rahmani4
, Sahar Rahmani4 

 , Soheil Kasaeian Naeini *5
, Soheil Kasaeian Naeini *5 




 , Atena Aghaee2
, Atena Aghaee2 

 , Seyed Rasoul Zakavi2
, Seyed Rasoul Zakavi2 

 , Mahdi Mottaghi1
, Mahdi Mottaghi1 

 , Maryam Emadzadeh3
, Maryam Emadzadeh3 

 , Sahar Rahmani4
, Sahar Rahmani4 

 , Soheil Kasaeian Naeini *5
, Soheil Kasaeian Naeini *5 


1- Kidney Transplantation Complications Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Nuclear Medicine Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Clinical Research Development Unit, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Kidney Transplantation Complications Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
5- Kidney Transplantation Complications Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. ,kasaeianns971@mums.ac.ir
2- Nuclear Medicine Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Clinical Research Development Unit, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Kidney Transplantation Complications Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
5- Kidney Transplantation Complications Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. ,
Full-Text [PDF 657 kb]
(1324 Downloads)
| Abstract (HTML) (2630 Views)
1. Introduction
Thyroid cancer is the most common endocrine malignancy. In 2021, it accounted for 2.3% of all new cancer diagnoses (1, 2). The American Cancer Society estimated that 43,800 new cases would be diagnosed with thyroid cancer in 2022 (1). Differentiated thyroid cancer (DTC), namely follicular and papillary thyroid cancers, account for more than 90% of thyroid cancers. The standard management of DTC is surgical tumor removal followed by radioactive iodine (RAI) ablation (3). Iodine-131 (I-131) can harm the radiation-sensitive tissues, which are mostly composed of labile cells (cells that are continuously divided). Although multiple studies have focused on the effects of radiation on the female reproductive system, male fertility-related complications have not been considered widely (4-6). While women have their oocyte bank at birth in a quiescent status, spermatogonia undergo continuous meiosis and are more vulnerable to radiation.
The β and γ radiations from RAI therapy can cause testicular damage (4). Spermatogonia are amongst the most radiation-sensitive cells. The instant injury to the testes arises from direct radiation from I-131 in the blood and its accumulation in the bladder. Pelvic irradiation in the presence of pelvic metastases also directly affects spermatogonia. The released derivatives of irradiated thyroid or cancer cells, thyroglobulins, and thyroxine into the blood cause delayed harmful effects on spermatognia (3). The estimated absorptive dose of testis after RAI is 27-54 mGy/ giga-becquerel (GBq) (7, 8). DNA alterations are expected following irradiation. A case report showed that following a single dose of 150 millicuries (mCi)/5.56 GBq I-131 therapy, the DNA fragmentation index returned to normal values after 3.25 months following RAI (3). Thus, RAI ablation can affect sperm quality, and previous studies showed hormonal changes after such treatment modality (7, 9).
Thyroid cancer tends to affect individuals at a younger age compared to other adult malignancies. The median age at diagnosis was 51 yr, while 36.1% of cases occur at < 44 yr (2); thus, a delicate evaluation of testes function is important for men with DTC, especially for those candidates for high-dose RAI cases or who suffer pelvic metastases. Several studies have also recommended sperm banking before iodine therapy, which is still controversial (3, 10).
We aimed to observe the effects of iodine therapy on semen parameters and the hormonal status of men with DTC.
2. Materials and Methods
2.1. Study design and participants
This prospective cohort study evaluated the hormonal status and sperm analysis of men with well-differentiated thyroid carcinoma before and after iodine therapy. From June to December 2020, we included (in a census manner) all males with well-differentiated thyroid carcinoma aged between 20-45 yr, who were then referred to the Ghaem and Imam-Reza hospitals of Mashhad University Medical Sciences, Mashhad, Iran. Exclusion criteria were considered as not willing to participate, developing varicocele during the experiment, having previous cardiac, renal, metabolic, hepatic, and neurologic diseases, and having a history of any infertility intervention (medical and surgical). 18 men entered the study and were followed for 12 months after iodine therapy. Baseline assessment of the levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and sperm quality parameters (count, motility, and morphology) were done after thyroidectomy and before levothyroxine cessation, 3 wk before iodine therapy (Figure 1). Participants' sperm count, motility, and morphology were evaluated based on the 2010 World Health Organization criteria. We also interviewed participants for any history of sexual dysfunction, previous infertility, or related treatments. All men underwent a baseline urologic examination to assess confounding factors, like the development or progression of varicocele during the study.
2.2. Ethical considerations
The Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran, approved this study (Code: IR.MUMS.MEDICAL.REC.1399.277). All participants gave written informed consent for participation in the study and the anonymous publication of the results.
2.3. Statistical analysis
We used the SPSS 24.0 software (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.) to analyze the results with a p-value < 0.05 as the significance cut off. The included cases were analyzed once as a whole and once in groups. We used the Shapiro-Wilk test for normality assessment. Repeated measures ANOVA was used to analyze quantitative values with normal distribution. We also used Friedman's test to analyze variables lacking normal distribution.
3. Results
Out of 18 men who met the inclusion criteria, 8 cases received low-dose and 10 cases received high-dose iodine therapy. The mean ages were 37.62 ± 10.43, 34.00 ± 9.39, and 35.61 ± 9.74 yr for low-dose, high-dose, and total participants, respectively. Physical examination was normal in all cases except for 4 with clinical varicocele grades II-III (2 men in each group). None of the participants experienced infertility problems. The median cumulative iodine dose was 150 mCi (interquartile range: 30-150).
The mean levels of LH, FSH, testosterone, and sperm quality parameters (count, motility, and morphology) at baseline and 3 and 12 months after iodine therapy are presented in table I. The trend of these 3 checkpoints (repeated measures ANOVA or Friedman's test) is shown in the p-value column. During the study, significant changes were observed in all the participants LH and FSH values. However, the analysis revealed no statistically significant difference after grouping these men. Interestingly, the sperm count changes were significant with high-dose iodine therapy; however, it normalized after 12 months of RAI. Analysis of sperm motility by paired t test at baseline and after 12 months showed a significant decline (p = 0.043).
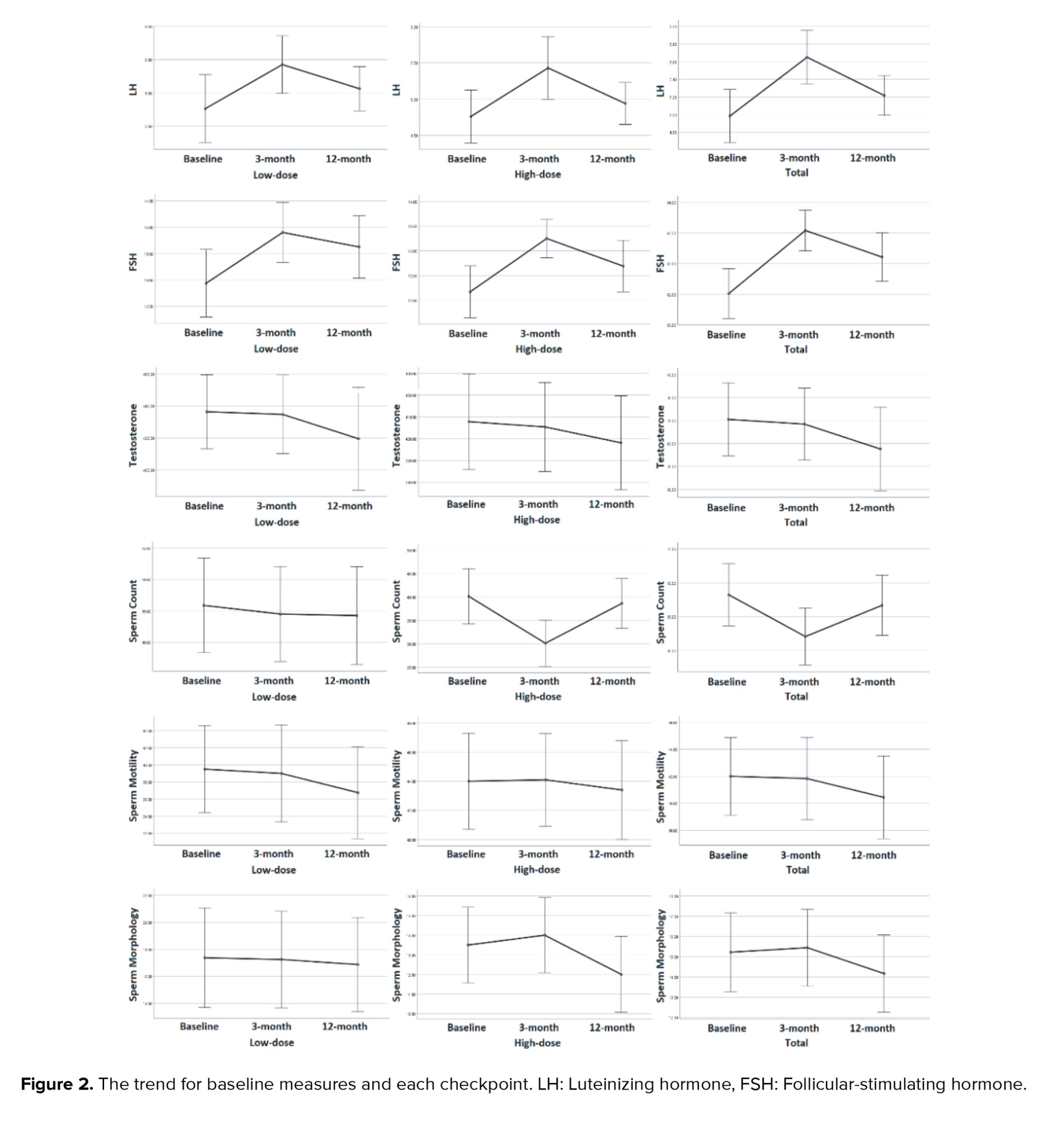
Figure 2 shows the trend for baseline measures and each checkpoint. The nadir and acme of each variable were unique chronologically; namely, the effects of iodine therapy were more significant in the 3-month follow-up for LH and FSH; however, its impact on morphology was more prominent at the 12-month follow-up.

4. Discussion
Our study aimed to evaluate the effects of iodine therapy on spermogram parameters in men with DTC. After 3 months, a significant increase in FSH and LH levels was detected at the first checkpoint, which returned to baseline at the last checkpoint. We also detected a significant decrease in sperm count, whereas no significant changes were observed in sperm motility and morphology.
RAI ablative therapy in managing DTC improves outcomes by targeting potential micrometastases. The treatment's main adverse effects (sialadenitis, gastrointestinal discomfort, leukemia, etc.) mostly involve rapid-cycling cells with many divisions (8). The germinal epithelium of the testis also consists of labile cells, and several studies have evaluated the effects of iodine therapy on sperm parameters (5, 6, 10, 11). Even an absorptive dose of 0.1 Gy can temporarily impair spermatogenesis (11). We detected a significant increase in FSH levels at the first checkpoint after 3 months. Although the changes seemed temporary, these measures tend to return to baseline at the last checkpoint. Multiple studies affirm a transient increase in FSH levels following iodine therapy; FSH tended to decrease toward normal levels after 9-12 months of RIA (9, 10). No statistical significance was observed in our subgroup analysis between low-dose (p = 0.081) and high-dose (p = 0.061) RAI in the magnitude of increased FSH levels. One has to interpret these results with caution, as several experiments evaluating higher radiation doses have declared a dose-dependent relation between RAI and FSH levels. Higher doses of RAI cause a more profound increase in FSH levels, but it is negligible as it mostly comes back toward normal levels (9, 10). Persistent FSH elevation was seen in cumulative doses higher than 14 GBq/378 mCi (10). However, another study with 25 participants did not show a dose-dependent effect on FSH levels; it should be noted that they compared radiation doses of 150-300 mCi with doses higher than 300 mCi (7, 8). A recent long-term study showed that RAI doses of 100 mCi/3.7 GBq or higher did not affect reproductive function, and sperm banking was not necessary routinely (4). However, men who desire to conceive within the first year of DTC ablation therapy should be informed about sperm quality and hormonal alterations after ablation.
LH and testosterone and the testosterone/LH ratio are indicators of Leydig cell function or testicular endocrine function (10). No significant differences were observed in testerone levels when LH levels were mirrored with the FSH changes. Such a trend has been observed in multiple studies (9, 10). Several studies similar to these surveys showed a significant increase in LH levels (from 3.0 ± 0.2 to 4.2 ± 0.3) with no changes in testosterone levels, suggesting a transient disturbance in Leydig cell function (9, 10). On the contrary, another study detected a significant LH increase in 9.6% of 52 cases who received 3.7-5.5 GBq radiation, while 22.7% of 22 cases who received ≥ 13 GBq radiation dose had elevated LH levels (10, 12). Another possible explanation for mentioned controversy on LH pattern is that Leydig cells are affected more than Sertoli cells in hypothyroidism (13); thus, individuals' adherence to thyroxine hormone replacement can potentially cause an impediment in LH trend assessment. As free testosterone remains in the normal range after RAI therapy, the exocrine functions of the testis seem to be more affected than endocrine roles (7). The impairment of testosterone production is expected at absorptive radiation doses greater than 20 Gy as the Leydig cells are more resistant to radiation (11). Future studies should focus on the exact effect of RAI on LH and testosterone levels.
We also observed changes in sperm count, motility, and morphology, of which sperm count changed significantly with an initial decrease followed by returning to normal after 12 months. However, sperm motility and morphology started to decline in the 12th month. Bourcigaux et al. found similar sperm concentration and motility results, but sperm morphology changes began after 3 months (10). In terms of long-term assessment, a recent study evaluated 51 men with DTC after a median of 5.8 yr (interquartile range: 3.0-9.5) after their last dose of I-131 with a cumulative dose of ≥ 3.7 GBq/100 mCi (4). They concluded that sperm count, progressive motility, and volume did not differ significantly from the general population and advised that semen banking should be considered individually. More extended damage is expected where azoospermia occurs with absorptive doses of 4 Gy (11).
The timing of hormonal measurement is crucial in assessing the fertility status of individuals with DTC treated with RAI. The TSH levels should be over 25 to ensure sufficient iodine absorption into the thyroid tissue(s); consequently, measuring FSH and LH when TSH is this high probably gives lower than expected results (14). We found one experiment that appropriately addressed this issue and performed hormonal analysis immediately before levothyroxine withdrawal (10), but some others have measured FSH and LH levels during the thyroxine withdrawal period (7, 8). Another finding of our observation was that the nadir and acme of each hormone occur at a specific checkpoint; as explained, iodine therapy affected LH and FSH at the 3-month follow-up. However, its impact on morphology was more prominent during the 12-month follow-up. Although it is transient, a thorough consultation with cases regarding fertility issues is needed if a patient desires to conceive within the first year of RAI therapy. It has also been shown previously for women that the risk of abortion increases within the first year of treatment (15).
4.1. Limitation and suggestions
The most significant limitation of the present study was its small sample size, probably secondary to not considering the psychological burden of the diagnosis on cases. Several cases refrained from participating because they did not receive proper psychological support after the cancer diagnosis, and asking them to participate in a study was relatively premature. Although this small sample size can compromise the validity of our conclusion, as it is mostly in line with similar studies of the female population (in terms of LH and FSH), we believe this effect is negligible. Other limitations were not including TSH, prolactin, inhibin B, and DNA fragmentation index in our fertility analysis. We suggest further studies in performing baseline hormonal assessment before the cessation of thyroid hormones for RAI therapy. Assessing baseline and follow-up of TSH levels helps determine individuals' adherence to thyroid hormone replacement and provides more reliable results. Due to insufficient data on the effects of iodine therapy on prolactin, evaluation of this hormone could be considered. Occupational status, previous history of medications, smoking, body mass index, and probable fever episodes in the last 3 months should also be considered.
5. Conclusion
Low-dose iodine therapy in men with DTC can cause short-term spermatogenesis defects, which are usually relieved within a year. Higher doses expectedly cause more profound damage, especially on sperm count. Men experience increased FSH and LH levels with no significant change in testosterone. Sperm count is affected more than sperm motility or morphology during the first year. Although such effects are transient, a thorough consultation regarding fertility issues is needed if a man desires to conceive within the first year of radioiodine treatment.
Acknowledgments
We acknowledge the support of the Clinical Research Development Unit of Ghaem hospital of Mashhad University of Medical Sciences, Mashhad, Iran, for their contribution to this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (371 Views)
1. Introduction
Thyroid cancer is the most common endocrine malignancy. In 2021, it accounted for 2.3% of all new cancer diagnoses (1, 2). The American Cancer Society estimated that 43,800 new cases would be diagnosed with thyroid cancer in 2022 (1). Differentiated thyroid cancer (DTC), namely follicular and papillary thyroid cancers, account for more than 90% of thyroid cancers. The standard management of DTC is surgical tumor removal followed by radioactive iodine (RAI) ablation (3). Iodine-131 (I-131) can harm the radiation-sensitive tissues, which are mostly composed of labile cells (cells that are continuously divided). Although multiple studies have focused on the effects of radiation on the female reproductive system, male fertility-related complications have not been considered widely (4-6). While women have their oocyte bank at birth in a quiescent status, spermatogonia undergo continuous meiosis and are more vulnerable to radiation.
The β and γ radiations from RAI therapy can cause testicular damage (4). Spermatogonia are amongst the most radiation-sensitive cells. The instant injury to the testes arises from direct radiation from I-131 in the blood and its accumulation in the bladder. Pelvic irradiation in the presence of pelvic metastases also directly affects spermatogonia. The released derivatives of irradiated thyroid or cancer cells, thyroglobulins, and thyroxine into the blood cause delayed harmful effects on spermatognia (3). The estimated absorptive dose of testis after RAI is 27-54 mGy/ giga-becquerel (GBq) (7, 8). DNA alterations are expected following irradiation. A case report showed that following a single dose of 150 millicuries (mCi)/5.56 GBq I-131 therapy, the DNA fragmentation index returned to normal values after 3.25 months following RAI (3). Thus, RAI ablation can affect sperm quality, and previous studies showed hormonal changes after such treatment modality (7, 9).
Thyroid cancer tends to affect individuals at a younger age compared to other adult malignancies. The median age at diagnosis was 51 yr, while 36.1% of cases occur at < 44 yr (2); thus, a delicate evaluation of testes function is important for men with DTC, especially for those candidates for high-dose RAI cases or who suffer pelvic metastases. Several studies have also recommended sperm banking before iodine therapy, which is still controversial (3, 10).
We aimed to observe the effects of iodine therapy on semen parameters and the hormonal status of men with DTC.
2. Materials and Methods
2.1. Study design and participants
This prospective cohort study evaluated the hormonal status and sperm analysis of men with well-differentiated thyroid carcinoma before and after iodine therapy. From June to December 2020, we included (in a census manner) all males with well-differentiated thyroid carcinoma aged between 20-45 yr, who were then referred to the Ghaem and Imam-Reza hospitals of Mashhad University Medical Sciences, Mashhad, Iran. Exclusion criteria were considered as not willing to participate, developing varicocele during the experiment, having previous cardiac, renal, metabolic, hepatic, and neurologic diseases, and having a history of any infertility intervention (medical and surgical). 18 men entered the study and were followed for 12 months after iodine therapy. Baseline assessment of the levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and sperm quality parameters (count, motility, and morphology) were done after thyroidectomy and before levothyroxine cessation, 3 wk before iodine therapy (Figure 1). Participants' sperm count, motility, and morphology were evaluated based on the 2010 World Health Organization criteria. We also interviewed participants for any history of sexual dysfunction, previous infertility, or related treatments. All men underwent a baseline urologic examination to assess confounding factors, like the development or progression of varicocele during the study.
2.2. Ethical considerations
The Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran, approved this study (Code: IR.MUMS.MEDICAL.REC.1399.277). All participants gave written informed consent for participation in the study and the anonymous publication of the results.
2.3. Statistical analysis
We used the SPSS 24.0 software (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.) to analyze the results with a p-value < 0.05 as the significance cut off. The included cases were analyzed once as a whole and once in groups. We used the Shapiro-Wilk test for normality assessment. Repeated measures ANOVA was used to analyze quantitative values with normal distribution. We also used Friedman's test to analyze variables lacking normal distribution.
3. Results
Out of 18 men who met the inclusion criteria, 8 cases received low-dose and 10 cases received high-dose iodine therapy. The mean ages were 37.62 ± 10.43, 34.00 ± 9.39, and 35.61 ± 9.74 yr for low-dose, high-dose, and total participants, respectively. Physical examination was normal in all cases except for 4 with clinical varicocele grades II-III (2 men in each group). None of the participants experienced infertility problems. The median cumulative iodine dose was 150 mCi (interquartile range: 30-150).
The mean levels of LH, FSH, testosterone, and sperm quality parameters (count, motility, and morphology) at baseline and 3 and 12 months after iodine therapy are presented in table I. The trend of these 3 checkpoints (repeated measures ANOVA or Friedman's test) is shown in the p-value column. During the study, significant changes were observed in all the participants LH and FSH values. However, the analysis revealed no statistically significant difference after grouping these men. Interestingly, the sperm count changes were significant with high-dose iodine therapy; however, it normalized after 12 months of RAI. Analysis of sperm motility by paired t test at baseline and after 12 months showed a significant decline (p = 0.043).
Figure 2 shows the trend for baseline measures and each checkpoint. The nadir and acme of each variable were unique chronologically; namely, the effects of iodine therapy were more significant in the 3-month follow-up for LH and FSH; however, its impact on morphology was more prominent at the 12-month follow-up.



4. Discussion
Our study aimed to evaluate the effects of iodine therapy on spermogram parameters in men with DTC. After 3 months, a significant increase in FSH and LH levels was detected at the first checkpoint, which returned to baseline at the last checkpoint. We also detected a significant decrease in sperm count, whereas no significant changes were observed in sperm motility and morphology.
RAI ablative therapy in managing DTC improves outcomes by targeting potential micrometastases. The treatment's main adverse effects (sialadenitis, gastrointestinal discomfort, leukemia, etc.) mostly involve rapid-cycling cells with many divisions (8). The germinal epithelium of the testis also consists of labile cells, and several studies have evaluated the effects of iodine therapy on sperm parameters (5, 6, 10, 11). Even an absorptive dose of 0.1 Gy can temporarily impair spermatogenesis (11). We detected a significant increase in FSH levels at the first checkpoint after 3 months. Although the changes seemed temporary, these measures tend to return to baseline at the last checkpoint. Multiple studies affirm a transient increase in FSH levels following iodine therapy; FSH tended to decrease toward normal levels after 9-12 months of RIA (9, 10). No statistical significance was observed in our subgroup analysis between low-dose (p = 0.081) and high-dose (p = 0.061) RAI in the magnitude of increased FSH levels. One has to interpret these results with caution, as several experiments evaluating higher radiation doses have declared a dose-dependent relation between RAI and FSH levels. Higher doses of RAI cause a more profound increase in FSH levels, but it is negligible as it mostly comes back toward normal levels (9, 10). Persistent FSH elevation was seen in cumulative doses higher than 14 GBq/378 mCi (10). However, another study with 25 participants did not show a dose-dependent effect on FSH levels; it should be noted that they compared radiation doses of 150-300 mCi with doses higher than 300 mCi (7, 8). A recent long-term study showed that RAI doses of 100 mCi/3.7 GBq or higher did not affect reproductive function, and sperm banking was not necessary routinely (4). However, men who desire to conceive within the first year of DTC ablation therapy should be informed about sperm quality and hormonal alterations after ablation.
LH and testosterone and the testosterone/LH ratio are indicators of Leydig cell function or testicular endocrine function (10). No significant differences were observed in testerone levels when LH levels were mirrored with the FSH changes. Such a trend has been observed in multiple studies (9, 10). Several studies similar to these surveys showed a significant increase in LH levels (from 3.0 ± 0.2 to 4.2 ± 0.3) with no changes in testosterone levels, suggesting a transient disturbance in Leydig cell function (9, 10). On the contrary, another study detected a significant LH increase in 9.6% of 52 cases who received 3.7-5.5 GBq radiation, while 22.7% of 22 cases who received ≥ 13 GBq radiation dose had elevated LH levels (10, 12). Another possible explanation for mentioned controversy on LH pattern is that Leydig cells are affected more than Sertoli cells in hypothyroidism (13); thus, individuals' adherence to thyroxine hormone replacement can potentially cause an impediment in LH trend assessment. As free testosterone remains in the normal range after RAI therapy, the exocrine functions of the testis seem to be more affected than endocrine roles (7). The impairment of testosterone production is expected at absorptive radiation doses greater than 20 Gy as the Leydig cells are more resistant to radiation (11). Future studies should focus on the exact effect of RAI on LH and testosterone levels.
We also observed changes in sperm count, motility, and morphology, of which sperm count changed significantly with an initial decrease followed by returning to normal after 12 months. However, sperm motility and morphology started to decline in the 12th month. Bourcigaux et al. found similar sperm concentration and motility results, but sperm morphology changes began after 3 months (10). In terms of long-term assessment, a recent study evaluated 51 men with DTC after a median of 5.8 yr (interquartile range: 3.0-9.5) after their last dose of I-131 with a cumulative dose of ≥ 3.7 GBq/100 mCi (4). They concluded that sperm count, progressive motility, and volume did not differ significantly from the general population and advised that semen banking should be considered individually. More extended damage is expected where azoospermia occurs with absorptive doses of 4 Gy (11).
The timing of hormonal measurement is crucial in assessing the fertility status of individuals with DTC treated with RAI. The TSH levels should be over 25 to ensure sufficient iodine absorption into the thyroid tissue(s); consequently, measuring FSH and LH when TSH is this high probably gives lower than expected results (14). We found one experiment that appropriately addressed this issue and performed hormonal analysis immediately before levothyroxine withdrawal (10), but some others have measured FSH and LH levels during the thyroxine withdrawal period (7, 8). Another finding of our observation was that the nadir and acme of each hormone occur at a specific checkpoint; as explained, iodine therapy affected LH and FSH at the 3-month follow-up. However, its impact on morphology was more prominent during the 12-month follow-up. Although it is transient, a thorough consultation with cases regarding fertility issues is needed if a patient desires to conceive within the first year of RAI therapy. It has also been shown previously for women that the risk of abortion increases within the first year of treatment (15).
4.1. Limitation and suggestions
The most significant limitation of the present study was its small sample size, probably secondary to not considering the psychological burden of the diagnosis on cases. Several cases refrained from participating because they did not receive proper psychological support after the cancer diagnosis, and asking them to participate in a study was relatively premature. Although this small sample size can compromise the validity of our conclusion, as it is mostly in line with similar studies of the female population (in terms of LH and FSH), we believe this effect is negligible. Other limitations were not including TSH, prolactin, inhibin B, and DNA fragmentation index in our fertility analysis. We suggest further studies in performing baseline hormonal assessment before the cessation of thyroid hormones for RAI therapy. Assessing baseline and follow-up of TSH levels helps determine individuals' adherence to thyroid hormone replacement and provides more reliable results. Due to insufficient data on the effects of iodine therapy on prolactin, evaluation of this hormone could be considered. Occupational status, previous history of medications, smoking, body mass index, and probable fever episodes in the last 3 months should also be considered.
5. Conclusion
Low-dose iodine therapy in men with DTC can cause short-term spermatogenesis defects, which are usually relieved within a year. Higher doses expectedly cause more profound damage, especially on sperm count. Men experience increased FSH and LH levels with no significant change in testosterone. Sperm count is affected more than sperm motility or morphology during the first year. Although such effects are transient, a thorough consultation regarding fertility issues is needed if a man desires to conceive within the first year of radioiodine treatment.
Acknowledgments
We acknowledge the support of the Clinical Research Development Unit of Ghaem hospital of Mashhad University of Medical Sciences, Mashhad, Iran, for their contribution to this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. National Cancer Institute. New research into thyroid cancer. 2021. Available at: https://www.cancer.org/cancer/thyroid-cancer/about/new-research.html.
2. National Cancer Institute. SEER stat fact sheets: Thyroid cancer. SEER cancer statistics review, 1975-2018, National Cancer Institute. 2021. Available at: https://seer.cancer.gov/statfacts/html/thyro.html.
3. Esquerré-Lamare C, Isus F, Moinard N, Bujan L. Sperm DNA fragmentation after radioiodine treatment for differentiated thyroid cancer. Basic Clin Androl 2015; 25: 8. [DOI:10.1186/s12610-015-0024-1] [PMID] [PMCID]
4. Nies M, Arts EGJM, van Velsen EF, Burgerhof JGM, Kobold ACM, Corssmit EPM, et al. Long-term male fertility after treatment with radioactive iodine for differentiated thyroid carcinoma. Eur J Endocrinol 2021; 185: 775-782. [DOI:10.1530/EJE-21-0315] [PMID]
5. van Velsen EFS, Visser WE, van den Berg SA, Kam BL, van Ginhoven TM, Massolt ET, et al. Longitudinal analysis of the effect of radioiodine therapy on ovarian reserve in females with differentiated thyroid cancer. Thyroid 2020; 30: 580-587. [DOI:10.1089/thy.2019.0504] [PMID]
6. Yaish I, Azem F, Gutfeld O, Silman Z, Serebro M, Sharon O, et al. A single radioactive iodine treatment has a deleterious effect on ovarian reserve in women with thyroid cancer: Results of a prospective pilot study. Thyroid 2018; 28: 522-527. [DOI:10.1089/thy.2017.0442] [PMID]
7. Wichers M, Benz E, Palmedo H, Biersack HJ, Grünwald F, Klingmüller D. Testicular function after radioiodine therapy for thyroid carcinoma. Eur J Nucl Med 2000; 27: 503-507. [DOI:10.1007/s002590050535] [PMID]
8. Fard-Esfahani A, Emami-Ardekani A, Fallahi B, Fard-Esfahani P, Beiki D, Hassanzadeh-Rad A, et al. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun 2014; 35: 808-817. [DOI:10.1097/MNM.0000000000000132] [PMID]
9. Canale D, Ceccarelli C, Caglieresi C, Moscatelli A, Gavioli S, Santini P, et al. Effects of radioiodine treatment for differentiated thyroid cancer on testis function. Clin Endocrinol 2015; 82: 295-299. [DOI:10.1111/cen.12514] [PMID]
10. Bourcigaux N, Rubino C, Berthaud I, Toubert ME, Donadille B, Leenhardt L, et al. Impact on testicular function of a single ablative activity of 3.7 GBq radioactive iodine for differentiated thyroid carcinoma. Hum Reprod 2018; 33: 1408-1416. [DOI:10.1093/humrep/dey222] [PMID]
11. Sayan M, Cassidy R, Butker EE, Nanda RH, Krishnamurti L, Khan MK, et al. Gonadal shielding technique to preserve fertility in male pediatric patients treated with total body irradiation for stem cell transplantation. Bone Marrow Transplant 2016; 51: 997-998. [DOI:10.1038/bmt.2016.25] [PMID]
12. Rosario PW, Xavier ACM. Recombinant human thyroid stimulating hormone in thyroid remnant ablation with 1.1 GBq 131iodine in low-risk patients. Am J Clin Oncol 2012; 35: 101-104. [DOI:10.1097/COC.0b013e318201a41a] [PMID]
13. Ambigapathy JS, Kamalanathan S, Sahoo J, Kumar R, Perumal NL. Effect of thyroxine replacement on leydig cell and sertoli cell function in men with hypothyroidism. Indian J Endocrinol Metab 2020; 24: 265-269. [DOI:10.4103/ijem.IJEM_69_20] [PMID] [PMCID]
14. Alahmar A, Dutta S, Sengupta P. Thyroid hormones in male reproduction and infertility. Asian Pac J Reprod 2019; 8: 203-210. [DOI:10.4103/2305-0500.268135]
15. Zhang L, Huang Y, Zheng Y, Cai L, Wen J, Chen G. The effect of I-131 therapy on pregnancy outcomes after thyroidectomy in patients with differentiated thyroid carcinoma: A meta-analysis. Endocrine 2021; 73: 301-307. [DOI:10.1007/s12020-021-02657-6] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




