Sun, Feb 1, 2026
[Archive]
Volume 21, Issue 5 (May 2023)
IJRM 2023, 21(5): 433-442 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zohrabi T, Sheikhha M H, Jambarsang S, Nadjarzadeh A, Aflatoonian A, Mozaffari-Khosravi H. Effect of dietary approaches to stop hypertension, and standard diets with and without curcumin on interleukin-1 alpha, 5-alpha reductase gene expressions, and androgenic and glycemic profile in polycystic ovary syndrome women undergoing in vitro fertilization treatment: A study protocol. IJRM 2023; 21 (5) :433-442
URL: http://ijrm.ir/article-1-2608-en.html
URL: http://ijrm.ir/article-1-2608-en.html
Tayebeh Zohrabi1 

 , Mohammad Hasan Sheikhha2
, Mohammad Hasan Sheikhha2 

 , Sara Jambarsang3
, Sara Jambarsang3 

 , Azadeh Nadjarzadeh1
, Azadeh Nadjarzadeh1 

 , Abbas Aflatoonian4
, Abbas Aflatoonian4 

 , Hassan Mozaffari-Khosravi *5
, Hassan Mozaffari-Khosravi *5 




 , Mohammad Hasan Sheikhha2
, Mohammad Hasan Sheikhha2 

 , Sara Jambarsang3
, Sara Jambarsang3 

 , Azadeh Nadjarzadeh1
, Azadeh Nadjarzadeh1 

 , Abbas Aflatoonian4
, Abbas Aflatoonian4 

 , Hassan Mozaffari-Khosravi *5
, Hassan Mozaffari-Khosravi *5 


1- Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences,Yazd, Iran.
2- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Center for Healthcare Data Modeling, Departments of Biostatistics and Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences,Yazd, Iran. ,h.mozaffari@ssu.ac.ir
2- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Center for Healthcare Data Modeling, Departments of Biostatistics and Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences,Yazd, Iran. ,
Keywords: Polycystic ovary syndrome, Dietary approaches to stop hypertension, Curcumin, Fertilization in vitro, Gene expression.
Full-Text [PDF 333 kb]
(1080 Downloads)
| Abstract (HTML) (1662 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder that can lead to major reproductive and metabolic complications, including infertility and insulin resistance. According to the 2003 Rotterdam criteria, the prevalence of PCOS in women of reproductive age is 4.4-18.6% (1). The Rotterdam consensus is the most widely accepted diagnostic criteria (2), by which women must meet at least 2 of the 3 following criteria: 1) confirmation of polycystic ovary by ultrasound; 2) clinical or biochemical hyperandrogenism; and 3) ovulatory dysfunction (oligo/anovulation) (3).
Hyperandrogenism and chronic inflammation have been suggested as the leading causes of pathophysiology and clinical manifestations of PCOS (4). Biochemical hyperandrogenism is defined as a high concentration of testosterone and a high level of free testosterone and free androgen index (3). Hyperandrogenism may be caused by increased 5α-reductase activity, a key enzyme with an essential role in regulating the peripheral conversion of testosterone to the most active androgen, 5α-dihydrotestosterone (5). The findings showed that the activity of 5α-reductase increased in PCOS, and this high activity is related to insulin resistance (6). There are 2 isozymes of 5α reductase, encoded by the 5α-reductase type1 and type2 genes (7). Findings from an animal study suggest that insulin-mediated changes in 5α-reductase type1 messenger ribonucleic acid (mRNA) expression can negatively affect the follicle development and ovulation (8). In addition, mild chronic inflammation, independent of obesity, has been confirmed in PCOS patients (4). One of the most important inflammatory mediators, interleukin-1 (IL-1), is a multifunctional cytokine with 3 different cytokines, including cytokines (α, β) and an IL-1 receptor antagonist. IL-1α gene polymorphism can be associated with PCOS and affect ovulation, fertilization, and implantation (9, 10).
Given the potential effects of chronic inflammation on the pathogenesis of PCOS through the pathway of insulin resistance and hyperandrogenism (11), new strategies have been proposed to improve the clinical symptoms of PCOS. According to evidence-based recommendations, lifestyle intervention (various components including diet, exercise, and behavioral approaches) is recommended (3).
The dietary approaches to stop hypertension (DASH) diet has been suggested as an effective treatment option (12). DASH diet, as a low-glycemic diet (13), with a high concentration of antioxidants, can have beneficial effects in reducing insulin resistance (14), inflammation (15), and free androgen index (16). This diet is rich in vegetables, low-fat dairy products, fruits, whole grains, poultry, fish, and nuts, but is limited in foods high in sodium, cholesterol, saturated fat, red and processed meat, refined grains, sweets, and sugary drinks (12). In the last decade, the antioxidative effects of plant polyphenols have been considered (17). Indeed, among natural phenols, the therapeutic properties of curcumin have been further investigated (18). Curcumin (diferuloylmethane) is a lipophilic yellow pigment extracted from turmeric rhizomes (Curcuma longa L.) (19). Curcumin supplementation improves glycemic control and lipid metabolism, and reduces radical oxygen species in PCOS patients (20, 21).
The anti‐inflammatory and antioxidant benefits of both the DASH diet and curcumin have been demonstrated by meta-analysis results (15, 22). Therefore, we plan to investigate the effect of the DASH diet in combination with curcumin supplements on IL-1α and 5α reductase gene expressions and improvement of insulin resistance and hyperandrogenism.
2. Materials and Methods
2.1. Trial design and study setting
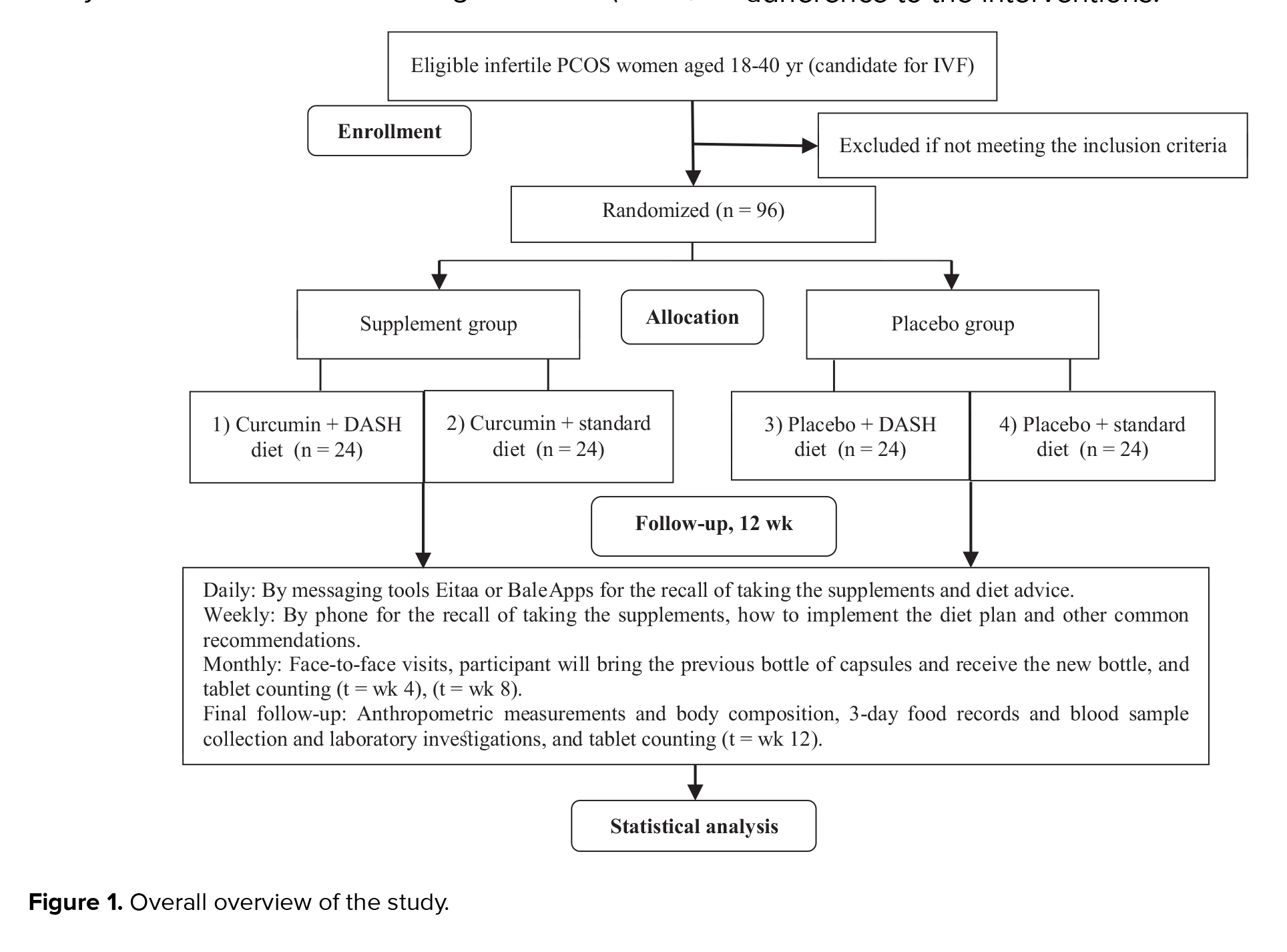
This randomized, placebo-controlled clinical trial will be conducted on PCOS women candidates for in vitro fertilization (IVF), using a convenience sampling method. Participants in a 2×2 factorial design will be randomly divided into 4 parallel treatment groups using block randomization: (1) curcumin supplement + DASH diet (2) curcumin supplement + standard diet (3) placebo + DASH diet, and (4) placebo + standard diet. The follow-up duration will be 12 wk. The study will be conducted at the Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. An overview of the study is presented in figure 1.
2.2. Eligibility criteria
Infertile women with PCOS (according to the Rotterdam criteria) (2) who were candidates for IVF treatment, without a previous history of IVF, will be included in this study. Eligibility criteria included body mass index (BMI) between 18.5-25 and BMI ≥ 25 kg/m² and aged between 18-40 yr.
The participants must not have any of the following:
Metabolic and endocrine abnormalities including thyroid dysfunction, Cushing's syndrome, diabetes or impaired glucose tolerance, hyperprolactinemia, congenital adrenal hyperplasia, liver disease, cardiovascular disease, kidney disease, hypertension, neurological diseases, any history of mental illness, cancer, autoimmune and inflammatory disorders (rheumatoid arthritis). Taking medications including contraceptives or any other drugs that may affect reproductive physiology (during the 3 months before the study); taking antidiabetic, antiobesity, estrogenic, and androgenic drugs, as well as taking supplements such as vitamin C, vitamin E, and omega-3 because of their anti-inflammatory and antioxidative effects, 3 months before or during the intervention; smoking or exposure to spouse's smoking; alcohol drinking; being pregnant; simultaneous participation in another study. Moreover, unwillingness to cooperate and nonadherence to dietary or supplementary interventions (compliance < 80%), allergy to supplements, and pregnancy of the participant during the study will be considered as drop-out criteria.
2.3. Interventions
Participants will be given 2 capsules of curcumin supplement per day (1 after breakfast and another after an evening meal with water) or 2 placebo capsules of curcumin per day at the same time for 12 wk. Curcumin capsules (BCM95/Curcugreen) and their placebos, with the same texture and appearance, were produced as 500 mg zero size veg capsules by “M/s Arjuna Natural Pvt Ltd., India”. The curcumin supplement was made from dried turmeric rhizomes by extraction with ethyl acetate. Curcuma longa L. commonly known as turmeric contains curcuminoids (curcumin, demethoxy curcumin, and bisdemethoxy curcumin) and essential oil of turmeric (ar-turmerone, curlone, and ar-curcumene). Curcuminoids are specific compounds with medicinal properties, of which the most important and active ingredient is the yellow coloring pigment curcumin; Ar-turmerone (the main bioactive components of the Curcuma longa L. essential oil), inhibits the P-glycoprotein pathway, thus improving the oral bioavailability and bioactivity of orally administered curcuminoids. BCM-95 w:as char:acterized by 95% total curcuminoid complex; the final formulation containing at least 65% curcumin and 86% total curcuminoids; and the essential oil containing not less than 45% ar-turmerone. Each 500-mg capsule contained 475 mg of curcuminoids and the essential oil of natural turmeric. Each placebo capsule contained 500 mg of roasted rice powder. The curcumin capsule actives will be analyzed by HPLC, heavy metals by ICP-MS; residual solvents by GCHS, pesticides by GCMS, and microbial indicators all of which were ensured to be in line with the EU standards (regulation (EC) No 1881/2006; directive 2009/32/CE; regulation (EC) No. 396/2005 and related amendments).
The intervention groups will receive 2 healthy dietary patterns (DASH or standard diet). These diets will be designed in the form of an individual diet containing 18% protein, 52% carbohydrates, and 30% total fat, which will be matched with the macronutrient plan used in the other original trial (23). Dietary programs will be planned after baseline assessments based on the current dietary intakes and body weight of each person. Daily calorie requirements will be estimated by calculating the resting energy expenditure and physical activity levels. If a person was overweight (BMI is 25.0 to < 30.0 kg/m²) or obese (≥ 30.0 kg/m²), the calculated energy will be reduced by 300 and 500 kcal, respectively. A 12-wk menu plan (7-day cycle menu with 21 meals) at 11 energy levels (1200, 1300, 1400, 1500, 1600, 1700, 1800, 1900, 2000, 2100, and 2200 kcal) for both dietary patterns will be constructed and analyzed. These will be considered as the basis for participants' diets. Nutritionist IV software (diet analysis, module version 3.5.2) will be used to calculate energy, food exchange, percent of kilocalories from macronutrients, and nutrient values in each daily menu plan. DASH diet will be designed with an emphasis on food rich in fiber, magnesium, potassium, calcium, and protein, such as vegetables, fruits, and low-fat dairy products; while foods high in saturated fat and sugar will be limited (12). The amount of sodium prescribed for each diet will be the same (2300 mg per day-approximately 1 teaspoon of salt). Overall, DASH and standard patterns will be designed using similar foods and recipes, but in different amounts, to achieve the specific characteristics of each diet. Both diets will be planned by the study dietitian and reviewed by the Chief investigator.
Participants will receive their specific diet through face-to-face individual counseling, initial assessment, and intervention for 45-60 min with a registered dietitian (follow-up visits for 15-30 min per month). They will also receive a bottle of supplement/placebo (on the 1st day, 30th day, and 60th day of visit), each bottle containing 64 capsules (providing supplement for 1 month and a grace period of 2 days). Administration instructions on taking the pills and what needs to be done in case a dose is missed were set for patients, including taking 2 curcumin/placebo capsules a day (one after breakfast and one after an evening meal with water) and taking the missed capsule the same day. The patients will be monitored for daily supplementations by contacting them via phone interviews and messaging (Eitaa or Bale apps) twice a day. Moreover, daily consumption of supplements/placebos will be recorded in the case report form given to participants. At each follow-up visit, a nutritionist will assess the participants adherence to the diet and supplementation.. The number of capsules taken will be calculated by counting the remaining capsules in each bottle. If the number of unused prescription capsules is more than 12 out of 60 capsules per month, for each participant, that patient will be classified in the non-adherent group.
All adverse events including unfavorable and/or unintended signs and any undesirable experience occurring to a patient will be reported in writing to the Medical Ethics Committee and the Data Monitoring Committee of the Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The participants, for whatever reason, can discontinue participating in the research study at any given time before or after signing the consent form. The researcher may also terminate the study to maintain safety and protect the participant from excessive risks and/or to keep the integrity of the research data due to inappropriate follow-up by the participant. In case of termination of the study by the investigator, the reasons for the termination will be explained to the participants.
2.4. Strategies to improve adherence to interventions
1) telephone follow-up; 2) weekly electronic counseling in order to improve patient’s participation by reducing barriers such as geographical distance and time constraints; 3) husbands of the participant will also receive free dietary consulting to improve compliance and family support.
2.5. Outcomes
The primary outcomes included gene expressions of IL-1α and 5α-reductase. The secondary outcomes consist serum levels of biochemical parameters comprising fasting blood sugar, fasting insulin, homeostasis model assessment of insulin resistance and quantitative insulin sensitivity check index, as well as serum androgen concentrations (testosterone, 5α-dihydrotestosterone) and anthropometric indices (height, weight, BMI, hip circumstance, waist circumstance, waist-hip ratio, waist-height ratio), body composition (muscle mass, fat mass, fat-free mass, and other factors such as trunk fat mass), physical activity, and dietary intake (energy and nutrient intake).
2.5.1. Demographic data and anthropometric measurements
A form including: full address, age, gender, income, employment, ethnicity, level of education, and medical history (dose and type of medicine and supplements) will be completed for each participant.
Participant’s weight, height, body circumferences (waist, hip), BMI, and waist-hip ratio as well as body composition analysis (fat mass, muscle mass, fat free mass) will be measured on the first and last day of the intervention. Weight will be measured to the nearest 0.5 kg (possible errors) using a pre-calibrated electronic scale (Seca, Germany). Height will be measured without shoes with an accuracy of 0.1 cm using a Seca wall stadiometer. Then BMI will be determined as weight (kg) divided by the square of their height (m²). Waist (cm) will be measured as the distance between the lowest rib and the iliac crest. Additionally, the hip circumference will be measured around the largest part of the hip. The waist-hip ratio will be the ratio of waist to hip circumference. The waist-height ratio will be measured by dividing the waist circumstance by the height. Body composition will be determined using body composition analyzer (Tanita BC-418, Tokyo, Japan), at the beginning of the study and after 12 wk of the study. All the measurements will be repeated by a trained assessor and recorded as average.
2.5.2. Biochemical measurements
After 2 wk of run-in period and considering 12-hr overnight fasting, 8 cc of blood samples will be collected in the morning from each participant, in the beginning, and at the end of the study.
5 cc of samples will be transferred to clot-activator tubes and then centrifuged for 30 min to separate the serum. Another 3 cc of blood samples will be collected in EDTA- coated tubes to seperate the plasma and buffy coat (the layer between red blood cells and plasma) and prepare buffy coat for gene expression assays. The allocated serum, plasma, and buffy coat will be stored immediately at -70°C. Before storage, the tripura isolation reagent will be added to the isolated buffy coat.
Serum levels of insulin, testosterone, and 5α-dihydrotestosterone will be evaluated using the enzyme-linked immunosorbent assay (ELIZA method), (ELISA kit, Monobind, California, United State). Fasting blood sugar will be measured by commercial kit using an enzymatic colorimetric method (Pars Azmoon, Tehran, Iran). homeostasis model assessment of insulin resistance will be calculated, using the following formula:
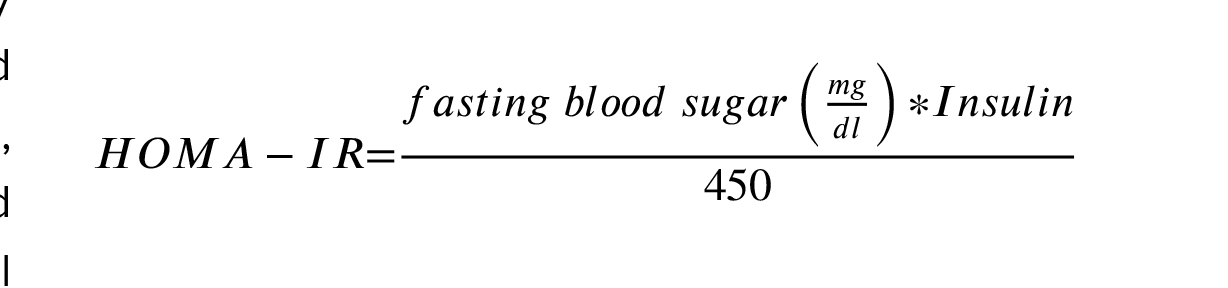
2.5.3. Gene expression assay
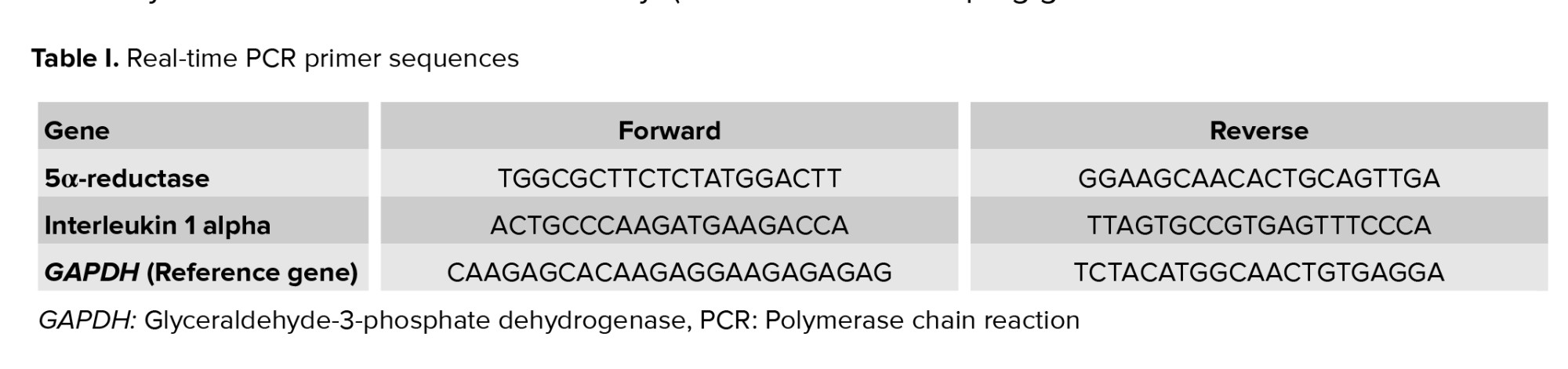
According to the kit protocol (Favorgen Biotech Corp., Taiwan), the buffy coat fraction of whole blood cells will be used for total RNA extraction. RNA concentration will be analyzed using spectrophotometer (DS-11, DeNovix) at 260 nm. Purity will be considered as an A260/A280 ratio of about 2 (24). cDNA will be synthesized from high-quality mRNA using a cDNA synthesis kit (GeneAll Biotechnology Co.). Forward and reverse primers are selected after reviewing previous studies (25-27) and checked by primer designing tools (Primer Blast, OLIGO 7 primer analysis software), as shown in table I.
IL-1α and 5α-reductase expression levels will be assessed by SYBR-green real-time PCR detection method using the kit protocol (Yekta Tajhiz Azma, IRAN), and the StepOne real-time PCR system (Applied Biosystems, Foster City, California, USA). GAPDH (glyceraldehyde phosphate dehydrogenase) will be chosen as the housekeeping gene.
2.5.4. Dietary intake
Food consumption information will be obtained through 3-day dietary records including 2 days per week and one weekend. Before the study, participants will be given adequate training on food consumption and will be provided with a guide including written examples for completing a questionnaire that simply explains how to accurately record food. The participants will be taught to use standard kitchen measuring tools to accurately determine the portion size. To reduce memory errors, they will be asked to make a detailed list of foods and beverages exactly when eating. The expert dietitian will review the completed dietary records, ingredient lists, and preparation methods, while will be blind to the study allocation. Each daily food record will be calculated by the Nutritionist IV software.
2.5.5. Physical activity
The metabolic equivalent of task-hours (MET-h)/day will be applied to assess physical activity questionnaire data (28).
2.6. Sample size
The sample size of 24 in each group was determined according to α = 0.05, the power of 80%, dropout rate of 20%, and considering a minimal clinically important difference of 0.969 and an effect size of 1, based on Heshmati et al. study (21) using the following formula:
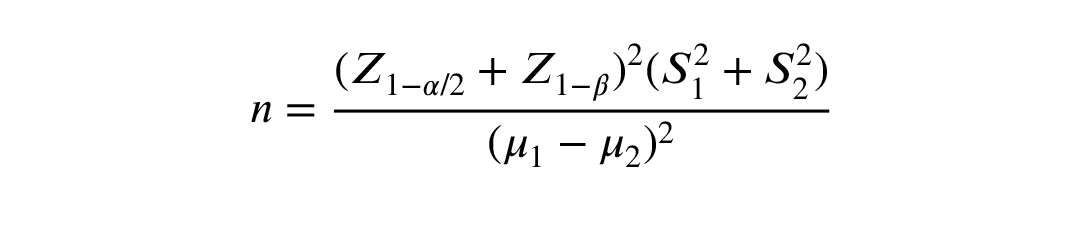
2.7. Recruitment
Unique codes will be assigned to perform allocation concealment and prevent "selection bias". Supplements and placebos capsules will be provided in the same appearance (in terms of color and shape) and packed in similar bottles. The bottles will be labeled with different marks (A and B) and placed in envelopes with serial numbers by someone not related to the study. Participants and researchers will not be aware of the bottles content. Intervention groups will also receive diets from a nutritionist who is not involved in this study.
The participants will be assigned to 4 groups according to the method of stratified permuted block randomization based on treatment conditions and BMI (BMI: 18.5-25 and BMI ≥ 25), in equal block sizes of 4. The random allocation list will be created by Package ‘blockrand’ in R software, version 4.0.2.
Randomization and allocation will be concealed from participants and researchers until finishing the study steps and data analysis. An independent biostatistician will generate the allocation sequence. 2 midwifes will give consecutive numbers to the eligible participants and refer them to a nutritionist. The sealed envelope containing the treatment code will be opened by the supervisor, after analyzing the final data or in an emergency condition.
All trial healthcare providers, data analysts, and outcome assessors will be blinded to intervention assignments. Participants may only be aware of the type of diet but will not be aware of the placebo or curcumin supplement content.
2.8. Statistical methods
The values of quantitative variables will be presented as the mean ± standard deviation, and qualitative variables will be expressed as numbers and percentages. The normality of data will be examined by the Kolmogorov-Smirnov test.
The frequency among study groups will be compared using the Chi-square test. Independent sample t test and 2-way ANOVA will be applied to estimate the mean of quantitative variable changes and interactions between study groups; Bonferroni correction will be considered for multiple comparisons; and analysis of covariance (ANCOVA) will be used to compare the differences in the mean of quantitative data between the studied groups, while taking into account the influence of possible confounding variables. Before statistical analysis, all data will be checked for accuracy and completeness. All statistical analysis will be conducted using SPSS 22 (SPSS, Inc, Chicago, IL, USA). A p-value < 0.05 will be defined as a statistically significant level.
The "intention-to-treat" and "per protocol analysis" approaches will be performed for data analysis. The "intention-to-treat"analysis considers all participants in their main randomized groups, regardless of whether they follow the prescribed intervention according to the protocol or not, and also ignores non-compliance and withdrawal. Per protocol analysis, which accurately reflects what has been done, will be conducted based on actual adherence to the interventions.
2.9. Participants and public involvement
Participants or the public will not participate in the setting, execution, measuring outcome, or dissemination plans of our study. The study results will be provided to patients upon request. Research findings will be reported in scientific journals.
2.10. Ethical considerations
This study will be conducted following the Declaration of Helsinki. This trial was approved on September 2020 by the Medical Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, (Code: IR.SSU.SPH.REC.1399.145).
All goals, working methods, and possible side effects of the interventions will be explained to all the participants before starting and entering the study by the main researcher, and an informed consent form will be obtained from the participants.
Participants will be assured that all their information will be kept confidential. The contact information of one of the researchers will be provided to the participants for 24-hr contact in case of any complications or problems. The study will have no financial burden on the participants.
2.11. Monitoring
Data monitoring and safety program are considered for this study. Project managers (HM, AA) and a research advisor (MS, SJ, and AN) will supervise and monitor the research process to ensure protocol compliance and data quality. Any withdrawals, lost to follow-up and adverse events will be assessed and reported. An independent trial Data Monitoring Committee will review the collected data and evaluate clinical trials for scientific validity, safety, and integrity.
2.12. Study strengths and limitations
This will be the first study to investigate the effects of the DASH diet or curcumin supplementation on IL-1α gene expression in human. This study will be the first clinical trial that investigates the co-administration of the DASH diet and curcumin supplementation in patients with PCOS. The frequency of PCOS women with BMI: 18.5-25 is probably low, which could increase study time and lead to bias in results.
Funding
This study is Ph.D. thesis and is funded by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant no. 8229). This trial is externally supported by Iran National Science foundation (INSF) fund after review and approval by the INSF Scientific Committee (under Project No. 99019137 dated July 6, 2021).
Acknowledgments
We would like to acknowledge "M/s Arjuna Natural Pvt Ltd., India", for providing the curcumin supplement (BCM95/Curcugreen) capsules as a gift. The authors would also like to thank INSF and Shahid Sadoughi University of Medical Sciences (Yazd Reproductive Sciences Institute and Gynecology Clinic of Research and Clinical Center for Infertility, Yazd, Iran).
Conflict of Interest
This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results. The authors have no conflict of interest to disclose.
Availability of data and materials
The datasets analyzed during the current study will be available from the corresponding author upon reasonable request.
Full-Text: (338 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder that can lead to major reproductive and metabolic complications, including infertility and insulin resistance. According to the 2003 Rotterdam criteria, the prevalence of PCOS in women of reproductive age is 4.4-18.6% (1). The Rotterdam consensus is the most widely accepted diagnostic criteria (2), by which women must meet at least 2 of the 3 following criteria: 1) confirmation of polycystic ovary by ultrasound; 2) clinical or biochemical hyperandrogenism; and 3) ovulatory dysfunction (oligo/anovulation) (3).
Hyperandrogenism and chronic inflammation have been suggested as the leading causes of pathophysiology and clinical manifestations of PCOS (4). Biochemical hyperandrogenism is defined as a high concentration of testosterone and a high level of free testosterone and free androgen index (3). Hyperandrogenism may be caused by increased 5α-reductase activity, a key enzyme with an essential role in regulating the peripheral conversion of testosterone to the most active androgen, 5α-dihydrotestosterone (5). The findings showed that the activity of 5α-reductase increased in PCOS, and this high activity is related to insulin resistance (6). There are 2 isozymes of 5α reductase, encoded by the 5α-reductase type1 and type2 genes (7). Findings from an animal study suggest that insulin-mediated changes in 5α-reductase type1 messenger ribonucleic acid (mRNA) expression can negatively affect the follicle development and ovulation (8). In addition, mild chronic inflammation, independent of obesity, has been confirmed in PCOS patients (4). One of the most important inflammatory mediators, interleukin-1 (IL-1), is a multifunctional cytokine with 3 different cytokines, including cytokines (α, β) and an IL-1 receptor antagonist. IL-1α gene polymorphism can be associated with PCOS and affect ovulation, fertilization, and implantation (9, 10).
Given the potential effects of chronic inflammation on the pathogenesis of PCOS through the pathway of insulin resistance and hyperandrogenism (11), new strategies have been proposed to improve the clinical symptoms of PCOS. According to evidence-based recommendations, lifestyle intervention (various components including diet, exercise, and behavioral approaches) is recommended (3).
The dietary approaches to stop hypertension (DASH) diet has been suggested as an effective treatment option (12). DASH diet, as a low-glycemic diet (13), with a high concentration of antioxidants, can have beneficial effects in reducing insulin resistance (14), inflammation (15), and free androgen index (16). This diet is rich in vegetables, low-fat dairy products, fruits, whole grains, poultry, fish, and nuts, but is limited in foods high in sodium, cholesterol, saturated fat, red and processed meat, refined grains, sweets, and sugary drinks (12). In the last decade, the antioxidative effects of plant polyphenols have been considered (17). Indeed, among natural phenols, the therapeutic properties of curcumin have been further investigated (18). Curcumin (diferuloylmethane) is a lipophilic yellow pigment extracted from turmeric rhizomes (Curcuma longa L.) (19). Curcumin supplementation improves glycemic control and lipid metabolism, and reduces radical oxygen species in PCOS patients (20, 21).
The anti‐inflammatory and antioxidant benefits of both the DASH diet and curcumin have been demonstrated by meta-analysis results (15, 22). Therefore, we plan to investigate the effect of the DASH diet in combination with curcumin supplements on IL-1α and 5α reductase gene expressions and improvement of insulin resistance and hyperandrogenism.
2. Materials and Methods
2.1. Trial design and study setting
This randomized, placebo-controlled clinical trial will be conducted on PCOS women candidates for in vitro fertilization (IVF), using a convenience sampling method. Participants in a 2×2 factorial design will be randomly divided into 4 parallel treatment groups using block randomization: (1) curcumin supplement + DASH diet (2) curcumin supplement + standard diet (3) placebo + DASH diet, and (4) placebo + standard diet. The follow-up duration will be 12 wk. The study will be conducted at the Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. An overview of the study is presented in figure 1.
2.2. Eligibility criteria
Infertile women with PCOS (according to the Rotterdam criteria) (2) who were candidates for IVF treatment, without a previous history of IVF, will be included in this study. Eligibility criteria included body mass index (BMI) between 18.5-25 and BMI ≥ 25 kg/m² and aged between 18-40 yr.
The participants must not have any of the following:
Metabolic and endocrine abnormalities including thyroid dysfunction, Cushing's syndrome, diabetes or impaired glucose tolerance, hyperprolactinemia, congenital adrenal hyperplasia, liver disease, cardiovascular disease, kidney disease, hypertension, neurological diseases, any history of mental illness, cancer, autoimmune and inflammatory disorders (rheumatoid arthritis). Taking medications including contraceptives or any other drugs that may affect reproductive physiology (during the 3 months before the study); taking antidiabetic, antiobesity, estrogenic, and androgenic drugs, as well as taking supplements such as vitamin C, vitamin E, and omega-3 because of their anti-inflammatory and antioxidative effects, 3 months before or during the intervention; smoking or exposure to spouse's smoking; alcohol drinking; being pregnant; simultaneous participation in another study. Moreover, unwillingness to cooperate and nonadherence to dietary or supplementary interventions (compliance < 80%), allergy to supplements, and pregnancy of the participant during the study will be considered as drop-out criteria.
2.3. Interventions
Participants will be given 2 capsules of curcumin supplement per day (1 after breakfast and another after an evening meal with water) or 2 placebo capsules of curcumin per day at the same time for 12 wk. Curcumin capsules (BCM95/Curcugreen) and their placebos, with the same texture and appearance, were produced as 500 mg zero size veg capsules by “M/s Arjuna Natural Pvt Ltd., India”. The curcumin supplement was made from dried turmeric rhizomes by extraction with ethyl acetate. Curcuma longa L. commonly known as turmeric contains curcuminoids (curcumin, demethoxy curcumin, and bisdemethoxy curcumin) and essential oil of turmeric (ar-turmerone, curlone, and ar-curcumene). Curcuminoids are specific compounds with medicinal properties, of which the most important and active ingredient is the yellow coloring pigment curcumin; Ar-turmerone (the main bioactive components of the Curcuma longa L. essential oil), inhibits the P-glycoprotein pathway, thus improving the oral bioavailability and bioactivity of orally administered curcuminoids. BCM-95 w:as char:acterized by 95% total curcuminoid complex; the final formulation containing at least 65% curcumin and 86% total curcuminoids; and the essential oil containing not less than 45% ar-turmerone. Each 500-mg capsule contained 475 mg of curcuminoids and the essential oil of natural turmeric. Each placebo capsule contained 500 mg of roasted rice powder. The curcumin capsule actives will be analyzed by HPLC, heavy metals by ICP-MS; residual solvents by GCHS, pesticides by GCMS, and microbial indicators all of which were ensured to be in line with the EU standards (regulation (EC) No 1881/2006; directive 2009/32/CE; regulation (EC) No. 396/2005 and related amendments).
The intervention groups will receive 2 healthy dietary patterns (DASH or standard diet). These diets will be designed in the form of an individual diet containing 18% protein, 52% carbohydrates, and 30% total fat, which will be matched with the macronutrient plan used in the other original trial (23). Dietary programs will be planned after baseline assessments based on the current dietary intakes and body weight of each person. Daily calorie requirements will be estimated by calculating the resting energy expenditure and physical activity levels. If a person was overweight (BMI is 25.0 to < 30.0 kg/m²) or obese (≥ 30.0 kg/m²), the calculated energy will be reduced by 300 and 500 kcal, respectively. A 12-wk menu plan (7-day cycle menu with 21 meals) at 11 energy levels (1200, 1300, 1400, 1500, 1600, 1700, 1800, 1900, 2000, 2100, and 2200 kcal) for both dietary patterns will be constructed and analyzed. These will be considered as the basis for participants' diets. Nutritionist IV software (diet analysis, module version 3.5.2) will be used to calculate energy, food exchange, percent of kilocalories from macronutrients, and nutrient values in each daily menu plan. DASH diet will be designed with an emphasis on food rich in fiber, magnesium, potassium, calcium, and protein, such as vegetables, fruits, and low-fat dairy products; while foods high in saturated fat and sugar will be limited (12). The amount of sodium prescribed for each diet will be the same (2300 mg per day-approximately 1 teaspoon of salt). Overall, DASH and standard patterns will be designed using similar foods and recipes, but in different amounts, to achieve the specific characteristics of each diet. Both diets will be planned by the study dietitian and reviewed by the Chief investigator.
Participants will receive their specific diet through face-to-face individual counseling, initial assessment, and intervention for 45-60 min with a registered dietitian (follow-up visits for 15-30 min per month). They will also receive a bottle of supplement/placebo (on the 1st day, 30th day, and 60th day of visit), each bottle containing 64 capsules (providing supplement for 1 month and a grace period of 2 days). Administration instructions on taking the pills and what needs to be done in case a dose is missed were set for patients, including taking 2 curcumin/placebo capsules a day (one after breakfast and one after an evening meal with water) and taking the missed capsule the same day. The patients will be monitored for daily supplementations by contacting them via phone interviews and messaging (Eitaa or Bale apps) twice a day. Moreover, daily consumption of supplements/placebos will be recorded in the case report form given to participants. At each follow-up visit, a nutritionist will assess the participants adherence to the diet and supplementation.. The number of capsules taken will be calculated by counting the remaining capsules in each bottle. If the number of unused prescription capsules is more than 12 out of 60 capsules per month, for each participant, that patient will be classified in the non-adherent group.
All adverse events including unfavorable and/or unintended signs and any undesirable experience occurring to a patient will be reported in writing to the Medical Ethics Committee and the Data Monitoring Committee of the Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The participants, for whatever reason, can discontinue participating in the research study at any given time before or after signing the consent form. The researcher may also terminate the study to maintain safety and protect the participant from excessive risks and/or to keep the integrity of the research data due to inappropriate follow-up by the participant. In case of termination of the study by the investigator, the reasons for the termination will be explained to the participants.
2.4. Strategies to improve adherence to interventions
1) telephone follow-up; 2) weekly electronic counseling in order to improve patient’s participation by reducing barriers such as geographical distance and time constraints; 3) husbands of the participant will also receive free dietary consulting to improve compliance and family support.
2.5. Outcomes
The primary outcomes included gene expressions of IL-1α and 5α-reductase. The secondary outcomes consist serum levels of biochemical parameters comprising fasting blood sugar, fasting insulin, homeostasis model assessment of insulin resistance and quantitative insulin sensitivity check index, as well as serum androgen concentrations (testosterone, 5α-dihydrotestosterone) and anthropometric indices (height, weight, BMI, hip circumstance, waist circumstance, waist-hip ratio, waist-height ratio), body composition (muscle mass, fat mass, fat-free mass, and other factors such as trunk fat mass), physical activity, and dietary intake (energy and nutrient intake).
2.5.1. Demographic data and anthropometric measurements
A form including: full address, age, gender, income, employment, ethnicity, level of education, and medical history (dose and type of medicine and supplements) will be completed for each participant.
Participant’s weight, height, body circumferences (waist, hip), BMI, and waist-hip ratio as well as body composition analysis (fat mass, muscle mass, fat free mass) will be measured on the first and last day of the intervention. Weight will be measured to the nearest 0.5 kg (possible errors) using a pre-calibrated electronic scale (Seca, Germany). Height will be measured without shoes with an accuracy of 0.1 cm using a Seca wall stadiometer. Then BMI will be determined as weight (kg) divided by the square of their height (m²). Waist (cm) will be measured as the distance between the lowest rib and the iliac crest. Additionally, the hip circumference will be measured around the largest part of the hip. The waist-hip ratio will be the ratio of waist to hip circumference. The waist-height ratio will be measured by dividing the waist circumstance by the height. Body composition will be determined using body composition analyzer (Tanita BC-418, Tokyo, Japan), at the beginning of the study and after 12 wk of the study. All the measurements will be repeated by a trained assessor and recorded as average.
2.5.2. Biochemical measurements
After 2 wk of run-in period and considering 12-hr overnight fasting, 8 cc of blood samples will be collected in the morning from each participant, in the beginning, and at the end of the study.
5 cc of samples will be transferred to clot-activator tubes and then centrifuged for 30 min to separate the serum. Another 3 cc of blood samples will be collected in EDTA- coated tubes to seperate the plasma and buffy coat (the layer between red blood cells and plasma) and prepare buffy coat for gene expression assays. The allocated serum, plasma, and buffy coat will be stored immediately at -70°C. Before storage, the tripura isolation reagent will be added to the isolated buffy coat.
Serum levels of insulin, testosterone, and 5α-dihydrotestosterone will be evaluated using the enzyme-linked immunosorbent assay (ELIZA method), (ELISA kit, Monobind, California, United State). Fasting blood sugar will be measured by commercial kit using an enzymatic colorimetric method (Pars Azmoon, Tehran, Iran). homeostasis model assessment of insulin resistance will be calculated, using the following formula:
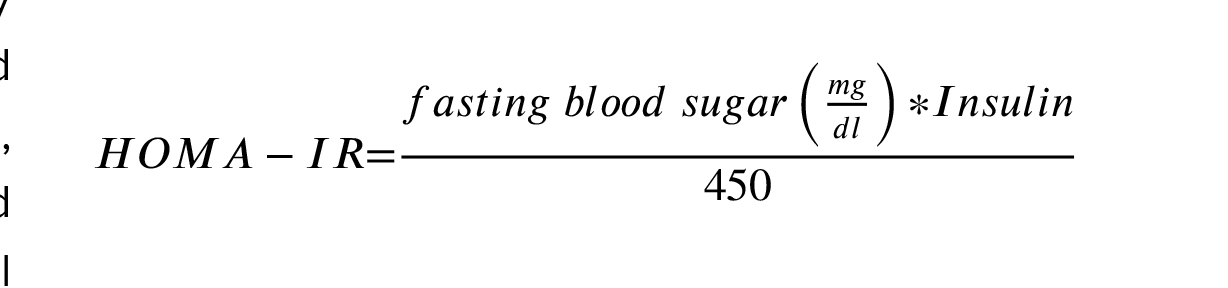
2.5.3. Gene expression assay
According to the kit protocol (Favorgen Biotech Corp., Taiwan), the buffy coat fraction of whole blood cells will be used for total RNA extraction. RNA concentration will be analyzed using spectrophotometer (DS-11, DeNovix) at 260 nm. Purity will be considered as an A260/A280 ratio of about 2 (24). cDNA will be synthesized from high-quality mRNA using a cDNA synthesis kit (GeneAll Biotechnology Co.). Forward and reverse primers are selected after reviewing previous studies (25-27) and checked by primer designing tools (Primer Blast, OLIGO 7 primer analysis software), as shown in table I.
IL-1α and 5α-reductase expression levels will be assessed by SYBR-green real-time PCR detection method using the kit protocol (Yekta Tajhiz Azma, IRAN), and the StepOne real-time PCR system (Applied Biosystems, Foster City, California, USA). GAPDH (glyceraldehyde phosphate dehydrogenase) will be chosen as the housekeeping gene.

2.5.4. Dietary intake
Food consumption information will be obtained through 3-day dietary records including 2 days per week and one weekend. Before the study, participants will be given adequate training on food consumption and will be provided with a guide including written examples for completing a questionnaire that simply explains how to accurately record food. The participants will be taught to use standard kitchen measuring tools to accurately determine the portion size. To reduce memory errors, they will be asked to make a detailed list of foods and beverages exactly when eating. The expert dietitian will review the completed dietary records, ingredient lists, and preparation methods, while will be blind to the study allocation. Each daily food record will be calculated by the Nutritionist IV software.
2.5.5. Physical activity
The metabolic equivalent of task-hours (MET-h)/day will be applied to assess physical activity questionnaire data (28).
2.6. Sample size
The sample size of 24 in each group was determined according to α = 0.05, the power of 80%, dropout rate of 20%, and considering a minimal clinically important difference of 0.969 and an effect size of 1, based on Heshmati et al. study (21) using the following formula:
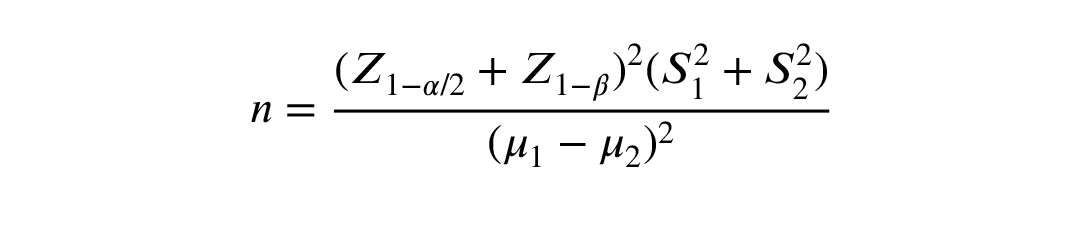
2.7. Recruitment
Unique codes will be assigned to perform allocation concealment and prevent "selection bias". Supplements and placebos capsules will be provided in the same appearance (in terms of color and shape) and packed in similar bottles. The bottles will be labeled with different marks (A and B) and placed in envelopes with serial numbers by someone not related to the study. Participants and researchers will not be aware of the bottles content. Intervention groups will also receive diets from a nutritionist who is not involved in this study.
The participants will be assigned to 4 groups according to the method of stratified permuted block randomization based on treatment conditions and BMI (BMI: 18.5-25 and BMI ≥ 25), in equal block sizes of 4. The random allocation list will be created by Package ‘blockrand’ in R software, version 4.0.2.
Randomization and allocation will be concealed from participants and researchers until finishing the study steps and data analysis. An independent biostatistician will generate the allocation sequence. 2 midwifes will give consecutive numbers to the eligible participants and refer them to a nutritionist. The sealed envelope containing the treatment code will be opened by the supervisor, after analyzing the final data or in an emergency condition.
All trial healthcare providers, data analysts, and outcome assessors will be blinded to intervention assignments. Participants may only be aware of the type of diet but will not be aware of the placebo or curcumin supplement content.
2.8. Statistical methods
The values of quantitative variables will be presented as the mean ± standard deviation, and qualitative variables will be expressed as numbers and percentages. The normality of data will be examined by the Kolmogorov-Smirnov test.
The frequency among study groups will be compared using the Chi-square test. Independent sample t test and 2-way ANOVA will be applied to estimate the mean of quantitative variable changes and interactions between study groups; Bonferroni correction will be considered for multiple comparisons; and analysis of covariance (ANCOVA) will be used to compare the differences in the mean of quantitative data between the studied groups, while taking into account the influence of possible confounding variables. Before statistical analysis, all data will be checked for accuracy and completeness. All statistical analysis will be conducted using SPSS 22 (SPSS, Inc, Chicago, IL, USA). A p-value < 0.05 will be defined as a statistically significant level.
The "intention-to-treat" and "per protocol analysis" approaches will be performed for data analysis. The "intention-to-treat"analysis considers all participants in their main randomized groups, regardless of whether they follow the prescribed intervention according to the protocol or not, and also ignores non-compliance and withdrawal. Per protocol analysis, which accurately reflects what has been done, will be conducted based on actual adherence to the interventions.

2.9. Participants and public involvement
Participants or the public will not participate in the setting, execution, measuring outcome, or dissemination plans of our study. The study results will be provided to patients upon request. Research findings will be reported in scientific journals.
2.10. Ethical considerations
This study will be conducted following the Declaration of Helsinki. This trial was approved on September 2020 by the Medical Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, (Code: IR.SSU.SPH.REC.1399.145).
All goals, working methods, and possible side effects of the interventions will be explained to all the participants before starting and entering the study by the main researcher, and an informed consent form will be obtained from the participants.
Participants will be assured that all their information will be kept confidential. The contact information of one of the researchers will be provided to the participants for 24-hr contact in case of any complications or problems. The study will have no financial burden on the participants.
2.11. Monitoring
Data monitoring and safety program are considered for this study. Project managers (HM, AA) and a research advisor (MS, SJ, and AN) will supervise and monitor the research process to ensure protocol compliance and data quality. Any withdrawals, lost to follow-up and adverse events will be assessed and reported. An independent trial Data Monitoring Committee will review the collected data and evaluate clinical trials for scientific validity, safety, and integrity.
2.12. Study strengths and limitations
This will be the first study to investigate the effects of the DASH diet or curcumin supplementation on IL-1α gene expression in human. This study will be the first clinical trial that investigates the co-administration of the DASH diet and curcumin supplementation in patients with PCOS. The frequency of PCOS women with BMI: 18.5-25 is probably low, which could increase study time and lead to bias in results.
Funding
This study is Ph.D. thesis and is funded by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant no. 8229). This trial is externally supported by Iran National Science foundation (INSF) fund after review and approval by the INSF Scientific Committee (under Project No. 99019137 dated July 6, 2021).
Acknowledgments
We would like to acknowledge "M/s Arjuna Natural Pvt Ltd., India", for providing the curcumin supplement (BCM95/Curcugreen) capsules as a gift. The authors would also like to thank INSF and Shahid Sadoughi University of Medical Sciences (Yazd Reproductive Sciences Institute and Gynecology Clinic of Research and Clinical Center for Infertility, Yazd, Iran).
Conflict of Interest
This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results. The authors have no conflict of interest to disclose.
Availability of data and materials
The datasets analyzed during the current study will be available from the corresponding author upon reasonable request.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Ding T, Hardiman PJ, Petersen I, Wang F-F, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: A systematic review and meta-analysis. Oncotarget 2017; 8: 96351-96358. [DOI:10.18632/oncotarget.19180] [PMID] [PMCID]
2. Yasmin A, Roychoudhury S, Paul Choudhury A, Ahmed ABF, Dutta S, Mottola F, et al. Polycystic ovary syndrome: An updated overview foregrounding impacts of ethnicities and geographic variations. Life 2022; 12: 1974. [DOI:10.3390/life12121974] [PMID] [PMCID]
3. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018; 33: 1602-1618. [DOI:10.1093/humrep/dey256] [PMID] [PMCID]
4. Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE-D, Amer S. The role of chronic inflammation in polycystic ovarian syndrome-A systematic review and meta-analysis. Int J Mol Sci 2021; 22: 2734. [DOI:10.3390/ijms22052734] [PMID] [PMCID]
5. Kannenberg F, Fobker M, Schulte E, Pierściński G, Kelsch R, Zitzmann M, et al. The simultaneous measurement of serum testosterone and 5α-dihydrotestosterone by gas chromatography-mass spectrometry (GC-MS). Clin Chim Acta 2018; 476: 15-24. [DOI:10.1016/j.cca.2017.10.030] [PMID]
6. Wu C, Wei K, Jiang Z. 5α-reductase activity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod Biol Endocrinol 2017; 15: 21. [DOI:10.1186/s12958-017-0242-9] [PMID] [PMCID]
7. Batista RL, Mendonca BB. Integrative and analytical review of the 5-alpha-reductase type 2 deficiency worldwide. Appl Clin Genet 2020; 13: 83-96. [DOI:10.2147/TACG.S198178] [PMID] [PMCID]
8. Wu C, Jiang F, Wei K, Jiang Z. Exercise activates the PI3K-AKT signal pathway by decreasing the expression of 5α-reductase type 1 in PCOS rats. Sci Rep 2018; 8: 7982. [DOI:10.1038/s41598-018-26210-0] [PMID] [PMCID]
9. Zafari Zangeneh F, Naghizadeh MM, Masoumi M. Polycystic ovary syndrome and circulating inflammatory markers. Int J Reprod BioMed 2017; 15: 375-382. [DOI:10.29252/ijrm.15.6.375] [PMID] [PMCID]
10. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018; 281: 8-27.
https://doi.org/10.1111/imr.12624 [DOI:10.1111/imr.12621]
11. Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci 2021; 22: 3789. [DOI:10.3390/ijms22073789] [PMID] [PMCID]
12. Foroozanfard F, Rafiei H, Samimi M, Gilasi HR, Gorjizadeh R, Heidar Z, et al. The effects of dietary approaches to stop hypertension diet on weight loss, anti‐müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: A randomized clinical trial. Clin Endocrinol 2017; 87: 51-58. [DOI:10.1111/cen.13333] [PMID]
13. Campbell AP. DASH eating plan: An eating pattern for diabetes management. Diabetes Spectr 2017; 30: 76-81. [DOI:10.2337/ds16-0084] [PMID] [PMCID]
14. Akhlaghi M. Dietary approaches to stop hypertension (DASH): Potential mechanisms of action against risk factors of the metabolic syndrome. Nutr Res Rev 2020; 33: 1-18. [DOI:10.1017/S0954422419000155] [PMID]
15. Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin Nutr 2018; 37: 542-550. [DOI:10.1016/j.clnu.2017.02.018] [PMID]
16. Azadi-Yazdi M, Karimi-Zarchi M, Salehi-Abargouei A, Fallahzadeh H, Nadjarzadeh A. Effects of dietary approach to stop hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: A randomised controlled trial. J Hum Nutr Diet 2017; 30: 275-283. [DOI:10.1111/jhn.12433] [PMID]
17. Leri M, Scuto M, Ontario ML, Calabrese V, Calabrese EJ, Bucciantini M, et al. Healthy effects of plant polyphenols: Molecular mechanisms. Int J Mol Sci 2020; 21: 1250. [DOI:10.3390/ijms21041250] [PMID] [PMCID]
18. Hewlings SJ, Kalman DS. Curcumin: A review of its effects on human health. Foods 2017; 6: 92. [DOI:10.3390/foods6100092] [PMID] [PMCID]
19. Urošević M, Nikolić L, Gajić I, Nikolić V, Dinić A, Miljković V. Curcumin: Biological activities and modern pharmaceutical forms. Antibiotics (Basel) 2022; 11: 135. [DOI:10.3390/antibiotics11020135] [PMID] [PMCID]
20. Chien Y-J, Chang C-Y, Wu M-Y, Chen C-H, Horng Y-S, Wu H-C. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: Systematic review with meta-analysis and trial sequential analysis. Nutrients 2021; 13: 684. [DOI:10.3390/nu13020684] [PMID] [PMCID]
21. Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, et al. The effects of Curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab Syndr 2020; 14: 77-82. [DOI:10.1016/j.dsx.2020.01.002] [PMID]
22. Tabrizi R, Vakili S, Akbari M, Mirhosseini N, Lankarani KB, Rahimi M, et al. The effects of curcumin‐containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta‐analysis of randomized controlled trials. Phytother Res 2019; 33: 253-262. [DOI:10.1002/ptr.6226] [PMID]
23. Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: A randomized controlled clinical trial. Horm Metab Res 2015; 47: 232-238. [DOI:10.1055/s-0034-1376990] [PMID]
24. Koetsier G, Cantor E. A practical guide to analyzing nucleic acid concentration and purity with microvolume spectrophotometers. New England Biolabs Inc 2019; 1-8.
25. Eggestøl HØ, Lunde HS, Knutsen TM, Haugland GT. Interleukin-1 ligands and receptors in lumpfish (Cyclopterus lumpus L.): Molecular characterization, phylogeny, gene expression, and transcriptome analyses. Front Immunol 2020; 11: 502. [DOI:10.3389/fimmu.2020.00502] [PMID] [PMCID]
26. Khantham C, Yooin W, Sringarm K, Sommano SR, Jiranusornkul S, Carmona FD, et al. effects on steroid 5-alpha reductase gene expression of thai rice bran extracts and molecular dynamics study on SRD5A2. Biology (Basel) 2021; 10: 319. [DOI:10.3390/biology10040319] [PMID] [PMCID]
27. Toupchian O, Abdollahi Sh, Salehi-Abargouei A, Heshmati J, Clark CCT, Sheikhha MH, et al. The effects of resveratrol supplementation on PPARα, p16, p53, p21 gene expressions, and sCD163/sTWEAK ratio in patients with type 2 diabetes mellitus: A double-blind controlled randomized trial. Phytother Res 2021; 35: 3205-3213. [DOI:10.1002/ptr.7031] [PMID]
28. de Almeida Mendes M, da Silva I, Ramires V, Reichert F, Martins R, Ferreira R, et al. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PLoS One 2018; 13: e0200701. [DOI:10.1371/journal.pone.0200701] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




