Fri, Feb 20, 2026
[Archive]
Volume 21, Issue 7 (July 2023)
IJRM 2023, 21(7): 541-550 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahramian H, Sherafatmanesh S, Asadi N, Bakhshi A, Eftekhari M H, Ekramzadeh M. Effects of single-dose and co-supplementation of vitamin D and omega-3 on metabolic profile in women with polycystic ovary syndrome: An RCT. IJRM 2023; 21 (7) :541-550
URL: http://ijrm.ir/article-1-2569-en.html
URL: http://ijrm.ir/article-1-2569-en.html
Hadis Bahramian1 

 , Saeed Sherafatmanesh2
, Saeed Sherafatmanesh2 

 , Nasrin Asadi3
, Nasrin Asadi3 

 , Ali Bakhshi4
, Ali Bakhshi4 

 , Mohammad Hassan Eftekhari5
, Mohammad Hassan Eftekhari5 

 , Maryam Ekramzadeh *6
, Maryam Ekramzadeh *6 




 , Saeed Sherafatmanesh2
, Saeed Sherafatmanesh2 

 , Nasrin Asadi3
, Nasrin Asadi3 

 , Ali Bakhshi4
, Ali Bakhshi4 

 , Mohammad Hassan Eftekhari5
, Mohammad Hassan Eftekhari5 

 , Maryam Ekramzadeh *6
, Maryam Ekramzadeh *6 


1- Student Research Committee, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Nutrition and Food Security Research Center, Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Clinical Biochemistry, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
6- Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran. Division of Nephrology and Hypertension, Lundquist Institute, Harbor-UCLA Medical Center, Torrance, CA 90502, USA. ,mekramzade@gmail.com
2- Nutrition and Food Security Research Center, Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Clinical Biochemistry, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
6- Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran. Division of Nephrology and Hypertension, Lundquist Institute, Harbor-UCLA Medical Center, Torrance, CA 90502, USA. ,
Keywords: Polycystic ovary syndrome, Vitamin D, Omega-3 fatty acid, Insulin, Sex hormone-binding globulin.
Full-Text [PDF 316 kb]
(1208 Downloads)
| Abstract (HTML) (1800 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine ailment, affecting 5-10% of females worldwide during their reproductive period (1). It is the main cause of infertility and unfavorable pregnancy consequences caused by dysfunctional phase of follicular maturation and ovulation, multicystic condition of ovaries, and hyperandrogenism (1). Furthermore, PCOS along with a group of risk factors is related to the development of cardiovascular disease and type 2 diabetes including insulin resistance, hyperglycemia, lipid profile abnormalities, and abdominal obesity (2).
Although the etiology of PCOS remains unclear, the underlying preventable and controllable mechanisms like obesity, hyperinsulinemia, and hyperandrogenism have provided new insights into the pathogenesis of this disease (3). It has been documented that individuals with PCOS can benefit from a combination of different nutrients, as well as improve their overall nutritional status (4). Hence, many researchers have focused on nutrition-based lifestyle modification interventions in women with PCOS as a global necessity.
Vitamin D (vit D) deficiency is one of the well-known health problems observed in nearly 67-85% of women with PCOS, worldwide (5). The effects of vit D are mediated by both genetic and cellular mechanisms. It controls gene transcription via the intracellular distribution of nuclear vit D receptors in several tissues, specifically the ovaries (6). There is accumulating evidence that shows that serum vit D levels may be negatively implicated in PCOS symptoms, including obesity, hyperlipidemia, insulin resistance, hyperandrogenism, diminished sex hormone binding globulin (SHBG) production, and higher risks of cardiovascular disease, and abortion (7, 8). Also, it has been reported that vit D deficiency may contribute to the dysregulation of calcium metabolism, which in turn led to advanced follicular arrest and menstrual disturbances in women with PCOS (9).
Moreover, it has been documented that the consumption of unsaturated fat-rich foods may result in the improvement of PCOS metabolic impairments (10). As a bioactive anti-inflammatory agent, it has been confirmed that omega-3 (O3) supplementation can exhibit beneficial effects in improving parameters such as fasting glucose, insulin resistance, triglyceride level, and ovulation function (11). Furthermore, this dietary supplement may be used for ameliorating insulin sensitivity by reducing the generation of inflammatory cytokines including tumor necrosis factor alpha and interleukin 6 (12).
Although the efficacy of single supplements (vit D and O3) has been evaluated in previous studies, the synergistic effects of both supplements in attenuating lipid and glycemic profiles have not been investigated in PCOS cases till now. Therefore, the present study aimed to determine the effects of single-dose and co-supplementation of vit D and O3 for the management of PCOS complications.
2. Materials and Methods
2.1. Study design and participants
This double-blind, randomized clinical trial was conducted on 80 PCOS women. An established diagnosis of PCOS based on Rotterdam diagnostic criteria and vit D deficiency (25-hydroxyvitamin D ≤ 20 nmol/L) was recruited after screening 107 participants (aged 18-45 yr) from outpatients visiting, 18.5 ≤ body mass index (BMI) ≤ 40 referred to Shahid Motahhari Clinic, Shiraz, Iran, from April to October 2017.
The exclusion criteria were listed as follows: having a background of chronic illnesses such as cancer, heart disease, diabetes, stroke, fibromyalgia, kidney or liver defects, having a history of food and drug allergies, starting the drug or surgical therapy for clinical symptoms associated with PCOS except for oral contraceptive pills, smoking or any drug addiction, pregnancy and lactation, being on a special diet in the last year, using any dietary supplement, having oral or injectable nutritional supplements containing vit D in the last 3 months, consuming nutritional supplements containing fish oil or O3 fatty acids in the last 3 months, having fish in the diet more than 3 servings per week during last 3 months, background of serious side effects or signs of poisoning from the study's supplements, and failure to follow the research protocol.
2.2. Sample size
With respect to the type I error of 5% (α = 0.05), and type II error of 20% from the prior investigation in PCOS cases (13), the sample size was calculated based on the following formula:

2.3. Randomization, blinding, and interventions
Computer-generated randomization was used to complete the randomization process, and a qualified clinician carried out the randomized allocation procedure and allocated individuals as 1: 1: 1: 1 to each of the study groups. The randomization sequence concealment continued until the end of the intervention course. The researcher and participants were blinded.
The participants were randomly divided into 4 groups (n = 20/each) for 8 wk by block randomization with a fixed block size of 4 for 8 wk as follows:
2.4. Outcomes
Measurements were performed by trained investigators before and after 8 wk of intervention, including height, weight, BMI, waist circumference (WC), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), fasting blood sugar (FBS), homeostasis model of insulin resistance index (HOMA-IR), serum insulin level, physical activity, and SHBG were compared between groups. All laboratory procedures were carried out following accepted laboratory practices under the supervision of competent professionals in the Nutrition and Food Science School's lab at Shiraz University of Medical Sciences, Shiraz, Iran.
2.5. Ethical considerations
All participants were informed about the study objectives and asked to sign the written consent form. The procedure of this study was conducted according to the Declaration of Helsinki. It was also approved by the Ethics Committee on Human Experimentation of Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR.SUMS.REC.1396.103). Furthermore, this trial was registered at the Iranian Registry of Clinical Trials and has been updated on September 19, 2022.
2.6. Statistical analysis
Statistical analysis was done using the SPSS software (Statistical Package for the Social Sciences, version 22.0, SPSS Inc, Chicago, Illinois, USA). First, the normal distribution of the data was assessed by Kolmogorov-Smirnov test. The Kruskal-Wallis test was used to compare the 4 groups regarding mean differences. Then, the Mann-Whitney U test was employed for comparing the means of quantitative data between groups. The Wilcoxon test was used to compare the changes in variables before and after the intervention in each group. All the differences were considered statistically significant at p ≤ 0.05.
3. Results
Table I summarizes the general characteristics of the research participants. At the beginning of the experiment, no statistically significant differences were identified among the 4 study groups, as shown in the table. 4 people were dropped from the research during the intervention phase because of non-compliance or heart surgery, and 76 individuals completed the trial (Figure 1). No serious side effects were detected in all study groups.
3.1. Anthropometric indices
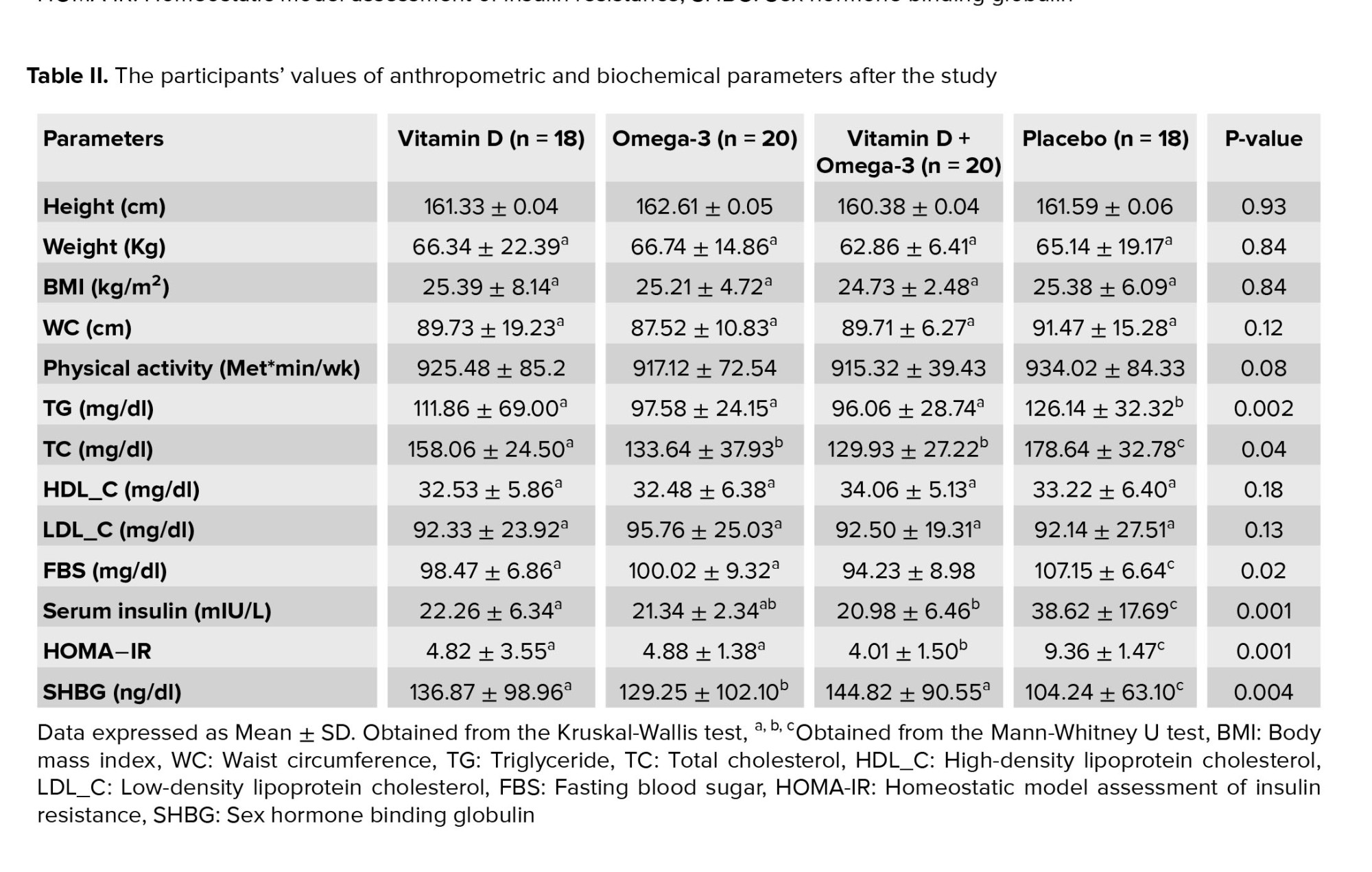
By the end of the study, no significant differences were observed between all study groups in terms of height, body weight, BMI, WC, and physical activity (Table II).
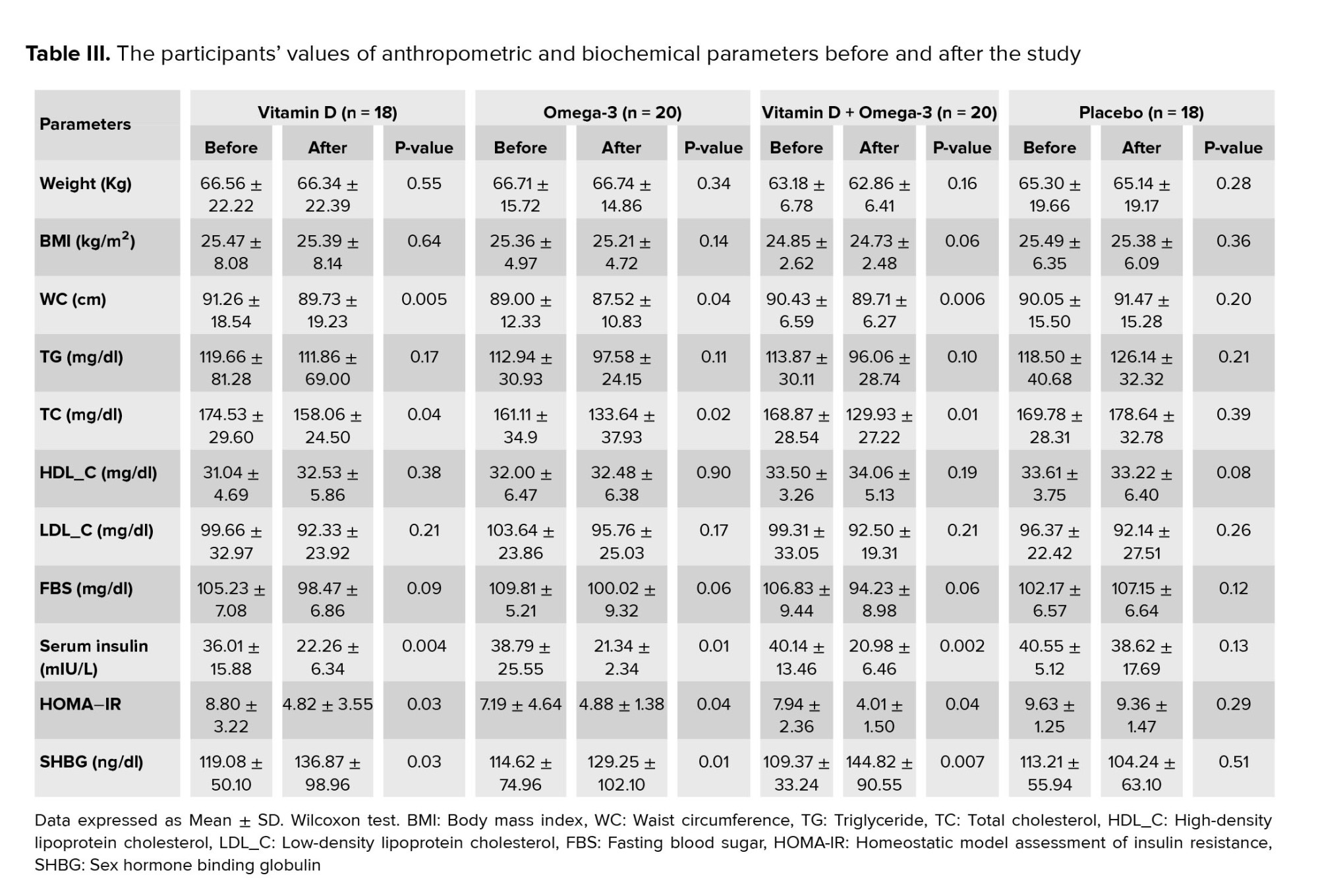
The mean values of anthropometric and biochemical parameters in each study group, before and after the study, have been presented in (Table III). After 8 wk intervention, the WC showed a significant decrease in vit D and O3 groups in comparison with the baseline levels. Also, compared to the values at the beginning of the study, a statistically significant reduction of WC was observed in vit D + O3 treated participants. However, at the end of the study, no significant difference was found in terms of body weight and BMI parameters in all the study groups compared to the baseline period.
3.2. Serum lipid profile
The effect of study treatments on the serum biochemical indices has been shown in (Table II). By the end of the experiment, no statistically significant difference was observed between any of the study groups regarding the serum HDL_C and LDL_C levels. Meanwhile, vit D, O3, and vit D + O3 treatments resulted in significantly lower TG levels as compared to cases treated with placebo [(p = 0.02), (p = 0.004), and (p = 0.004), respectively]. Furthermore, compared to the placebo group, all the vit D, O3, and vit D + O3 treatments showed a significant decrease in TC concentration after the study [(p = 0.04), (p = 0.01), and (p = 0.008), respectively].
In addition, compared to the baseline course, serum TC was remarkably decreased in vit D, O3, and vit D + O3 supplemented groups. Nevertheless, there were no significant changes in other serum lipid parameters at the end of the study when compared to the baseline (Table III).
3.3. FBS, serum insulin, and HOMA−IR parameters
By the end of the study, vit D, O3, and vit D + O3 interventions led to a significantly lower FBS level in comparison with the individuals treated with placebo [(p = 0.01), (p = 0.03), and (p = 0.007), respectively]. Moreover, a lower concentration of serum insulin was detected in the vit D, O3, and vit D + O3 groups in comparison with the amounts in the placebo group [(p = 0.002), (p = 0.002), and (p = 0.001), respectively]. As well, compared to the placebo group, interventions with vit D, O3, and vit D + O3 provided significantly lower HOMA-IR levels [(p = 0.002), (p = 0.007), and (p = 0.001), respectively]. In this regard, compared to the vit D and O3 treatments, the oral co-supplementation with vit D + O3 was accompanied by a synergistically significant reduction in serum FBS [(p = 0.04), (p = 0.03), respectively] and HOMA-IR [(p = 0.02), (p = 0.01), respectively] levels (Table II).
Moreover, in comparison with the baseline period, serum insulin was notably decreased in the vit D, O3, and vit D + O3 treated groups when compared to the baseline. Furthermore, a statistically significant reduction was seen regarding the HOMA-IR level in the vit D, O3, and vit D + O3 supplemented groups when compared to the amounts at the beginning of the experiment (Table III).
3.4. Serum SHBG hormone
In comparison to the placebo group, all vit D, O3, and vit D + O3 treatments showed a significant increase in SHBG concentration after the study [(p = 0.001), (p = 0.02), and (p = 0.02), respectively] (Table II).
Besides, there were notable enhancements in the SHBG parameter for the vit D, O3, and vit D + O3 groups compared with the values at the beginning of the trial (Table III).


4. Discussion
To the best of our knowledge, this study is a worthwhile effort to investigate the effects of single-dose and co-supplementation of vit D and O3 on anthropometric factors, lipid and glycemic profile, and SHBG status in women with PCOS.
PCOS is known as a metabolic disorder closely associated with hyperglycemia, insulin resistance, and dyslipidemia (2). The current study revealed that a single dose and co-supplementation of vit D and O3 for 8 wk resulted in improved HOMA-IR and serum TG, TC, FBS, insulin, and SHBG concentrations in PCOS participants.
Several studies have been conducted to investigate the occurrence of vit D deficiency in PCOS and its consequences on lipid and glycemic disorders (16, 17). It has been reported that inadequate vit D status would dramatically decrease vitamin D receptor activity, which in turn would result in higher circulating cholesterol levels through insulin-induced gene 2/sterol regulatory element-binding protein-2/3-hydroxy-3-methylglutaryl-CoA reductase-dependent signaling pathways (18). According to previous experiments, the effect of vit D on blood lipids may be due to the inhibition of parathyroid hormone (PTH) secretion, as elevated PTH has been shown to have a positive association with higher WC (19), suppressed lipolysis (20), and insulin resistance (21). Lower glucose uptake, as well as an increase in body fat accumulation, may result in ovarian and adrenal hyperandrogenism (22). Hypovitaminosis D may adversely contribute to hyperandrogenism through 2 different mechanisms: (1) upregulation of androgen synthesis in theca cells due to stimulation of the luteinizing hormone; (2) inhibition of SHBG production in the liver (23). Vit D may increase insulin secretion and stimulate glucose transfer via activation of the genes related to insulin receptor synthesis and production of insulin-regulated glucose transporter type-4 in the cell membrane (24). Moreover, this vitamin increases the production of the peroxisome proliferator-activated receptor gamma gene, which in turn enhances insulin sensitivity and fatty acid metabolism (24). Meanwhile, it has been reported in previous trials that adding O3 to vit D supplementation might enhance its beneficial impact on the metabolic profile of PCOS individuals (25). Several mechanisms have been proposed for the effect of O3 on glycemic indices and serum lipids, such as: (1) activation of AMP-activated protein kinase in the liver, white adipose tissues, and muscles which can reduce lipogenesis, improve the oxidation of fatty acids, and also promote glucose transfer into the cells, (2) O3 may increase insulin sensitivity and decrease adipocyte proliferation due to its multiple anti-inflammatory properties (26). In one study, the researchers found O3 as an effective agent in raising SHBG in non-obese PCOS women after 6-month therapy (27). Furthermore, several experiments have been conducted regarding the effects of vit D and O3 co-supplementation on different serum biochemical indices in other diseases. Consistent with the current findings, in a 6-wk investigation regarding the benefits of taking 50,000 IU of vit D every 2 wk and 1000 mg of O3 twice daily in women with gestational diabetes, it was found that co-supplementation with vit D and O3 led to an improvement in terms of glycemic regulation and triglyceride levels while having little effect on other serum lipids (28). Similar to the results of the present study, Gurol et al. (29) reported that vit D and O3 co-supplementation could be synergistically effective in lowering FBS concentration in pancreatic islets in transplanted rats. Additionally, another trial study investigated the impact of combined therapy with O3 (4-10 gr of fish oil/daily) and vit D (2000 IU/daily) on serum lipid profiles in individuals with subclinical arteriosclerosis. After 18 months follow-up, they found that the participants had lower TC, TG, and LDL-C levels, as well as a higher HDL-C concentration. The fact that their trial used a higher dose of O3 and vit D along with a longer study period may account for the inconsistency between LDL-C and HDL-C outcomes in the aforementioned study and the present experiment (30).
Our results should shed light while considering the main limitations. The intervention period appears to be insufficient to detect changes in other study indices, such as anthropometric parameters. As a result of limited funding, other important biochemical parameters related to the PCOS cases, such as androstenedione, dehydroepiandrosterone sulfate, PTH, and Ca2+, were not assessed. Future investigations are thus required to assess other particular indicators that may elucidate the advantageous underlying processes relating to the benefits of supplementation with vit D and O3 on PCOS complications.
5. Conclusion
Findings of the current study indicated that a single dose and co-supplementation of vit D and O3 for 8 wk was associated with beneficial effects on serum TG, TC, insulin, and SHBG concentrations. A positive synergistic effect of vit D and O3 co-administration was found regarding glycemic control and insulin resistance (FBS and HOMA-IR levels) among women suffering from PCOS.
Acknowledgments
The authors would like to thank Mrs. Najmeh Maayeshi for her editorial assistance. This work was supported by the Shiraz University of Medical Sciences, Shiraz, Iran (grant number: 1396-01-84-14295].
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (397 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine ailment, affecting 5-10% of females worldwide during their reproductive period (1). It is the main cause of infertility and unfavorable pregnancy consequences caused by dysfunctional phase of follicular maturation and ovulation, multicystic condition of ovaries, and hyperandrogenism (1). Furthermore, PCOS along with a group of risk factors is related to the development of cardiovascular disease and type 2 diabetes including insulin resistance, hyperglycemia, lipid profile abnormalities, and abdominal obesity (2).
Although the etiology of PCOS remains unclear, the underlying preventable and controllable mechanisms like obesity, hyperinsulinemia, and hyperandrogenism have provided new insights into the pathogenesis of this disease (3). It has been documented that individuals with PCOS can benefit from a combination of different nutrients, as well as improve their overall nutritional status (4). Hence, many researchers have focused on nutrition-based lifestyle modification interventions in women with PCOS as a global necessity.
Vitamin D (vit D) deficiency is one of the well-known health problems observed in nearly 67-85% of women with PCOS, worldwide (5). The effects of vit D are mediated by both genetic and cellular mechanisms. It controls gene transcription via the intracellular distribution of nuclear vit D receptors in several tissues, specifically the ovaries (6). There is accumulating evidence that shows that serum vit D levels may be negatively implicated in PCOS symptoms, including obesity, hyperlipidemia, insulin resistance, hyperandrogenism, diminished sex hormone binding globulin (SHBG) production, and higher risks of cardiovascular disease, and abortion (7, 8). Also, it has been reported that vit D deficiency may contribute to the dysregulation of calcium metabolism, which in turn led to advanced follicular arrest and menstrual disturbances in women with PCOS (9).
Moreover, it has been documented that the consumption of unsaturated fat-rich foods may result in the improvement of PCOS metabolic impairments (10). As a bioactive anti-inflammatory agent, it has been confirmed that omega-3 (O3) supplementation can exhibit beneficial effects in improving parameters such as fasting glucose, insulin resistance, triglyceride level, and ovulation function (11). Furthermore, this dietary supplement may be used for ameliorating insulin sensitivity by reducing the generation of inflammatory cytokines including tumor necrosis factor alpha and interleukin 6 (12).
Although the efficacy of single supplements (vit D and O3) has been evaluated in previous studies, the synergistic effects of both supplements in attenuating lipid and glycemic profiles have not been investigated in PCOS cases till now. Therefore, the present study aimed to determine the effects of single-dose and co-supplementation of vit D and O3 for the management of PCOS complications.
2. Materials and Methods
2.1. Study design and participants
This double-blind, randomized clinical trial was conducted on 80 PCOS women. An established diagnosis of PCOS based on Rotterdam diagnostic criteria and vit D deficiency (25-hydroxyvitamin D ≤ 20 nmol/L) was recruited after screening 107 participants (aged 18-45 yr) from outpatients visiting, 18.5 ≤ body mass index (BMI) ≤ 40 referred to Shahid Motahhari Clinic, Shiraz, Iran, from April to October 2017.
The exclusion criteria were listed as follows: having a background of chronic illnesses such as cancer, heart disease, diabetes, stroke, fibromyalgia, kidney or liver defects, having a history of food and drug allergies, starting the drug or surgical therapy for clinical symptoms associated with PCOS except for oral contraceptive pills, smoking or any drug addiction, pregnancy and lactation, being on a special diet in the last year, using any dietary supplement, having oral or injectable nutritional supplements containing vit D in the last 3 months, consuming nutritional supplements containing fish oil or O3 fatty acids in the last 3 months, having fish in the diet more than 3 servings per week during last 3 months, background of serious side effects or signs of poisoning from the study's supplements, and failure to follow the research protocol.
2.2. Sample size
With respect to the type I error of 5% (α = 0.05), and type II error of 20% from the prior investigation in PCOS cases (13), the sample size was calculated based on the following formula:

2.3. Randomization, blinding, and interventions
Computer-generated randomization was used to complete the randomization process, and a qualified clinician carried out the randomized allocation procedure and allocated individuals as 1: 1: 1: 1 to each of the study groups. The randomization sequence concealment continued until the end of the intervention course. The researcher and participants were blinded.
The participants were randomly divided into 4 groups (n = 20/each) for 8 wk by block randomization with a fixed block size of 4 for 8 wk as follows:
- Vit D group: received 1 vit D capsule (Zahravi Pharmaceutical Company, Tehran, Iran) (50,000 IU/weekly) + 2 placebo capsules (paraffin oil; daily).
- O3 group: received 2 O3 capsules (Zahravi Pharmaceutical Company, Tehran, Iran) daily (each one contained 360 mg eicosapentaenoic acid and 240 mg docosahexaenoic acid) + 1 placebo capsule (paraffin oil; weekly).
- Vit D + O3 group: received 1 vit D capsule (50,000 IU/weekly) + 2, O3 capsules daily (each one contained 360 mg eicosapentaenoic acid and 240 mg docosahexaenoic acid).
- Placebo group: received 1 placebo capsule (paraffin oil; weekly) + 2 placebo capsules (paraffin oil; daily).
2.4. Outcomes
Measurements were performed by trained investigators before and after 8 wk of intervention, including height, weight, BMI, waist circumference (WC), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), fasting blood sugar (FBS), homeostasis model of insulin resistance index (HOMA-IR), serum insulin level, physical activity, and SHBG were compared between groups. All laboratory procedures were carried out following accepted laboratory practices under the supervision of competent professionals in the Nutrition and Food Science School's lab at Shiraz University of Medical Sciences, Shiraz, Iran.
2.5. Ethical considerations
All participants were informed about the study objectives and asked to sign the written consent form. The procedure of this study was conducted according to the Declaration of Helsinki. It was also approved by the Ethics Committee on Human Experimentation of Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR.SUMS.REC.1396.103). Furthermore, this trial was registered at the Iranian Registry of Clinical Trials and has been updated on September 19, 2022.
2.6. Statistical analysis
Statistical analysis was done using the SPSS software (Statistical Package for the Social Sciences, version 22.0, SPSS Inc, Chicago, Illinois, USA). First, the normal distribution of the data was assessed by Kolmogorov-Smirnov test. The Kruskal-Wallis test was used to compare the 4 groups regarding mean differences. Then, the Mann-Whitney U test was employed for comparing the means of quantitative data between groups. The Wilcoxon test was used to compare the changes in variables before and after the intervention in each group. All the differences were considered statistically significant at p ≤ 0.05.
3. Results
Table I summarizes the general characteristics of the research participants. At the beginning of the experiment, no statistically significant differences were identified among the 4 study groups, as shown in the table. 4 people were dropped from the research during the intervention phase because of non-compliance or heart surgery, and 76 individuals completed the trial (Figure 1). No serious side effects were detected in all study groups.
3.1. Anthropometric indices
By the end of the study, no significant differences were observed between all study groups in terms of height, body weight, BMI, WC, and physical activity (Table II).
The mean values of anthropometric and biochemical parameters in each study group, before and after the study, have been presented in (Table III). After 8 wk intervention, the WC showed a significant decrease in vit D and O3 groups in comparison with the baseline levels. Also, compared to the values at the beginning of the study, a statistically significant reduction of WC was observed in vit D + O3 treated participants. However, at the end of the study, no significant difference was found in terms of body weight and BMI parameters in all the study groups compared to the baseline period.
3.2. Serum lipid profile
The effect of study treatments on the serum biochemical indices has been shown in (Table II). By the end of the experiment, no statistically significant difference was observed between any of the study groups regarding the serum HDL_C and LDL_C levels. Meanwhile, vit D, O3, and vit D + O3 treatments resulted in significantly lower TG levels as compared to cases treated with placebo [(p = 0.02), (p = 0.004), and (p = 0.004), respectively]. Furthermore, compared to the placebo group, all the vit D, O3, and vit D + O3 treatments showed a significant decrease in TC concentration after the study [(p = 0.04), (p = 0.01), and (p = 0.008), respectively].
In addition, compared to the baseline course, serum TC was remarkably decreased in vit D, O3, and vit D + O3 supplemented groups. Nevertheless, there were no significant changes in other serum lipid parameters at the end of the study when compared to the baseline (Table III).
3.3. FBS, serum insulin, and HOMA−IR parameters
By the end of the study, vit D, O3, and vit D + O3 interventions led to a significantly lower FBS level in comparison with the individuals treated with placebo [(p = 0.01), (p = 0.03), and (p = 0.007), respectively]. Moreover, a lower concentration of serum insulin was detected in the vit D, O3, and vit D + O3 groups in comparison with the amounts in the placebo group [(p = 0.002), (p = 0.002), and (p = 0.001), respectively]. As well, compared to the placebo group, interventions with vit D, O3, and vit D + O3 provided significantly lower HOMA-IR levels [(p = 0.002), (p = 0.007), and (p = 0.001), respectively]. In this regard, compared to the vit D and O3 treatments, the oral co-supplementation with vit D + O3 was accompanied by a synergistically significant reduction in serum FBS [(p = 0.04), (p = 0.03), respectively] and HOMA-IR [(p = 0.02), (p = 0.01), respectively] levels (Table II).
Moreover, in comparison with the baseline period, serum insulin was notably decreased in the vit D, O3, and vit D + O3 treated groups when compared to the baseline. Furthermore, a statistically significant reduction was seen regarding the HOMA-IR level in the vit D, O3, and vit D + O3 supplemented groups when compared to the amounts at the beginning of the experiment (Table III).
3.4. Serum SHBG hormone
In comparison to the placebo group, all vit D, O3, and vit D + O3 treatments showed a significant increase in SHBG concentration after the study [(p = 0.001), (p = 0.02), and (p = 0.02), respectively] (Table II).
Besides, there were notable enhancements in the SHBG parameter for the vit D, O3, and vit D + O3 groups compared with the values at the beginning of the trial (Table III).




4. Discussion
To the best of our knowledge, this study is a worthwhile effort to investigate the effects of single-dose and co-supplementation of vit D and O3 on anthropometric factors, lipid and glycemic profile, and SHBG status in women with PCOS.
PCOS is known as a metabolic disorder closely associated with hyperglycemia, insulin resistance, and dyslipidemia (2). The current study revealed that a single dose and co-supplementation of vit D and O3 for 8 wk resulted in improved HOMA-IR and serum TG, TC, FBS, insulin, and SHBG concentrations in PCOS participants.
Several studies have been conducted to investigate the occurrence of vit D deficiency in PCOS and its consequences on lipid and glycemic disorders (16, 17). It has been reported that inadequate vit D status would dramatically decrease vitamin D receptor activity, which in turn would result in higher circulating cholesterol levels through insulin-induced gene 2/sterol regulatory element-binding protein-2/3-hydroxy-3-methylglutaryl-CoA reductase-dependent signaling pathways (18). According to previous experiments, the effect of vit D on blood lipids may be due to the inhibition of parathyroid hormone (PTH) secretion, as elevated PTH has been shown to have a positive association with higher WC (19), suppressed lipolysis (20), and insulin resistance (21). Lower glucose uptake, as well as an increase in body fat accumulation, may result in ovarian and adrenal hyperandrogenism (22). Hypovitaminosis D may adversely contribute to hyperandrogenism through 2 different mechanisms: (1) upregulation of androgen synthesis in theca cells due to stimulation of the luteinizing hormone; (2) inhibition of SHBG production in the liver (23). Vit D may increase insulin secretion and stimulate glucose transfer via activation of the genes related to insulin receptor synthesis and production of insulin-regulated glucose transporter type-4 in the cell membrane (24). Moreover, this vitamin increases the production of the peroxisome proliferator-activated receptor gamma gene, which in turn enhances insulin sensitivity and fatty acid metabolism (24). Meanwhile, it has been reported in previous trials that adding O3 to vit D supplementation might enhance its beneficial impact on the metabolic profile of PCOS individuals (25). Several mechanisms have been proposed for the effect of O3 on glycemic indices and serum lipids, such as: (1) activation of AMP-activated protein kinase in the liver, white adipose tissues, and muscles which can reduce lipogenesis, improve the oxidation of fatty acids, and also promote glucose transfer into the cells, (2) O3 may increase insulin sensitivity and decrease adipocyte proliferation due to its multiple anti-inflammatory properties (26). In one study, the researchers found O3 as an effective agent in raising SHBG in non-obese PCOS women after 6-month therapy (27). Furthermore, several experiments have been conducted regarding the effects of vit D and O3 co-supplementation on different serum biochemical indices in other diseases. Consistent with the current findings, in a 6-wk investigation regarding the benefits of taking 50,000 IU of vit D every 2 wk and 1000 mg of O3 twice daily in women with gestational diabetes, it was found that co-supplementation with vit D and O3 led to an improvement in terms of glycemic regulation and triglyceride levels while having little effect on other serum lipids (28). Similar to the results of the present study, Gurol et al. (29) reported that vit D and O3 co-supplementation could be synergistically effective in lowering FBS concentration in pancreatic islets in transplanted rats. Additionally, another trial study investigated the impact of combined therapy with O3 (4-10 gr of fish oil/daily) and vit D (2000 IU/daily) on serum lipid profiles in individuals with subclinical arteriosclerosis. After 18 months follow-up, they found that the participants had lower TC, TG, and LDL-C levels, as well as a higher HDL-C concentration. The fact that their trial used a higher dose of O3 and vit D along with a longer study period may account for the inconsistency between LDL-C and HDL-C outcomes in the aforementioned study and the present experiment (30).
Our results should shed light while considering the main limitations. The intervention period appears to be insufficient to detect changes in other study indices, such as anthropometric parameters. As a result of limited funding, other important biochemical parameters related to the PCOS cases, such as androstenedione, dehydroepiandrosterone sulfate, PTH, and Ca2+, were not assessed. Future investigations are thus required to assess other particular indicators that may elucidate the advantageous underlying processes relating to the benefits of supplementation with vit D and O3 on PCOS complications.
5. Conclusion
Findings of the current study indicated that a single dose and co-supplementation of vit D and O3 for 8 wk was associated with beneficial effects on serum TG, TC, insulin, and SHBG concentrations. A positive synergistic effect of vit D and O3 co-administration was found regarding glycemic control and insulin resistance (FBS and HOMA-IR levels) among women suffering from PCOS.
Acknowledgments
The authors would like to thank Mrs. Najmeh Maayeshi for her editorial assistance. This work was supported by the Shiraz University of Medical Sciences, Shiraz, Iran (grant number: 1396-01-84-14295].
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Koneru A, Priyanka S. Polycystic ovary syndrome (PCOS) and sexual dysfunctions. J Psychosexual Health 2019; 1: 154-158. [DOI:10.1177/2631831819861471]
2. Sherafatmanesh S, Ekramzadeh M, Tanideh N, Golmakani M-T, Koohpeyma F. The effects of thylakoid-rich spinach extract and aqueous extract of caraway (Carum carvi L.) in letrozole-induced polycystic ovarian syndrome rats. BMC Complement Med Ther 2020; 20: 249. [DOI:10.1186/s12906-020-03044-w] [PMID] [PMCID]
3. Walters KA, Gilchrist RB, Ledger WL, Teede HJ, Handelsman DJ, Campbell RE. New perspectives on the pathogenesis of PCOS: Neuroendocrine origins. Trends Endocrinol Metab 2018; 29: 841-852. [DOI:10.1016/j.tem.2018.08.005] [PMID]
4. Maktabi M, Jamilian M, Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 2018; 182: 21-28. [DOI:10.1007/s12011-017-1085-0] [PMID]
5. Menichini D, Facchinetti F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: A review. Gynecol Endocrinol 2020; 36: 1-5. [DOI:10.1080/09513590.2019.1625881] [PMID]
6. Renke G, Starling-Soares B, Baesso T, Petronio R, Aguiar D, Paes R. Effects of vitamin D on cardiovascular risk and oxidative stress. Nutrients 2023; 15: 769. [DOI:10.3390/nu15030769] [PMID] [PMCID]
7. He C, Lin Z, Robb SW, Ezeamama AE. Serum vitamin D levels and polycystic ovary syndrome: A systematic review and meta-analysis. Nutrients 2015; 7: 4555-4577. [DOI:10.3390/nu7064555] [PMID] [PMCID]
8. Li HWR, Brereton RE, Anderson RA, Wallace AM, Ho CKM. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011; 60: 1475-1481. [DOI:10.1016/j.metabol.2011.03.002] [PMID]
9. El-Bahya AAZ, Radwanb RA, Gadc MZ, Abdel Maksoudc SM. A closer insight into the role of vitamin D in polycystic ovary syndrome (Pcos). Global J Pharmaceu Sci 2018; 6: 79-87. [DOI:10.19080/GJPPS.2018.06.555692]
10. Phelan N, O'Connor A, Kyaw Tun T, Correia N, Boran G, Roche HM, et al. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: Results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am J Clin Nutr 2011; 93: 652-662. [DOI:10.3945/ajcn.110.005538] [PMID]
11. Yang K, Zeng L, Bao T, Ge J. Effectiveness of omega-3 fatty acid for polycystic ovary syndrome: A systematic review and meta-analysis. Reprod Biol Endocrinol 2018; 16: 27. [DOI:10.1186/s12958-018-0346-x] [PMID] [PMCID]
12. Monk JM, Turk HF, Liddle DM, De Boer AA, Power KA, Ma DWL, et al. n-3 polyunsaturated fatty acids and mechanisms to mitigate inflammatory paracrine signaling in obesity-associated breast cancer. Nutrients 2014; 6: 4760-4793. [DOI:10.3390/nu6114760] [PMID] [PMCID]
13. Mohammadi E, Rafraf M, Farzadi L, Asghari-Jafarabadi M, Sabour S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac J Clin Nutr 2012; 21: 511-518.
14. Irani M, Seifer DB, Grazi RV, Julka N, Bhatt D, Kalgi B, et al. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: A randomized placebo-controlled trial. J Clin Endocrinol Metab 2015; 100: 4307-4314. [DOI:10.1210/jc.2015-2580] [PMID]
15. Hallal PC, Victora CG. Reliability and validity of the international physical activity questionnaire (IPAQ). Med Sci Sports Exerc 2004; 36: 556. [DOI:10.1249/01.MSS.0000117161.66394.07] [PMID]
16. Rashidi H, Ghaderian SB, Moradi L. The effect of vitamin D3 on improving lipid profile, fasting glucose and insulin resistance in polycystic ovary syndrome women with vitamin D deficiency. Middle East Fertil Soc J 2018; 23: 178-183. [DOI:10.1016/j.mefs.2017.11.002]
17. Wehr E, Trummer O, Giuliani A, Gruber H-J, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 2011; 164: 741-749. [DOI:10.1530/EJE-11-0134] [PMID]
18. Li S, He Y, Lin S, Hao L, Ye Y, Lv L, et al. Increase of circulating cholesterol in vitamin D deficiency is linked to reduced vitamin D receptor activity via the Insig‐2/SREBP‐2 pathway. Mol Nutr Food Res 2016; 60: 798-809. [DOI:10.1002/mnfr.201500425] [PMID]
19. Kayaniyil S, Vieth R, Harris SB, Retnakaran R, Knight JA, Gerstein HC, et al. Association of 25 (OH) D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab 2011; 96: 168-175. [DOI:10.1210/jc.2010-1439] [PMID]
20. Gao H, Li Y, Yan W, Gao F. The effect of vitamin D supplementation on blood lipids in patients with polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Int J Endocrinol 2021; 2021: 8849688. [DOI:10.1155/2021/8849688] [PMID] [PMCID]
21. Gerveieeha Z, Siassi F, Qorbani M, Ziaeian F, Sotoudeh G. The effect of different amounts of vitamin D supplementation on serum calcidiol, anthropometric status, and body composition in overweight or obese nursing women: A study protocol for a randomized placebo-controlled clinical trial. Trials 2019; 20: 542. [DOI:10.1186/s13063-019-3622-y] [PMID] [PMCID]
22. Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Zolfaghari Z, et al. Abdominal obesity can induce both systemic and follicular fluid oxidative stress independent from polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2015; 184: 112-116. [DOI:10.1016/j.ejogrb.2014.11.008] [PMID]
23. Berry S, Seidler K, Neil J. Vitamin D deficiency and female infertility: A mechanism review examining the role of vitamin D in ovulatory dysfunction as a symptom of polycystic ovary syndrome. J Reprod Immunol 2022; 151: 103633. [DOI:10.1016/j.jri.2022.103633] [PMID]
24. Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol 2018; 175: 177-189. [DOI:10.1016/j.jsbmb.2016.09.017] [PMID]
25. Jamilian M, Samimi M, Mirhosseini N, Afshar Ebrahimi F, Aghadavod E, Talaee R, et al. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J Affect Disord 2018; 238: 32-38. [DOI:10.1016/j.jad.2018.05.027] [PMID]
26. Flachs P, Rossmeisl M, Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res 2014; 63 (Suppl.): S93-S119. [DOI:10.33549/physiolres.932715] [PMID]
27. Oner G, Muderris II. Efficacy of omega-3 in the treatment of polycystic ovary syndrome. J Obstet Gynaecol 2013; 33: 289-291. [DOI:10.3109/01443615.2012.751365] [PMID]
28. Jamilian M, Samimi M, Afshar Ebrahimi F, Hashemi T, Taghizadeh M, Razavi M, et al. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J Clin Lipidol 2017; 11: 459-468. [DOI:10.1016/j.jacl.2017.01.011] [PMID]
29. Gurol AO, Okten-Kursun A, Kasapoglu P, Suzergoz F, Kucuksezer UC, Cevik A, et al. The synergistic effect of ω3 and Vit D3 on glycemia and TNF-α in islet transplantation. Cell Mol Biol 2016; 62: 90-98.
30. Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther 2009; 16: 326-332. [DOI:10.1097/MJT.0b013e31817a8f3c] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




