Sun, Feb 15, 2026
[Archive]
Volume 21, Issue 4 (April 2023)
IJRM 2023, 21(4): 285-294 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saleh S, Ghanaatpisheh A, Haghshenas H, Parvin N, Mikaeiliagah E, Kargar Jahromi H et al . The effect of leaf hydroalcoholic extract of Ephedra pachyclada infertility in male rats treated with cyclophosphamide: An experimental study. IJRM 2023; 21 (4) :285-294
URL: http://ijrm.ir/article-1-2635-en.html
URL: http://ijrm.ir/article-1-2635-en.html
Shiva Saleh1 

 , Aref Ghanaatpisheh1
, Aref Ghanaatpisheh1 

 , Hoda Haghshenas1
, Hoda Haghshenas1 

 , Negar Parvin1
, Negar Parvin1 

 , Elmira Mikaeiliagah2
, Elmira Mikaeiliagah2 

 , Hossein Kargar Jahromi *3
, Hossein Kargar Jahromi *3 

 , Bahareh Ebrahimi4
, Bahareh Ebrahimi4 




 , Aref Ghanaatpisheh1
, Aref Ghanaatpisheh1 

 , Hoda Haghshenas1
, Hoda Haghshenas1 

 , Negar Parvin1
, Negar Parvin1 

 , Elmira Mikaeiliagah2
, Elmira Mikaeiliagah2 

 , Hossein Kargar Jahromi *3
, Hossein Kargar Jahromi *3 

 , Bahareh Ebrahimi4
, Bahareh Ebrahimi4 


1- Student Research Committee, Jahrom University of Medical Sciences, Jahrom, Iran.
2- Stem Cell and Tissue Engineering Laboratory, Department of Orthopedics, West Virginia University, Morgantown, USA. Department of Biology, Ardabil Branch, Islamic Azad University, Ardabil, Iran.
3- Research Center for Noncommunicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran. ,h.kargar@jums.ac.ir
4- Shiraz Geriatric Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Stem Cell and Tissue Engineering Laboratory, Department of Orthopedics, West Virginia University, Morgantown, USA. Department of Biology, Ardabil Branch, Islamic Azad University, Ardabil, Iran.
3- Research Center for Noncommunicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran. ,
4- Shiraz Geriatric Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 9700 kb]
(1112 Downloads)
| Abstract (HTML) (1569 Views)
1. Introduction
Cancer, is known to be the second cause of mortality in over 100 countries, affecting life expectancy and patient's quality of life. From the past decades till now, rational use of effective anti-cancer agents for treatment was felted (1); among all discovered drugs, cyclophosphamide (CP), a nitrogen mustard anti-cancer agent, has been consumed as a chemotherapeutic drug for more than 50 yr after its discovery (2-3). Despite CP’s clinical benefits, some significant adverse effects of CP, such as nephrotoxicity (4), cardiotoxicity (3), severe bladder bleeding, and gonadotoxicity had limited its clinical indication (5-7). In the presence of cytochrome P450, CP metabolizes into 2 toxic compounds, acrolein, and phosphoramide. Acrolein is an unsaturated and electrophilic reactive aldehyde that generates noxious reactive oxygen species (ROS) and next, affects the neighboring tissue (8, 9). Studies have defined the acrolein-induced excessive ROS effects on testicular microvascular, endocrine signaling, and germ-cell apoptosis which causes testicular infertility (10). Besides them, CP increases the incidence of early menopause and infertility in males and females; this type of infertility is often transitory, but it can potentially be permanent (11, 12). In a study by Watcho et al., CP-treated Wistar rats had significant reduction in seminal vesicle weight, testosterone level, sperm count, motility, and viability; furthermore, sperm morphological abnormality was also detected (13). In addition to morphological and qualitative changes in sperm caused by CP, there are also studies that have shown DNA damage and nuclear maturations (14).
In the last few years, considerable interest in the use of medicinal plants with the aim of cancer treatment or reducing anticancer agents’ side effects has been increased. CP applies its effect primarily by persuading oxidative stress and changing gene expression in spermatocytes vastly unlike stages of development (15); therefore, these studies mostly rely on the antioxidant role of plants and consider antioxidants as affective factors (13, 14). For example, in a study by Can et al., useful Hypericum Triquetrifolium Turra against CP gonadotoxicity had elevated total oxidant status (TOS), and malondialdehyde (MDA) in the CP group (16).
Ephedra (EP) is a major genus of the Ephedraceae family, found in dry and waterless regions of the globe. Some members of this genus are extensively used in traditional medicine to ameliorate and regulate asthma’s symptoms, common cold, influenza, fever, and headache (17). Due to ephedrine-type alkaloids and their pharmacological characteristics, the chemical ingredients of EP species have been the subject of investigation for decades (10). Its role in cardiovascular and central nervous systems, nephrotoxicity and hepatotoxicity, and cancers such as breast cancer were examined in different studies (18-20). Despite the known antioxidative function of EP (21, 22), in only one in vivo study, EP had significantly bring back testicular weight and activity, spermatozoid characteristics, serum and tissue hormonal levels, and antioxidant protection. This study had shown significantly activated gonadotropin-releasing hormone (GnRH) signaling pathway and meiosis-related protein levels by EP in rat model of adriamycin injury (23).
Considering today’s high prevalence of cancer and CP role as an essential anticancer drug, special attention should be paid to this drug’s side effects. To compensate for the side effects of these chemotheraputic drugs, herbs, especially those with antioxidative function would be useful. We turned to use EP, a plant with proven antioxidative functions. This study sought to determine EP hydroalcoholic extract role on testicular tissue and the pituitary-gonadal axis in CP-treated male rats.
2. Materials and Methods
2.1. EP collection and extraction
EP was collected from Yasuj, Iran, and then the plant’s stems were dried at room temperature and powdered. EP powder was mixed with ethanol (70%) solution at a chamber temperature (35 ± 5°C) and after 4 days “the extract was filtered using a sterile cloth sheet. The extract was dried under reduced pressure at below 45°C with a rotary evaporator. This extract were stored at 4°C” (24).
2.2. Determination of extract purification
At first, 1 ml of phenol solution was combined with 200 µl EP pachyclada extract and “stored in dark for 6 min, then 2 ml of NaCo3 (7%) was added and kept for 120 min. The solution absorbance was measured at a wavelength of 765 ɲm” and finally, the total phenolic amount of extract was calculated using the standard curve of gallic acid and reported 9.28 mg/(GAE)4/g (25). Total flavonoid measurement was calculated by the method of Dharmadasa et al. and 44.32 mg of (QE) 5/g extract was reported based on the quercetin standard curve (26).
2.3. Experiment design
In this experimental study, 48 Wistar male rats (180-200 g) were divided into 6 groups. The animals were kept in the animal laboratory of Jahrom University of Medical Sciences, Jahrom, Iran with standard laboratory conditions of 12 hr light/dark cycles and 50-55% of humidity.
According to the previous studies, EP extract was prescribed with concentrations of 250, 500, and 1000 mg/kg by gavage (24). The CP (Baxter, Germany) concentration was determined 5 mg/kg (for 28 days) (27); the control group did not receive any treatment during the experiment, and the sham group received distilled water and alcohol-based on body weight by gavage. Experimental group 1 received only 5 mg/kg CP, and experimental groups received CP and EP extract 250, 500, and 1000 mg/kg.
2.4. Biochemical analysis
On the 29th day, the blood of the weighed animals’ was drawn from their heart (under anesthesia using Xylazine (10 mg/kg of body weight) and Ketamine (50 mg/kg of body weight)). Their serums were collected via centrifugation (for 15 min and 3000 rpm) and then kept at -20°C until laboratory analysis. To measure the luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, GnRH, TOS, MDA, total antioxidant capacity (TAC), and plasma glutathione peroxidase (GSH-PX), the ELIZA kits for rats were used (Shanghai Crystal day Biotech Co., China).
2.5. Histological examination
For histological examination, testis tissue (left and right) was rapidly removed and placed in a 10% formalin container. After 48 hr, the testis tissue was removed from the solution and after routine tissue processing; paraffin sections of 5 μm thickness were prepared as serial sections. Sections of each testis were stained by the H&E method. The mean number of spermatogonia, spermatocytes, spermatids, Leydig, and Sertoli cells were assessed in the testis by stereology method. To count the cells, the optical dissector method and unbiased counting frame were used. The counting frame was randomly placed on the fields of view selected from each slice (10,000× magnification), from each side (top and bottom) up to a depth of 5 µ, sections with artifacts were considered, and counting was not done in those sections. Cells selected by the frame were counted at other depths (average 130-150 of each cell). The desired depths were obtained with a microcator (MT12, Germany). Then, the numerical density of the cells was obtained and multiplied by the final volume of the testis, in order to achieve the estimated cell count.
2.6. Ethical considerations
This study was in agreement with ethical issues of animal laboratory Ethics of Jahrom University of Medical Science, Jahrom, Iran (Code: IR.JUMS.REC.1396.018).
2.7. Statistical analysis
SPSS software (version 24) was used for data analysis. At first, the normality of the data was measured using Shapiro-Wilk normality test. Data were analyzed by Independent-samples t test using SPSS software version 16 and the significance level was considered 0.05.
3. Results
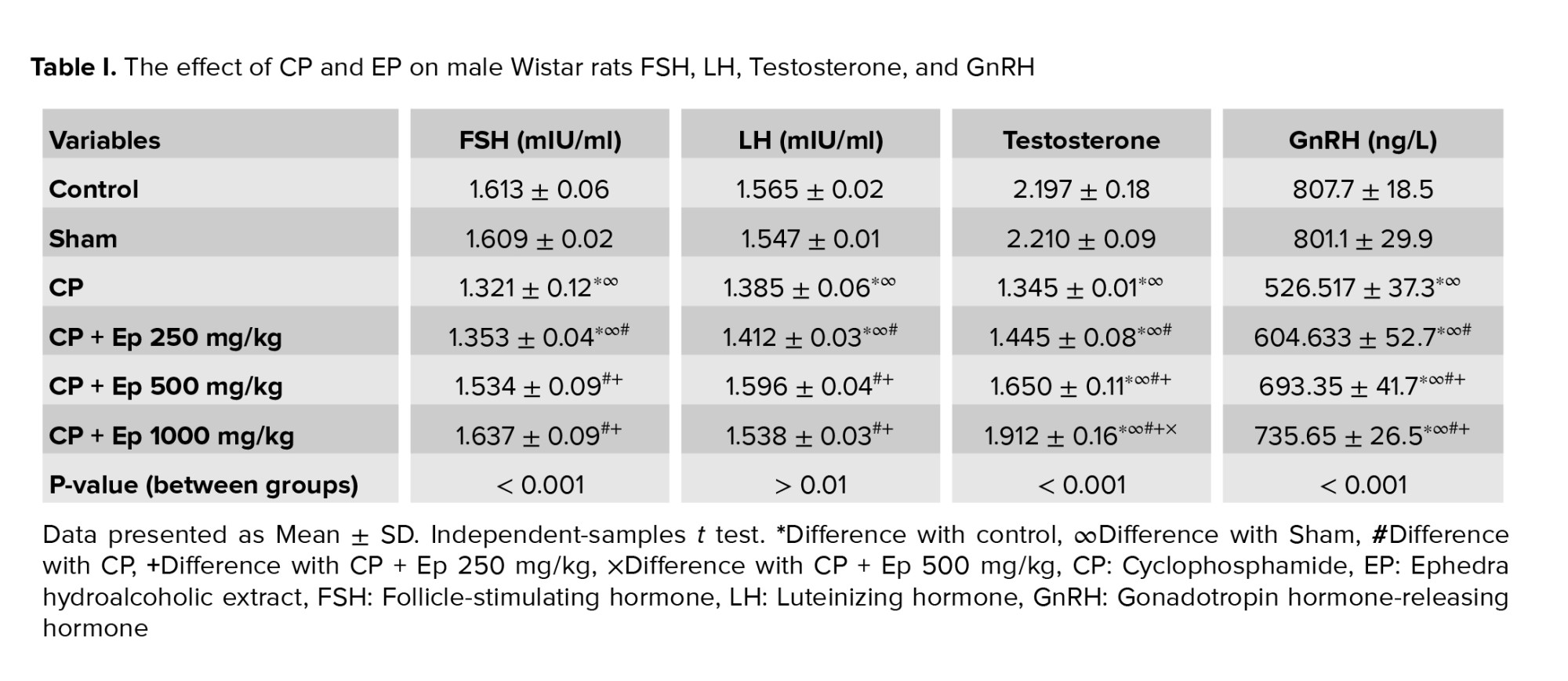
3.1. Changes in serum LH, FSH, testosterone, and GnRH
Data analysis showed that CP had affected serum FSH and LH and using EP had altered these; using higher doses of EP (500 and 1000 mg/kg) were not significantly different with the control. All experimental groups showed a significant decrease in serum testosterone compared to the control group at p < 0.05. The mean testosterone concentration in CP and EP 500 mg/kg did not significantly differ from the CP group; however, the higher doses were significantly different from CP group and the best effect belonged to the highest dose.
Serum GnRH concentration showed a significant increase in all EP groups compared to the control and CP groups. The mean serum concentration of GnRH in experimental groups containing 500 and 1000 mg/kg EP was significantly higher than the group treated with 250 mg/kg EP (p < 0.05).
On comparing CP groups with EP extract ones, it was found that 1000 mg/kg EP had more significant effects on boosting serum-assessed parameters (Table I).
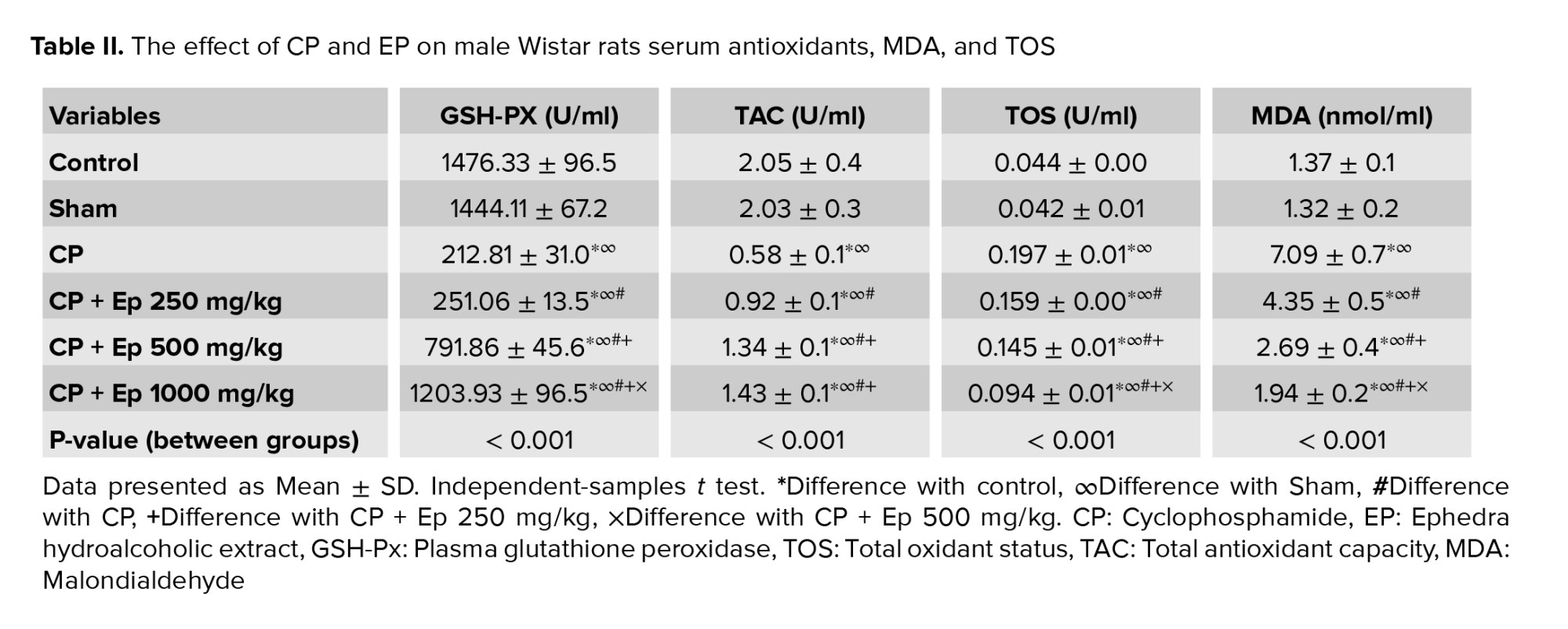
3.2. Changes in serum antioxidants, MDA, and TOS
Data analysis showed a significant reduction of serum TAC and GSH-PX in groups that used CP compared to the control. EP (all doses) significantly increased TAC concentration compared to the CP group (p < 0.05); however, GSH-PX data showed this significant increase only in higher doses of EP.
A significant difference was observed in serum sets of TOS and MDA in all experimental groups compared to the control group. In addition, serum levels of TOS and MDA in EP groups compared to the CP group showed a significant reduction at the p < 0.05 (Table II). Each group means with at least one shared letter had no significant difference.
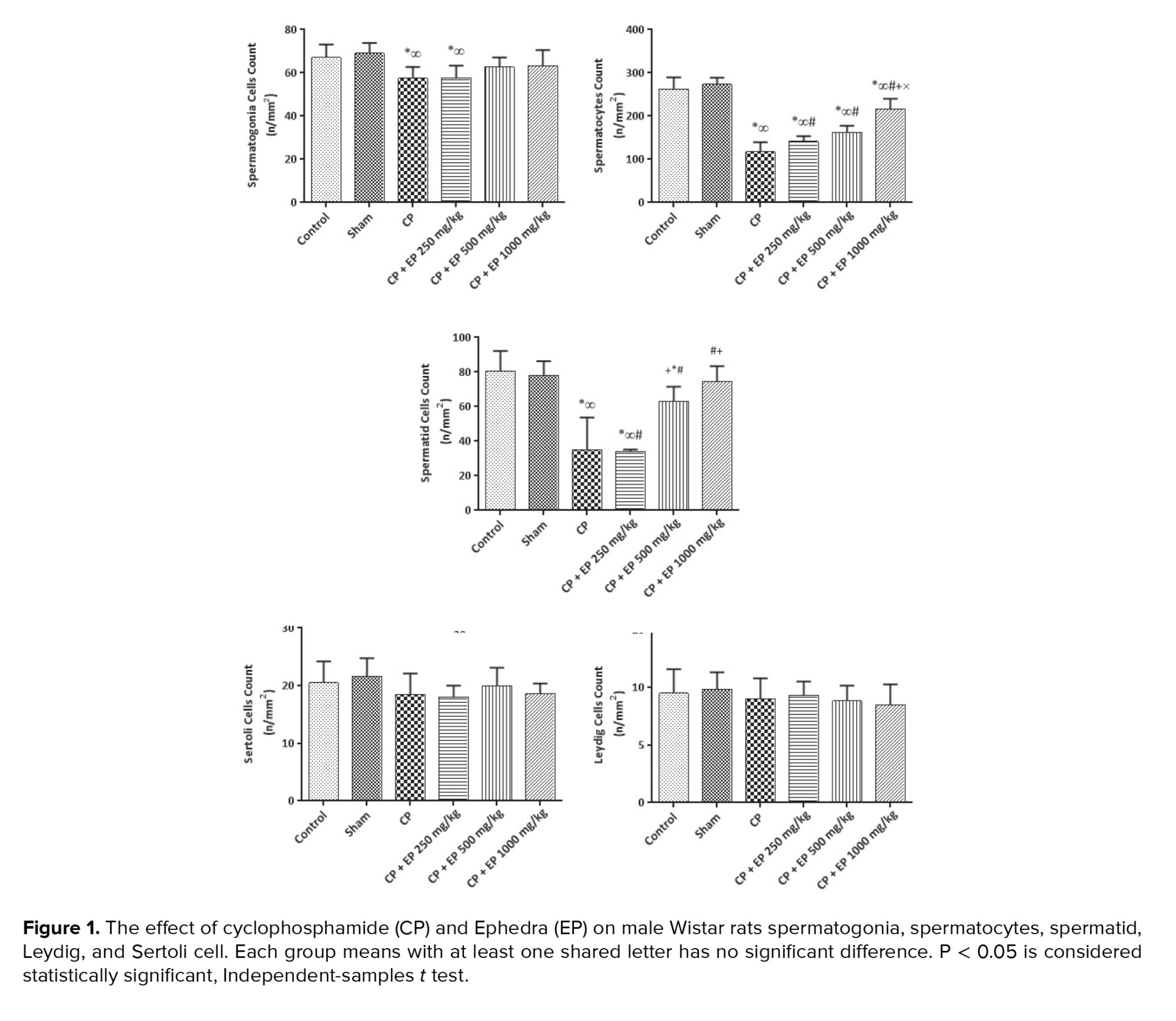
Changes in the mean number of spermatogonia, spermatocytes, spermatid, Leydig, and Sertoli cells:
This study showed that despite the role of CP on the mean number of spermatogonia (p < 0.05), experimental groups, received higher doses of EP with no significant difference with the control group. No significant difference was observed between the CP groups and the 250 mg/kg EP group. All experimental groups showed a significant decrease in spermatocyte count compared to the control group at p < 0.05, but using EP affected this significantly compared to the CP group. Moreover, no significant difference was observed between 500 and 1000 mg/kg EP. The mean changes of spermatid cells in CP groups except the group treated with 1000 mg/kg EP was significantly lower than the control group. Higher doses significantly increased the spermatid cells count compared to the CP and CP+250 mg/kg EP groups. The mean number of Leydig and Sertoli cells in all study groups did not significantly differ compared to the control group and EP did not affect them (Figure 1).
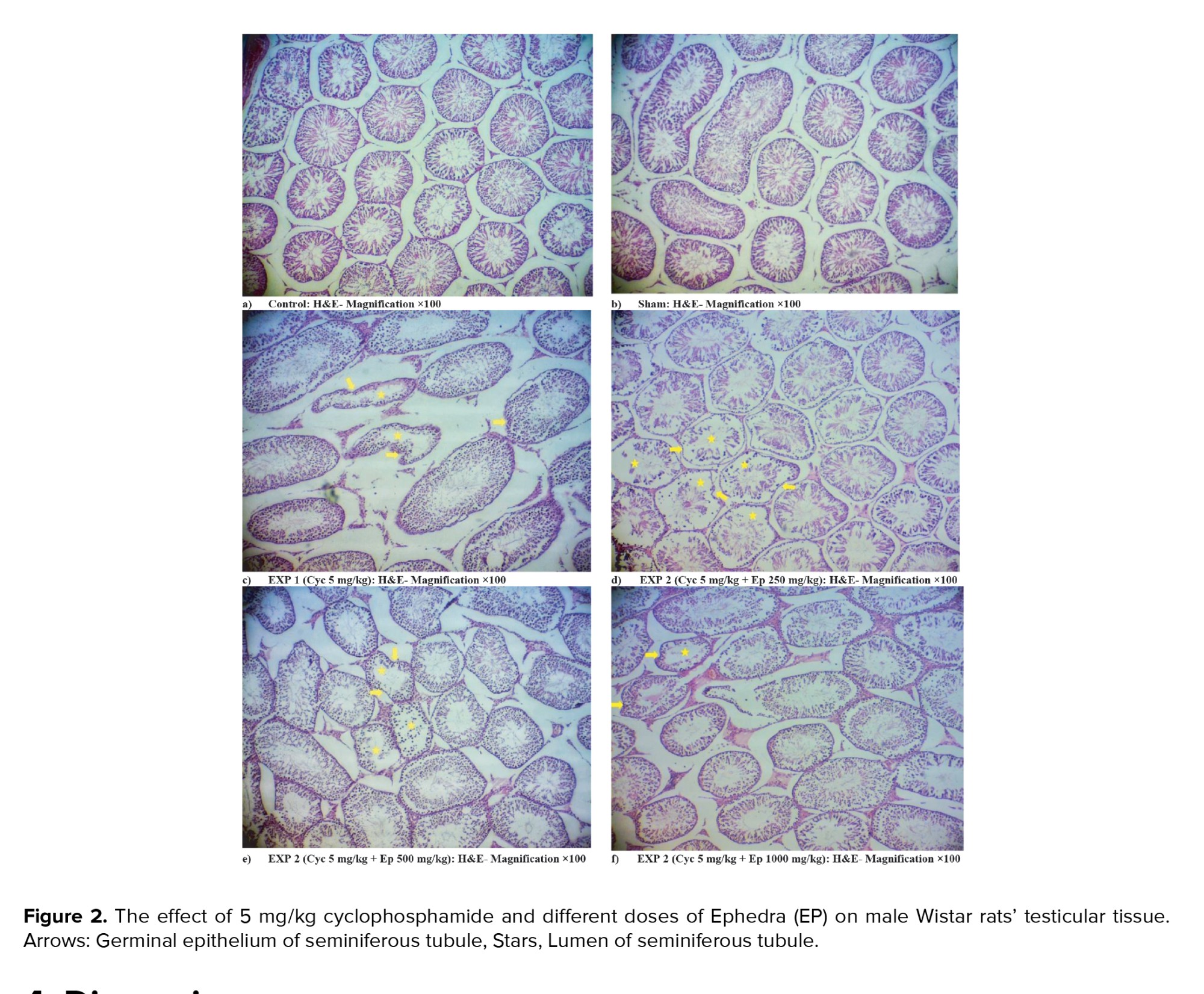
3.3. Testicular tissue histological results
The histological examination of the testicular tissue showed that no tissue changes were observed in the control and sham groups. In the CP group, cell disorganization and disorders inside the seminiferous tubule, and wrinkling of the germinal epithelium were observed. In groups receiving EP with different doses, tissue disorders showed less severity than the CP group receiving CP. By increasing the concentration of EP, the intensity of these changes decreased so the lowest amount of tissue disorders was observed in the EP 1000 mg/kg group (Figure 2).
4. Discussion
Gonadotoxicity has become one of the most notable and commonly encountered side effects of extended CP use, so there is an imperative and crucial need to identify therapeutic strategies to decrease or regulate the reproductive potential of cancer patients subjected to CP therapy (28). CP is metabolized by cytochrome P450 to 2 stable toxic composites: Acrolein, an α, β-unsaturated aldehyde that has an extremely electrophilic feature, and phosphoramide mustard. ROS mainly induces Acrolein, and Acrolein, induces ROS and oxidative damage via DNA cross-linking and interfering in rapidly proliferating organs and tissues such as testicular tissue, which is known well (29, 30). For example, in a study, it was shown that CP induced gonadotoxicity would be affected by using Nerolidol, a sesquiterpene with potent antioxidative properties. Nerolidol acted as a gonadoprotective molecule and increased testosterone level, sperm count, as well as it also reduced MPO (myeloperoxidase) expression and improved CP-induced histological abnormalities in testes, seminal vesicles, and even epididymis (7). Different studies had shown the beneficial effect of herbs on CP-induced tissue damages, such as gonadotoxicity (31, 32).
This study revealed the gonadoprotective effects of the leaf hydroalcoholic extract of EP pachyclada counter to gonadotoxicity caused by CP in male rats by indicating its effect on serum concentration of FSH, testosterone, and LH. The results showed that the hydroalcoholic extract of EP significantly increased the mean serum levels of FSH, LH, and testosterone; EP also affected testicular spermatocytes, spermatozoids, and spermatids in a dose-dependent manner, especially in 1000 mg/kg EP group. EP has been shown to have significant free radical scavenging potential (33) and its benefits on various problems such as blood parameters in diabetic rats (34), cancer cells viability, and inflammatory factors were investigated; however, as mentioned in the introduction section, only one study showed EP benefits in gonadotoxicity.
The testosterone creation is a complicated pathway that relies on the balances between FSH, LH, GnRH, and vital factors necessary for activating Leydig cells and, eventually, spermatogenesis (35). In the meantime, ROS and oxidative stress link to steroidogenic enzyme decline, such as 17- and 3-hydroxysteroid dehydrogenase enzymes, which leads to a reduction in testosterone and LH synthesis (36, 37). In our study, we had a significant reduction of LH, FSH, and testosterone in groups treated only with CP, while this outcome is supported by the fact we had mentioned before. In the previous study, EP had a significant increase in serum and tissue hormonal levels, and had activated the GnRH signaling pathway (23).
CP-induced ROS disrupts spermatogenesis through the testicular-endocrine pathway, altering the synthesis of FSH and LH, resulting in low testosterone levels (7, 38). The influence of dietary antioxidant agents in oxidative situations is discussed in many studies (39-41). A study by Ebokaiwe et al., evaluated the effect of quercetin, with antioxidant features, on CP-induced gonadotoxicity. This study revealed a significant increase in the activities of tryptophan 2, 3-dioxygenase, indoleamine 2, 3-dioxygenases, an elevated interferon-γ, interleukin 6, and testis MDA and quercetin co-administration significantly preventing their elevation (39).
In another study, the protective potential of Irbesartan against CP-induced gonadotoxicity was discussed. This study showed that CP treatment raised MDA, caspase-1, and interleukin 18 levels, as well as NF-B, NLRP3, B-cell lymphoma-2 associated X protein, caspase-3, and MT staining in testicular tissue compared to the normal control group. In comparison to the normal control group, there was a decrease in GSH, superoxide dismutase, PPAR expression, B-cell lymphoma-2 staining, circulating testosterone, and the quantity and quality of epididymal sperm. However, using Irbesartan with anti-inflammatory, anti-apoptotic, and antioxidative features regulated these altered biomarkers, and revealed the possible protective effect against testicular damage (42).
Many other studies had proved the CP-induced gonadotoxicity and the benefit of herbs, with antioxidative properties on this (43, 44). These facts support our experiment's results that CP has a potent feature in the diminution of testosterone in plasma and regulating the testicular and gamete cells.
Investigating the effect of hydroalcoholic extract of EP on sperm parameters, such as viability and sperm DNA integrity can increase our knowledge about this plant. It also suggests that further studies should be conducted on rats that have been exposed to chemotherapy.
5. Conclusion
As a result, leaf hydroalcoholic extract of EP pachyclada can be utilized to mitigate the adverse effects of CP, especially in higher doses. The EP extracts’ therapeutic effects are based on its antioxidative function and resilience against the ROS status and oxidative stress in CP toxicity that diminishes steroidogenesis, testosterone production, and sperm functional parameters.
Acknowledgments
The authors are grateful to Deputy of Research of Jahrom University of Medical Sciences for their financial support. This study was approved by the Student Research Committee of Jahrom University of Medical Sciences, Jahrom, Iran (Grant no: 1396.18).
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (343 Views)
1. Introduction
Cancer, is known to be the second cause of mortality in over 100 countries, affecting life expectancy and patient's quality of life. From the past decades till now, rational use of effective anti-cancer agents for treatment was felted (1); among all discovered drugs, cyclophosphamide (CP), a nitrogen mustard anti-cancer agent, has been consumed as a chemotherapeutic drug for more than 50 yr after its discovery (2-3). Despite CP’s clinical benefits, some significant adverse effects of CP, such as nephrotoxicity (4), cardiotoxicity (3), severe bladder bleeding, and gonadotoxicity had limited its clinical indication (5-7). In the presence of cytochrome P450, CP metabolizes into 2 toxic compounds, acrolein, and phosphoramide. Acrolein is an unsaturated and electrophilic reactive aldehyde that generates noxious reactive oxygen species (ROS) and next, affects the neighboring tissue (8, 9). Studies have defined the acrolein-induced excessive ROS effects on testicular microvascular, endocrine signaling, and germ-cell apoptosis which causes testicular infertility (10). Besides them, CP increases the incidence of early menopause and infertility in males and females; this type of infertility is often transitory, but it can potentially be permanent (11, 12). In a study by Watcho et al., CP-treated Wistar rats had significant reduction in seminal vesicle weight, testosterone level, sperm count, motility, and viability; furthermore, sperm morphological abnormality was also detected (13). In addition to morphological and qualitative changes in sperm caused by CP, there are also studies that have shown DNA damage and nuclear maturations (14).
In the last few years, considerable interest in the use of medicinal plants with the aim of cancer treatment or reducing anticancer agents’ side effects has been increased. CP applies its effect primarily by persuading oxidative stress and changing gene expression in spermatocytes vastly unlike stages of development (15); therefore, these studies mostly rely on the antioxidant role of plants and consider antioxidants as affective factors (13, 14). For example, in a study by Can et al., useful Hypericum Triquetrifolium Turra against CP gonadotoxicity had elevated total oxidant status (TOS), and malondialdehyde (MDA) in the CP group (16).
Ephedra (EP) is a major genus of the Ephedraceae family, found in dry and waterless regions of the globe. Some members of this genus are extensively used in traditional medicine to ameliorate and regulate asthma’s symptoms, common cold, influenza, fever, and headache (17). Due to ephedrine-type alkaloids and their pharmacological characteristics, the chemical ingredients of EP species have been the subject of investigation for decades (10). Its role in cardiovascular and central nervous systems, nephrotoxicity and hepatotoxicity, and cancers such as breast cancer were examined in different studies (18-20). Despite the known antioxidative function of EP (21, 22), in only one in vivo study, EP had significantly bring back testicular weight and activity, spermatozoid characteristics, serum and tissue hormonal levels, and antioxidant protection. This study had shown significantly activated gonadotropin-releasing hormone (GnRH) signaling pathway and meiosis-related protein levels by EP in rat model of adriamycin injury (23).
Considering today’s high prevalence of cancer and CP role as an essential anticancer drug, special attention should be paid to this drug’s side effects. To compensate for the side effects of these chemotheraputic drugs, herbs, especially those with antioxidative function would be useful. We turned to use EP, a plant with proven antioxidative functions. This study sought to determine EP hydroalcoholic extract role on testicular tissue and the pituitary-gonadal axis in CP-treated male rats.
2. Materials and Methods
2.1. EP collection and extraction
EP was collected from Yasuj, Iran, and then the plant’s stems were dried at room temperature and powdered. EP powder was mixed with ethanol (70%) solution at a chamber temperature (35 ± 5°C) and after 4 days “the extract was filtered using a sterile cloth sheet. The extract was dried under reduced pressure at below 45°C with a rotary evaporator. This extract were stored at 4°C” (24).
2.2. Determination of extract purification
At first, 1 ml of phenol solution was combined with 200 µl EP pachyclada extract and “stored in dark for 6 min, then 2 ml of NaCo3 (7%) was added and kept for 120 min. The solution absorbance was measured at a wavelength of 765 ɲm” and finally, the total phenolic amount of extract was calculated using the standard curve of gallic acid and reported 9.28 mg/(GAE)4/g (25). Total flavonoid measurement was calculated by the method of Dharmadasa et al. and 44.32 mg of (QE) 5/g extract was reported based on the quercetin standard curve (26).
2.3. Experiment design
In this experimental study, 48 Wistar male rats (180-200 g) were divided into 6 groups. The animals were kept in the animal laboratory of Jahrom University of Medical Sciences, Jahrom, Iran with standard laboratory conditions of 12 hr light/dark cycles and 50-55% of humidity.
According to the previous studies, EP extract was prescribed with concentrations of 250, 500, and 1000 mg/kg by gavage (24). The CP (Baxter, Germany) concentration was determined 5 mg/kg (for 28 days) (27); the control group did not receive any treatment during the experiment, and the sham group received distilled water and alcohol-based on body weight by gavage. Experimental group 1 received only 5 mg/kg CP, and experimental groups received CP and EP extract 250, 500, and 1000 mg/kg.
2.4. Biochemical analysis
On the 29th day, the blood of the weighed animals’ was drawn from their heart (under anesthesia using Xylazine (10 mg/kg of body weight) and Ketamine (50 mg/kg of body weight)). Their serums were collected via centrifugation (for 15 min and 3000 rpm) and then kept at -20°C until laboratory analysis. To measure the luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, GnRH, TOS, MDA, total antioxidant capacity (TAC), and plasma glutathione peroxidase (GSH-PX), the ELIZA kits for rats were used (Shanghai Crystal day Biotech Co., China).
2.5. Histological examination
For histological examination, testis tissue (left and right) was rapidly removed and placed in a 10% formalin container. After 48 hr, the testis tissue was removed from the solution and after routine tissue processing; paraffin sections of 5 μm thickness were prepared as serial sections. Sections of each testis were stained by the H&E method. The mean number of spermatogonia, spermatocytes, spermatids, Leydig, and Sertoli cells were assessed in the testis by stereology method. To count the cells, the optical dissector method and unbiased counting frame were used. The counting frame was randomly placed on the fields of view selected from each slice (10,000× magnification), from each side (top and bottom) up to a depth of 5 µ, sections with artifacts were considered, and counting was not done in those sections. Cells selected by the frame were counted at other depths (average 130-150 of each cell). The desired depths were obtained with a microcator (MT12, Germany). Then, the numerical density of the cells was obtained and multiplied by the final volume of the testis, in order to achieve the estimated cell count.
2.6. Ethical considerations
This study was in agreement with ethical issues of animal laboratory Ethics of Jahrom University of Medical Science, Jahrom, Iran (Code: IR.JUMS.REC.1396.018).
2.7. Statistical analysis
SPSS software (version 24) was used for data analysis. At first, the normality of the data was measured using Shapiro-Wilk normality test. Data were analyzed by Independent-samples t test using SPSS software version 16 and the significance level was considered 0.05.
3. Results
3.1. Changes in serum LH, FSH, testosterone, and GnRH
Data analysis showed that CP had affected serum FSH and LH and using EP had altered these; using higher doses of EP (500 and 1000 mg/kg) were not significantly different with the control. All experimental groups showed a significant decrease in serum testosterone compared to the control group at p < 0.05. The mean testosterone concentration in CP and EP 500 mg/kg did not significantly differ from the CP group; however, the higher doses were significantly different from CP group and the best effect belonged to the highest dose.
Serum GnRH concentration showed a significant increase in all EP groups compared to the control and CP groups. The mean serum concentration of GnRH in experimental groups containing 500 and 1000 mg/kg EP was significantly higher than the group treated with 250 mg/kg EP (p < 0.05).
On comparing CP groups with EP extract ones, it was found that 1000 mg/kg EP had more significant effects on boosting serum-assessed parameters (Table I).
3.2. Changes in serum antioxidants, MDA, and TOS
Data analysis showed a significant reduction of serum TAC and GSH-PX in groups that used CP compared to the control. EP (all doses) significantly increased TAC concentration compared to the CP group (p < 0.05); however, GSH-PX data showed this significant increase only in higher doses of EP.
A significant difference was observed in serum sets of TOS and MDA in all experimental groups compared to the control group. In addition, serum levels of TOS and MDA in EP groups compared to the CP group showed a significant reduction at the p < 0.05 (Table II). Each group means with at least one shared letter had no significant difference.
Changes in the mean number of spermatogonia, spermatocytes, spermatid, Leydig, and Sertoli cells:
This study showed that despite the role of CP on the mean number of spermatogonia (p < 0.05), experimental groups, received higher doses of EP with no significant difference with the control group. No significant difference was observed between the CP groups and the 250 mg/kg EP group. All experimental groups showed a significant decrease in spermatocyte count compared to the control group at p < 0.05, but using EP affected this significantly compared to the CP group. Moreover, no significant difference was observed between 500 and 1000 mg/kg EP. The mean changes of spermatid cells in CP groups except the group treated with 1000 mg/kg EP was significantly lower than the control group. Higher doses significantly increased the spermatid cells count compared to the CP and CP+250 mg/kg EP groups. The mean number of Leydig and Sertoli cells in all study groups did not significantly differ compared to the control group and EP did not affect them (Figure 1).
3.3. Testicular tissue histological results
The histological examination of the testicular tissue showed that no tissue changes were observed in the control and sham groups. In the CP group, cell disorganization and disorders inside the seminiferous tubule, and wrinkling of the germinal epithelium were observed. In groups receiving EP with different doses, tissue disorders showed less severity than the CP group receiving CP. By increasing the concentration of EP, the intensity of these changes decreased so the lowest amount of tissue disorders was observed in the EP 1000 mg/kg group (Figure 2).




4. Discussion
Gonadotoxicity has become one of the most notable and commonly encountered side effects of extended CP use, so there is an imperative and crucial need to identify therapeutic strategies to decrease or regulate the reproductive potential of cancer patients subjected to CP therapy (28). CP is metabolized by cytochrome P450 to 2 stable toxic composites: Acrolein, an α, β-unsaturated aldehyde that has an extremely electrophilic feature, and phosphoramide mustard. ROS mainly induces Acrolein, and Acrolein, induces ROS and oxidative damage via DNA cross-linking and interfering in rapidly proliferating organs and tissues such as testicular tissue, which is known well (29, 30). For example, in a study, it was shown that CP induced gonadotoxicity would be affected by using Nerolidol, a sesquiterpene with potent antioxidative properties. Nerolidol acted as a gonadoprotective molecule and increased testosterone level, sperm count, as well as it also reduced MPO (myeloperoxidase) expression and improved CP-induced histological abnormalities in testes, seminal vesicles, and even epididymis (7). Different studies had shown the beneficial effect of herbs on CP-induced tissue damages, such as gonadotoxicity (31, 32).
This study revealed the gonadoprotective effects of the leaf hydroalcoholic extract of EP pachyclada counter to gonadotoxicity caused by CP in male rats by indicating its effect on serum concentration of FSH, testosterone, and LH. The results showed that the hydroalcoholic extract of EP significantly increased the mean serum levels of FSH, LH, and testosterone; EP also affected testicular spermatocytes, spermatozoids, and spermatids in a dose-dependent manner, especially in 1000 mg/kg EP group. EP has been shown to have significant free radical scavenging potential (33) and its benefits on various problems such as blood parameters in diabetic rats (34), cancer cells viability, and inflammatory factors were investigated; however, as mentioned in the introduction section, only one study showed EP benefits in gonadotoxicity.
The testosterone creation is a complicated pathway that relies on the balances between FSH, LH, GnRH, and vital factors necessary for activating Leydig cells and, eventually, spermatogenesis (35). In the meantime, ROS and oxidative stress link to steroidogenic enzyme decline, such as 17- and 3-hydroxysteroid dehydrogenase enzymes, which leads to a reduction in testosterone and LH synthesis (36, 37). In our study, we had a significant reduction of LH, FSH, and testosterone in groups treated only with CP, while this outcome is supported by the fact we had mentioned before. In the previous study, EP had a significant increase in serum and tissue hormonal levels, and had activated the GnRH signaling pathway (23).
CP-induced ROS disrupts spermatogenesis through the testicular-endocrine pathway, altering the synthesis of FSH and LH, resulting in low testosterone levels (7, 38). The influence of dietary antioxidant agents in oxidative situations is discussed in many studies (39-41). A study by Ebokaiwe et al., evaluated the effect of quercetin, with antioxidant features, on CP-induced gonadotoxicity. This study revealed a significant increase in the activities of tryptophan 2, 3-dioxygenase, indoleamine 2, 3-dioxygenases, an elevated interferon-γ, interleukin 6, and testis MDA and quercetin co-administration significantly preventing their elevation (39).
In another study, the protective potential of Irbesartan against CP-induced gonadotoxicity was discussed. This study showed that CP treatment raised MDA, caspase-1, and interleukin 18 levels, as well as NF-B, NLRP3, B-cell lymphoma-2 associated X protein, caspase-3, and MT staining in testicular tissue compared to the normal control group. In comparison to the normal control group, there was a decrease in GSH, superoxide dismutase, PPAR expression, B-cell lymphoma-2 staining, circulating testosterone, and the quantity and quality of epididymal sperm. However, using Irbesartan with anti-inflammatory, anti-apoptotic, and antioxidative features regulated these altered biomarkers, and revealed the possible protective effect against testicular damage (42).
Many other studies had proved the CP-induced gonadotoxicity and the benefit of herbs, with antioxidative properties on this (43, 44). These facts support our experiment's results that CP has a potent feature in the diminution of testosterone in plasma and regulating the testicular and gamete cells.
Investigating the effect of hydroalcoholic extract of EP on sperm parameters, such as viability and sperm DNA integrity can increase our knowledge about this plant. It also suggests that further studies should be conducted on rats that have been exposed to chemotherapy.
5. Conclusion
As a result, leaf hydroalcoholic extract of EP pachyclada can be utilized to mitigate the adverse effects of CP, especially in higher doses. The EP extracts’ therapeutic effects are based on its antioxidative function and resilience against the ROS status and oxidative stress in CP toxicity that diminishes steroidogenesis, testosterone production, and sperm functional parameters.
Acknowledgments
The authors are grateful to Deputy of Research of Jahrom University of Medical Sciences for their financial support. This study was approved by the Student Research Committee of Jahrom University of Medical Sciences, Jahrom, Iran (Grant no: 1396.18).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Oncology
References
1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev 2016; 25: 16-27. [DOI:10.1158/1055-9965.EPI-15-0578] [PMID]
2. Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical stem cell transplantation with posttransplant cyclophosphamide therapy vs other donor transplantations in adults with hematologic cancers: A systematic review and meta-analysis. JAMA Oncol 2019; 5: 1739-1748. [DOI:10.1001/jamaoncol.2019.3541] [PMID] [PMCID]
3. Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, Ali SM, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci 2019; 218: 112-131. [DOI:10.1016/j.lfs.2018.12.018] [PMID]
4. Sinanoglu O, Yener AN, Ekici S, Midi A, Aksungar FB. The protective effects of spirulina in cyclophosphamide induced nephrotoxicity and urotoxicity in rats. Urology 2012; 80: 1392. e1-e6. [DOI:10.1016/j.urology.2012.06.053] [PMID]
5. Akomolafe SF, Aluko BT. Protective effect of curcumin on fertility in cyclophosphamide exposed rats: Involvement of multiple pathways. J Food Biochem 2020; 44: e13095. [DOI:10.1111/jfbc.13095] [PMID]
6. Nagappa M, Netravathi M, Taly AB, Sinha S, Bindu PS, Mahadevan A. Long-term efficacy and limitations of cyclophosphamide in myasthenia gravis. J Clin Neurosci 2014; 21: 1909-1914. [DOI:10.1016/j.jocn.2014.03.019] [PMID]
7. Iqubal A, Syed MA, Najmi AK, Ali J, Haque SE. Ameliorative effect of nerolidol on cyclophosphamide‐induced gonadal toxicity in Swiss Albino mice: Biochemical‐, histological‐and immunohistochemical‐based evidences. Andrologia 2020; 52: e13535. [DOI:10.1111/and.13535] [PMID]
8. Qin W-S, Deng Y-H, Cui FC. Sulforaphane protects against acrolein-induced oxidative stress and inflammatory responses: Modulation of Nrf-2 and COX-2 expression. Arch Med Sci 2016; 12: 871-880. [DOI:10.5114/aoms.2016.59919] [PMID] [PMCID]
9. Esfandyari S, Aleyasin A, Noroozi Z, Taheri M, Khodarahmian M, Eslami M, et al. The protective effect of sulforaphane against oxidative stress through activation of Nrf2/ARE pathway in human granulosa cells. Cell J 2021; 23: 692-700.
10. Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem 2012; 287: 33048-33060. [DOI:10.1074/jbc.M112.366690] [PMID] [PMCID]
11. Harward LE, Mitchell K, Pieper C, Copland S, Criscione-Schreiber LG, Clowse MEB. The impact of cyclophosphamide on menstruation and pregnancy in women with rheumatologic disease. Lupus 2013; 22: 81-86. [DOI:10.1177/0961203312468624] [PMID]
12. Fusco R, Trovato Salinaro A, Siracusa R, D'Amico R, Impellizzeri D, Scuto M, et al. Hidrox(®) counteracts cyclophosphamide-induced male infertility through NRF2 pathways in a mouse model. Antioxidants (Basel) 2021; 10: 778. [DOI:10.3390/antiox10050778] [PMID] [PMCID]
13. Watcho P, Mpeck IR, Deeh Defo PB, Wankeu-Nya M, Ngadjui E, Bonsou Fozin GR, et al. Cyclophosphamide-induced reproductive toxicity: Beneficial effects of Helichrysum odoratissimum (Asteraceae) in male Wistar rats. J Integr Med 2019; 17: 366-373. [DOI:10.1016/j.joim.2019.07.002] [PMID]
14. Bakhtiary Z, Shahrooz R, Ahmadi A, Zarei L. Evaluation of antioxidant effects of crocin on sperm quality in cyclophosphamide treated adult mice. Vet Res Forum 2014; 5: 213-218.
15. Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin Drug Metab Toxicol 2017; 13: 525-536. [DOI:10.1080/17425255.2017.1277205] [PMID]
16. Can S, Yıldız SC, Keskin C, Şahintürk V, Cengiz M, Appak Başköy S, et al. Investigation into the protective effects of Hypericum Triquetrifolium Turra seed against cyclophosphamide-induced testicular injury in Sprague Dawley rats. Drug Chem Toxicol 2022; 45: 1679-1686. [DOI:10.1080/01480545.2020.1856130] [PMID]
17. González-Juárez DE, Escobedo-Moratilla A, Flores J, Hidalgo-Figueroa S, Martínez-Tagüeña N, Morales-Jiménez J, et al. A review of the Ephedra genus: Distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules 2020; 25: 3283. [DOI:10.3390/molecules25143283] [PMID] [PMCID]
18. Sioud F, Toumia IB, Lahmer A, Khlifi R, Dhaouefi Z, Maatouk M, et al. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ Sci Pollut Res Int 2020; 27: 12792-12801. [DOI:10.1007/s11356-020-07904-3] [PMID]
19. Danciu C, Muntean D, Alexa E, Farcas C, Oprean C, Zupko I, et al. Phytochemical characterization and evaluation of the antimicrobial, antiproliferative and pro-apoptotic potential of Ephedra alata Decne. Hydroalcoholic extract against the MCF-7 breast cancer cell line. Molecules 2018; 24: 13. [DOI:10.3390/molecules24010013] [PMID] [PMCID]
20. Al-saraireh YM, Youssef AMM, Alshammari FOFO, Al-Sarayreh SA, Al-Shuneigat JM, Alrawashdeh HM, et al. Phytochemical characterization and anti-cancer properties of extract of Ephedra foeminea (Ephedraceae) aerial parts. Trop J Pharm Res 2021; 20: 1675-1681. [DOI:10.4314/tjpr.v20i8.18]
21. Rustaiyan A, Javidnia K, Farjam MH, Aboee-Mehrizi F, Ezzatzadeh E. Antimicrobial and antioxidant activity of the Ephedra sarcocarpa growing in Iran. J Med Plant Res 2011; 5: 4251-4255.
22. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Scientific World Journal 2017; 2017: 5873648. [DOI:10.1155/2017/5873648] [PMID] [PMCID]
23. Fu Y, Yuan P, Zheng Y, Gao L, Wei Y, Chen Y, et al. Ephedra herb reduces adriamycin-induced testicular toxicity by upregulating the gonadotropin-releasing hormone signalling pathway. Biomed Pharmacother 2022; 150: 113061. [DOI:10.1016/j.biopha.2022.113061] [PMID]
24. Ghasemi Pirbalouti A, Amirmohammadi M, Azizi Sh, Craker L. Healing effect of hydro-alcoholic extract of Ephedra pachyclada Boiss. In experimental gastric ulcer in rat. Acta Pol Pharm 2013; 70: 1003-1009.
25. Alizadeh Behbahani B, Falah F, Lavi Arab F, Vasiee M, Tabatabaee Yazdi F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid Based Complement Alternat Med 2020; 2020: 5190603. [DOI:10.1155/2020/5190603] [PMID] [PMCID]
26. Dharmadasa RM, Abeysinghe DC, Dissanayake DMN, Abeywardhane KW, Fernando NS. Leaf essential oil composition, antioxidant activity, total phenolic content and total flavonoid content of Pimenta dioica (L.) Merr (Myrtaceae): A superior quality spice grown in Sri Lanka. Universal Journal of Agricultural Research 2015; 3: 49-52. [DOI:10.13189/ujar.2015.030203]
27. Marcozzi S, Rossi V, Salvatore G, Di Rella F, De Felici M, Klinger FG. Distinct effects of epirubicin, cisplatin and cyclophosphamide on ovarian somatic cells of prepuberal ovaries. Aging (Albany NY) 2019; 11: 10532-10556. [DOI:10.18632/aging.102476] [PMID] [PMCID]
28. Unal F, Yuksel MA, Boran B, Temel Yuksel I, Abali R. The role of N-Acetylcysteine in preventing cyclophosphamide-induced gonadotoxicity: An experimental study in rats. J Obstet Gynaecol 2016; 36: 372-375. [DOI:10.3109/01443615.2015.1065236] [PMID]
29. Iqubal A, Sharma S, Ansari MA, Najmi AK, Syed MA, Ali J, et al. Nerolidol attenuates cyclophosphamide-induced cardiac inflammation, apoptosis and fibrosis in Swiss Albino mice. Eur J Pharmacol 2019; 863: 172666. [DOI:10.1016/j.ejphar.2019.172666] [PMID]
30. Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr HR, Aldhaheri SR, Najafi T, et al. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med 2017; 110: 11-18. [DOI:10.1016/j.freeradbiomed.2017.05.006] [PMID] [PMCID]
31. Wang Y, Zou Z, Jaisi A, Olatunji OJ. Unravelling the protective effects of emodin against cyclophosphamide induced gonadotoxicity in male Wistar rats. Drug Des Devel Ther 2021; 15: 4403-4411. [DOI:10.2147/DDDT.S333383] [PMID] [PMCID]
32. Briseño-Bugarín J, Hernández-Ochoa I, Araujo-Padilla X, Mojica-Villegas MA, Montaño-González RI, Gutiérrez-Salmeán G, et al. Phycobiliproteins ameliorate gonadal toxicity in male mice treated with cyclophosphamide. Nutrients 2021; 13: 2616. [DOI:10.3390/nu13082616] [PMID] [PMCID]
33. Khan A, Jan G, Khan A, Jan FG, Bahadur A, Danish M. In vitro antioxidant and antimicrobial activities of Ephedra gerardiana (root and stem) crude extract and fractions. Evid Based Complement Alternat Med 2017; 2017: 4040254. [DOI:10.1155/2017/4040254] [PMID] [PMCID]
34. Abu Hajleh MN, Khleifat KM, Alqaraleh M, Al-Hraishat E, Al-Limoun MO, Qaralleh H, et al. Antioxidant and antihyperglycemic effects of Ephedra foeminea aqueous extract in streptozotocin-induced diabetic rats. Nutrients 2022; 14: 2338. [DOI:10.3390/nu14112338] [PMID] [PMCID]
35. Creasy DM, Chapin RE. Male reproductive system. In: Haschek WM, Rousseau CG, Wallig MA. Haschek and Rousseaux's handbook of toxicologic pathology. Netherlands: Elsevier Academic Press; 2013: 2493-2598. [DOI:10.1016/B978-0-12-415759-0.00059-5]
36. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Men's Health 2014; 32: 1-17. [DOI:10.5534/wjmh.2014.32.1.1] [PMID] [PMCID]
37. Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl 2014; 16: 31-38. [DOI:10.4103/1008-682X.122203] [PMID] [PMCID]
38. Potnuri AG, Allakonda L, Lahkar M. Crocin attenuates cyclophosphamide induced testicular toxicity by preserving glutathione redox system. Biomed Pharmacother 2018; 101: 174-180. [DOI:10.1016/j.biopha.2018.02.068] [PMID]
39. Ebokaiwe AP, Obasi DO, Njoku RC, Osawe S. Cyclophosphamide-induced testicular oxidative-inflammatory injury is accompanied by altered immunosuppressive indoleamine 2, 3-dioxygenase in Wister rats: Influence of dietary quercetin. Andrologia 2022; 54: e14341. [DOI:10.1111/and.14341] [PMID]
40. Ebrahimi B, Matavos-Aramyan H, Keshtgar S. The cryoprotective effect of vitamins on human spermatozoa quality: A systematic review and meta-analysis. Cell Tissue Bank 2022; 23: 213-225. [DOI:10.1007/s10561-021-09953-5] [PMID]
41. Veskoukis AS, Tsatsakis AM, Kouretas D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012; 17: 11-21. [DOI:10.1007/s12192-011-0293-3] [PMID] [PMCID]
42. Abu-Risha SE, Mousa MA, Elsisi AE. Protective role of irbesartan against cyclophosphamide-induced testicular damage in rats via up-regulating PPAR-γ signaling and ameliorating NF-κB/NLRP3/IL-18 inflammatory axis. Life Sci 2022; 289: 120218. [DOI:10.1016/j.lfs.2021.120218] [PMID]
43. Poojary KK, Nayak G, Vasani A, Kumari S, Dcunha R, Kunhiraman JP, et al. Curcumin nanocrystals attenuate cyclophosphamide-induced testicular toxicity in mice. Toxicol Appl Pharmacol 2021; 433: 115772. [DOI:10.1016/j.taap.2021.115772] [PMID]
44. Barik G, Chaturvedula L, Bobby Z. Role of oxidative stress and antioxidants in male infertility: An interventional study. J Hum Reprod Sci 2019; 12: 204-209. [DOI:10.4103/jhrs.JHRS_135_18] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





