Sat, Jul 12, 2025
[Archive]
Volume 21, Issue 4 (April 2023)
IJRM 2023, 21(4): 277-284 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hegazy A A, Al-Qtaitat A I, Hegazy R A. A new hypothesis may explain human parthenogenesis and ovarian teratoma: A review study. IJRM 2023; 21 (4) :277-284
URL: http://ijrm.ir/article-1-2666-en.html
URL: http://ijrm.ir/article-1-2666-en.html
1- Faculty of Dentistry, Zarqa University, Zarqa City, Jordan. Faculty of Medicine, Zagazig University, Zagazig City, Egypt. , ahegazy@zu.edu.jo, ahegazy@zu.edu.eg
2- Faculty of Dentistry, Zarqa University, Zarqa City, Jordan. Faculty of Medicine, Mutah University, Alkarak, Jordan.
3- Faculty of Medicine, Zagazig University, Zagazig City, Egypt.
2- Faculty of Dentistry, Zarqa University, Zarqa City, Jordan. Faculty of Medicine, Mutah University, Alkarak, Jordan.
3- Faculty of Medicine, Zagazig University, Zagazig City, Egypt.
Full-Text [PDF 1216 kb]
(1063 Downloads)
| Abstract (HTML) (1539 Views)
1. Introduction
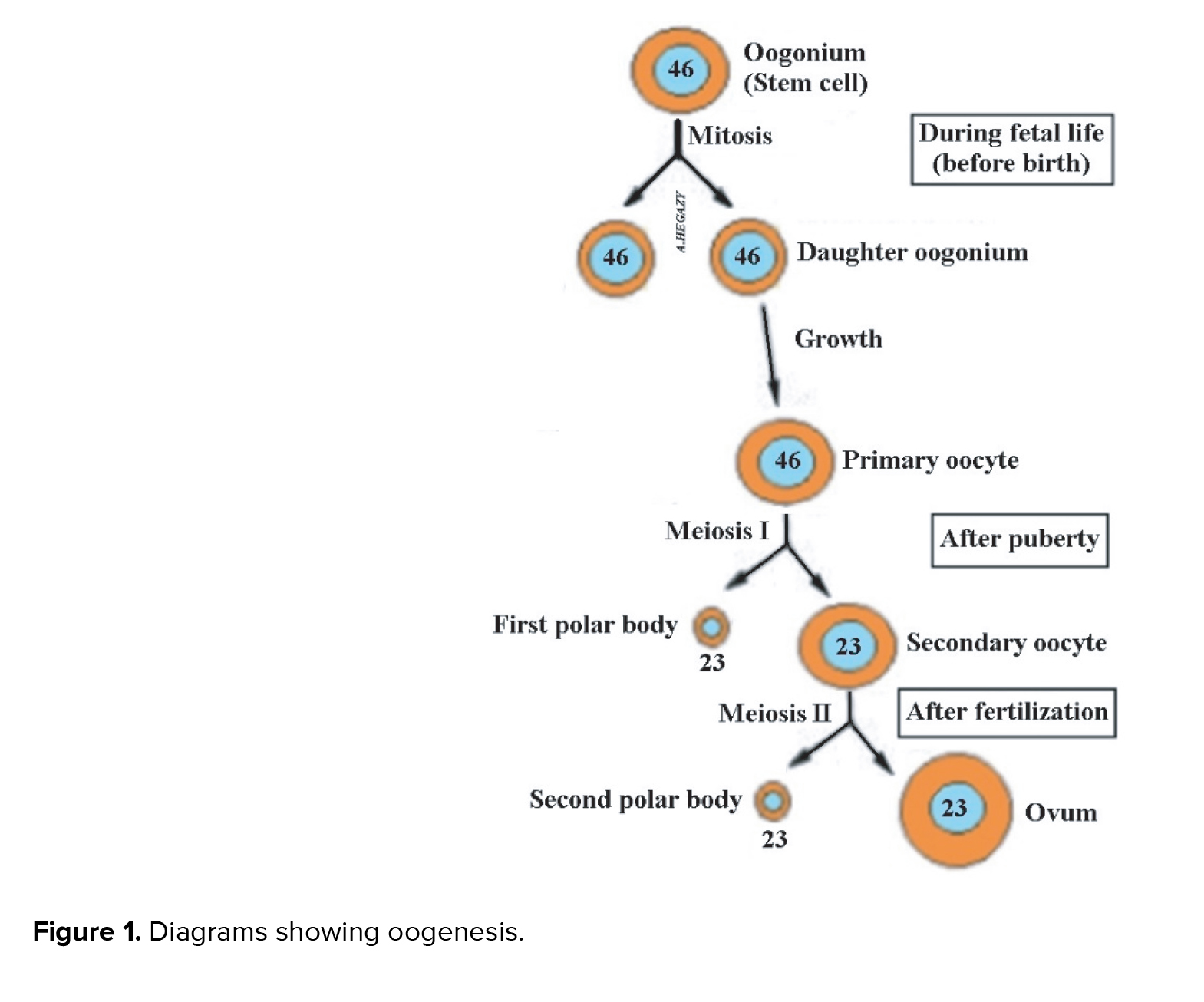
Parthenogenesis (PG) is an asexual reproduction in which a female can produce an embryo without fertilizing an egg with sperm. In Greek, it means the virgin creation. It occurs naturally in some jawed vertebrates such as the whiptail lizard, but in mammals, it is an unnatural event (1). However, PG may not be a rare event in humans as we thought, but it may occur spontaneously resulting in the formation of teratoma in the ovary (2). The secondary oocyte (SO), which is ovulated at each ovarian cycle, usually stops in metaphase II until it is fertilized by the sperm (Figure 1). Once the sperm enters the oocyte, the egg completes meiosis II to form the ovum, whose nucleus fuses with the sperm nucleus to form the zygote (3). This process is called sexual reproduction. However, under idiopathic abnormal circumstances, a spontaneous exit of the oocyte from metaphase-II could occur without apparent stimulation. This is known as oocyte spontaneous activation (4). Such spontaneous parthenogenetic activation may occur in human oocytes but mostly leads to tumor formation, including ovarian teratoma (OT) (5, 6). Some authors postulate that PG occurs in humans through the formation of OTs (2, 6, 7). However, they did not explain how this could happen. Although reproduction in most mammals occurs through mating between male and female, it has been hypothesized that presence of rare cases of PG in humans that result in normal and viable individuals go unnoticed due to the absence of congenital anomalies (8). Parthenogenetic activation by artificial manipulation has been conducted in mice with the creation of an early stage of fetal development (9). However, other authors stated that the creation and birth of the animal through oocyte parthenogenic activation using genetic modification technologies is possible (10).
This article aims to find the cause and mechanism of spontaneous PG and OT. Accordingly, we attempted to propose a new explanation for the occurrence of spontaneous PG in humans, which appears to occur more frequently than previously reported.
2. Materials and Methods
We searched the literature available in international journals and various scientific websites including Google Scholar, PubMed, Scopus, Web of Science, Springer, and Elsevier to find an explanation or pathogenesis for this phenomenon in humans. In this process, we used the title words as well as all subtitles and key words of the current article. For inclusion in this review, studies were case reports or original research dealing with spontaneous PG and its associated causes, risk factors, and proposed mechanism. Search limitations were to only include humans and English-language publications. We retrieved studies published up to December 2022. Then, we analyzed and discussed the results of previous relevant studies and added our proposal for an explanation for this rare event in humans.
3. Results and Discussion
PG or the so-called asexual reproduction that results in normal, viable offspring, is a rare event in humans (11). Previous literature on the causes of occurrence of these cases was sparse with no specific cause or mechanism. However, previous studies in their trial of finding an explanation for idiopathic OT, attributed these cases to spontaneous PG (2, 6, 7). This means that PG is not as rare as previously thought but can go unnoticed with an OT outcome or even a full-term birth, particularly, in the presence of a male partner.
3.1. Specious variations in sexual and asexual reproduction
Sexual reproduction occurs through the fertilization of a mature oocyte by a sperm, which leads to the restoration of the diploid number of the oocyte. Once the egg regains the 46 chromosomes, cell division by mitosis begins to form a new organism. In this type of reproduction, there will be diversity within the human species as well as sex determination. Species divergence occurs as half of the offspring's chromosomes come from the mother and the other from the father. On the other hand, sexual determination occurs through the sex chromosome carried by the sperm. In the case of X-chromosome sperm, the offspring will be female, while in the case of Y-chromosome sperm the creature will be male (3). This variation is not found in the case of PG where all chromosomes originate from the mother's side only; therefore, the new organism will be female “of the same sex as the mother”. In the process of PG, the female body finds a unique way to fill in the genes that sperm normally provide. However, the new organism may not typically be like its mother due to the exchange of chromosomes that occurs through the process of oogenesis (Figure 2).
3.2. Reported cases of spontaneous PG
Spontaneous cases of PG have been reported in infertile women treated with assisted reproductive technologies (Table I) (6, 12-15). The diagnosis was made based on the findings that biopsy samples of embryos were exclusively compatible with those from the maternal side. It has been hypothesized that spontaneous stimulation of oocytes, or what is called PG, may be the factor involved in recurrent miscarriage and the inability to maintain a pregnancy (14). A case of recurrent miscarriage was reported with either in-vivo or in-vitro fertilization (IVF). Cytogenetic analysis of conceptus outcome samples, matched exclusively for the maternal side, may indicate that PG occurred spontaneously after insemination. On the other hand, other authors reported a case of PG embryo retrieved from an IVF patient with a history of infertility and OTs (6). They suggested presence of a possible link between spontaneous PG and OT development. Ye and co-authors also reported a case of PG in a 39-yr-old infertile woman (15). They supposed that occurrence of SG may be involved in failure to maintain pregnancy in IVF cases. Similarly, another case of a 38-yr-old infertile woman with a history of salpingectomy was also reported (14). It has been hypothesized that bilateral salpingectomy or advanced female age may result in spontaneous activation of oocytes (12, 14, 15). Other authors have suggested oocyte abnormalities in some infertile women that predispose to spontaneous PG (13, 14). Infertility was documented in all the cases mentioned. However, it is not clear whether exposure of the oocyte to sperm without fertilization or entry has a role in the spontaneous activation of the oocyte.

3.3. Proposed automixis mechanism of PG
Fertilization in sexual reproduction occurs initially through the membrane of the SO that is normally prepared to penetrate through the rupture of the sperm cap, in order to release its enclosed enzymes such as acrosin- and trypsin-like substances that aid in sperm entry. The sperm capable of perforating the egg membrane is considered to be the healthiest to enable the new offspring to withstand the hardships of life in the future. After entering the fertilized sperm, the SO completes its second division to produce 2 cells: a large cell called the ovum whose nucleus fuses with the sperm nucleus to form the zygote, and the small SO which degenerates (Figure 1) (3).
On the other hand, after the first meiosis of a primary oocyte with a diploid number “23 pairs” of chromosomes that occurs cyclically after female sexual puberty, 2 cells emerge, SO and first polar body (FPB). Each cell contains a haploid number of chromosomes "23". The process of release of SO from the surface of the ovary known as ovulation occurs once in each female reproductive cycle. The 2 cells “SO and FPB” are placed together within the zona pellucida (Figure 3).
Under normal conditions, FPB decomposes. However, we suggest that in some cases the 2 cells may fuse to form a single cell that restores the diploid number of chromosomes and initiates cell division to form a new organism. This may occur because of an aberration in the egg cell membrane, which may fuse with the membrane of FPB. The proposed automixis mechanism of PG was previously documented in animals such as sharks and aphids (16, 17). This condition is considered asexual reproduction but with no clonal offspring (17). This is because it depends on oogenesis, the process by which cross-over and exchange of DNA between chromosomes can occur during meiosis (Figure 2) (18).
Therefore, PG differs from cloning in that the offspring is not as typical as its mother, while in cloning through the intracytoplasmic transfer of the somatic cell nucleus the new organism is typical with the donor (3). In this respect, the individual offspring of a typical parthenogenetic reproduction will be different from its mother and each other. Spontaneous PG in humans either degrades or leads to formation of OT (Figure 4), but it cannot develop into a term due to abnormal genomic imprinting and lack of paternal genes (4, 19). This is in addition to the aberrant location in the ovary. On the other hand, some authors have suggested another mechanism of PG. They attributed this to human embryonic stem cells of haploid oocytes that may have properties of pluripotent stem cells including self-renewal and a molecular signature of pluripotency (20).



3.3. Proposed automixis mechanism of PG
Fertilization in sexual reproduction occurs initially through the membrane of the SO that is normally prepared to penetrate through the rupture of the sperm cap, in order to release its enclosed enzymes such as acrosin- and trypsin-like substances that aid in sperm entry. The sperm capable of perforating the egg membrane is considered to be the healthiest to enable the new offspring to withstand the hardships of life in the future. After entering the fertilized sperm, the SO completes its second division to produce 2 cells: a large cell called the ovum whose nucleus fuses with the sperm nucleus to form the zygote, and the small SO which degenerates (Figure 1) (3).
On the other hand, after the first meiosis of a primary oocyte with a diploid number “23 pairs” of chromosomes that occurs cyclically after female sexual puberty, 2 cells emerge, SO and first polar body (FPB). Each cell contains a haploid number of chromosomes "23". The process of release of SO from the surface of the ovary known as ovulation occurs once in each female reproductive cycle. The 2 cells “SO and FPB” are placed together within the zona pellucida (Figure 3).
Under normal conditions, FPB decomposes. However, we suggest that in some cases the 2 cells may fuse to form a single cell that restores the diploid number of chromosomes and initiates cell division to form a new organism. This may occur because of an aberration in the egg cell membrane, which may fuse with the membrane of FPB. The proposed automixis mechanism of PG was previously documented in animals such as sharks and aphids (16, 17). This condition is considered asexual reproduction but with no clonal offspring (17). This is because it depends on oogenesis, the process by which cross-over and exchange of DNA between chromosomes can occur during meiosis (Figure 2) (18).
Therefore, PG differs from cloning in that the offspring is not as typical as its mother, while in cloning through the intracytoplasmic transfer of the somatic cell nucleus the new organism is typical with the donor (3). In this respect, the individual offspring of a typical parthenogenetic reproduction will be different from its mother and each other. Spontaneous PG in humans either degrades or leads to formation of OT (Figure 4), but it cannot develop into a term due to abnormal genomic imprinting and lack of paternal genes (4, 19). This is in addition to the aberrant location in the ovary. On the other hand, some authors have suggested another mechanism of PG. They attributed this to human embryonic stem cells of haploid oocytes that may have properties of pluripotent stem cells including self-renewal and a molecular signature of pluripotency (20).
Full-Text: (818 Views)
1. Introduction
Parthenogenesis (PG) is an asexual reproduction in which a female can produce an embryo without fertilizing an egg with sperm. In Greek, it means the virgin creation. It occurs naturally in some jawed vertebrates such as the whiptail lizard, but in mammals, it is an unnatural event (1). However, PG may not be a rare event in humans as we thought, but it may occur spontaneously resulting in the formation of teratoma in the ovary (2). The secondary oocyte (SO), which is ovulated at each ovarian cycle, usually stops in metaphase II until it is fertilized by the sperm (Figure 1). Once the sperm enters the oocyte, the egg completes meiosis II to form the ovum, whose nucleus fuses with the sperm nucleus to form the zygote (3). This process is called sexual reproduction. However, under idiopathic abnormal circumstances, a spontaneous exit of the oocyte from metaphase-II could occur without apparent stimulation. This is known as oocyte spontaneous activation (4). Such spontaneous parthenogenetic activation may occur in human oocytes but mostly leads to tumor formation, including ovarian teratoma (OT) (5, 6). Some authors postulate that PG occurs in humans through the formation of OTs (2, 6, 7). However, they did not explain how this could happen. Although reproduction in most mammals occurs through mating between male and female, it has been hypothesized that presence of rare cases of PG in humans that result in normal and viable individuals go unnoticed due to the absence of congenital anomalies (8). Parthenogenetic activation by artificial manipulation has been conducted in mice with the creation of an early stage of fetal development (9). However, other authors stated that the creation and birth of the animal through oocyte parthenogenic activation using genetic modification technologies is possible (10).
This article aims to find the cause and mechanism of spontaneous PG and OT. Accordingly, we attempted to propose a new explanation for the occurrence of spontaneous PG in humans, which appears to occur more frequently than previously reported.

2. Materials and Methods
We searched the literature available in international journals and various scientific websites including Google Scholar, PubMed, Scopus, Web of Science, Springer, and Elsevier to find an explanation or pathogenesis for this phenomenon in humans. In this process, we used the title words as well as all subtitles and key words of the current article. For inclusion in this review, studies were case reports or original research dealing with spontaneous PG and its associated causes, risk factors, and proposed mechanism. Search limitations were to only include humans and English-language publications. We retrieved studies published up to December 2022. Then, we analyzed and discussed the results of previous relevant studies and added our proposal for an explanation for this rare event in humans.
3. Results and Discussion
PG or the so-called asexual reproduction that results in normal, viable offspring, is a rare event in humans (11). Previous literature on the causes of occurrence of these cases was sparse with no specific cause or mechanism. However, previous studies in their trial of finding an explanation for idiopathic OT, attributed these cases to spontaneous PG (2, 6, 7). This means that PG is not as rare as previously thought but can go unnoticed with an OT outcome or even a full-term birth, particularly, in the presence of a male partner.
3.1. Specious variations in sexual and asexual reproduction
Sexual reproduction occurs through the fertilization of a mature oocyte by a sperm, which leads to the restoration of the diploid number of the oocyte. Once the egg regains the 46 chromosomes, cell division by mitosis begins to form a new organism. In this type of reproduction, there will be diversity within the human species as well as sex determination. Species divergence occurs as half of the offspring's chromosomes come from the mother and the other from the father. On the other hand, sexual determination occurs through the sex chromosome carried by the sperm. In the case of X-chromosome sperm, the offspring will be female, while in the case of Y-chromosome sperm the creature will be male (3). This variation is not found in the case of PG where all chromosomes originate from the mother's side only; therefore, the new organism will be female “of the same sex as the mother”. In the process of PG, the female body finds a unique way to fill in the genes that sperm normally provide. However, the new organism may not typically be like its mother due to the exchange of chromosomes that occurs through the process of oogenesis (Figure 2).
3.2. Reported cases of spontaneous PG
Spontaneous cases of PG have been reported in infertile women treated with assisted reproductive technologies (Table I) (6, 12-15). The diagnosis was made based on the findings that biopsy samples of embryos were exclusively compatible with those from the maternal side. It has been hypothesized that spontaneous stimulation of oocytes, or what is called PG, may be the factor involved in recurrent miscarriage and the inability to maintain a pregnancy (14). A case of recurrent miscarriage was reported with either in-vivo or in-vitro fertilization (IVF). Cytogenetic analysis of conceptus outcome samples, matched exclusively for the maternal side, may indicate that PG occurred spontaneously after insemination. On the other hand, other authors reported a case of PG embryo retrieved from an IVF patient with a history of infertility and OTs (6). They suggested presence of a possible link between spontaneous PG and OT development. Ye and co-authors also reported a case of PG in a 39-yr-old infertile woman (15). They supposed that occurrence of SG may be involved in failure to maintain pregnancy in IVF cases. Similarly, another case of a 38-yr-old infertile woman with a history of salpingectomy was also reported (14). It has been hypothesized that bilateral salpingectomy or advanced female age may result in spontaneous activation of oocytes (12, 14, 15). Other authors have suggested oocyte abnormalities in some infertile women that predispose to spontaneous PG (13, 14). Infertility was documented in all the cases mentioned. However, it is not clear whether exposure of the oocyte to sperm without fertilization or entry has a role in the spontaneous activation of the oocyte.

3.3. Proposed automixis mechanism of PG
Fertilization in sexual reproduction occurs initially through the membrane of the SO that is normally prepared to penetrate through the rupture of the sperm cap, in order to release its enclosed enzymes such as acrosin- and trypsin-like substances that aid in sperm entry. The sperm capable of perforating the egg membrane is considered to be the healthiest to enable the new offspring to withstand the hardships of life in the future. After entering the fertilized sperm, the SO completes its second division to produce 2 cells: a large cell called the ovum whose nucleus fuses with the sperm nucleus to form the zygote, and the small SO which degenerates (Figure 1) (3).
On the other hand, after the first meiosis of a primary oocyte with a diploid number “23 pairs” of chromosomes that occurs cyclically after female sexual puberty, 2 cells emerge, SO and first polar body (FPB). Each cell contains a haploid number of chromosomes "23". The process of release of SO from the surface of the ovary known as ovulation occurs once in each female reproductive cycle. The 2 cells “SO and FPB” are placed together within the zona pellucida (Figure 3).
Under normal conditions, FPB decomposes. However, we suggest that in some cases the 2 cells may fuse to form a single cell that restores the diploid number of chromosomes and initiates cell division to form a new organism. This may occur because of an aberration in the egg cell membrane, which may fuse with the membrane of FPB. The proposed automixis mechanism of PG was previously documented in animals such as sharks and aphids (16, 17). This condition is considered asexual reproduction but with no clonal offspring (17). This is because it depends on oogenesis, the process by which cross-over and exchange of DNA between chromosomes can occur during meiosis (Figure 2) (18).
Therefore, PG differs from cloning in that the offspring is not as typical as its mother, while in cloning through the intracytoplasmic transfer of the somatic cell nucleus the new organism is typical with the donor (3). In this respect, the individual offspring of a typical parthenogenetic reproduction will be different from its mother and each other. Spontaneous PG in humans either degrades or leads to formation of OT (Figure 4), but it cannot develop into a term due to abnormal genomic imprinting and lack of paternal genes (4, 19). This is in addition to the aberrant location in the ovary. On the other hand, some authors have suggested another mechanism of PG. They attributed this to human embryonic stem cells of haploid oocytes that may have properties of pluripotent stem cells including self-renewal and a molecular signature of pluripotency (20).



3.3. Proposed automixis mechanism of PG
Fertilization in sexual reproduction occurs initially through the membrane of the SO that is normally prepared to penetrate through the rupture of the sperm cap, in order to release its enclosed enzymes such as acrosin- and trypsin-like substances that aid in sperm entry. The sperm capable of perforating the egg membrane is considered to be the healthiest to enable the new offspring to withstand the hardships of life in the future. After entering the fertilized sperm, the SO completes its second division to produce 2 cells: a large cell called the ovum whose nucleus fuses with the sperm nucleus to form the zygote, and the small SO which degenerates (Figure 1) (3).
On the other hand, after the first meiosis of a primary oocyte with a diploid number “23 pairs” of chromosomes that occurs cyclically after female sexual puberty, 2 cells emerge, SO and first polar body (FPB). Each cell contains a haploid number of chromosomes "23". The process of release of SO from the surface of the ovary known as ovulation occurs once in each female reproductive cycle. The 2 cells “SO and FPB” are placed together within the zona pellucida (Figure 3).
Under normal conditions, FPB decomposes. However, we suggest that in some cases the 2 cells may fuse to form a single cell that restores the diploid number of chromosomes and initiates cell division to form a new organism. This may occur because of an aberration in the egg cell membrane, which may fuse with the membrane of FPB. The proposed automixis mechanism of PG was previously documented in animals such as sharks and aphids (16, 17). This condition is considered asexual reproduction but with no clonal offspring (17). This is because it depends on oogenesis, the process by which cross-over and exchange of DNA between chromosomes can occur during meiosis (Figure 2) (18).
Therefore, PG differs from cloning in that the offspring is not as typical as its mother, while in cloning through the intracytoplasmic transfer of the somatic cell nucleus the new organism is typical with the donor (3). In this respect, the individual offspring of a typical parthenogenetic reproduction will be different from its mother and each other. Spontaneous PG in humans either degrades or leads to formation of OT (Figure 4), but it cannot develop into a term due to abnormal genomic imprinting and lack of paternal genes (4, 19). This is in addition to the aberrant location in the ovary. On the other hand, some authors have suggested another mechanism of PG. They attributed this to human embryonic stem cells of haploid oocytes that may have properties of pluripotent stem cells including self-renewal and a molecular signature of pluripotency (20).
Type of Study: Review Article |
Subject:
Reproductive Biology
References
1. Bos-Mikich A, Bressan FF, Ruggeri RR, Watanabe Y, Meirelles FV. Parthenogenesis and human assisted reproduction. Stem Cells International 2016; 1970843: 1-8. [DOI:10.1155/2016/1970843] [PMID] [PMCID]
2. de Carli GJ, Pereira TC. On human parthenogenesis. Med Hypotheses 2017; 106: 57-60. [DOI:10.1016/j.mehy.2017.07.008] [PMID]
3. Hegazy A. Clinical embryology for medical students and postgraduate doctors. Berlin: Lap Lambert Academic Publishing; 2014: 13-37.
4. Cui W. Oocyte spontaneous activation: An overlooked cellular event that impairs female fertility in mammals. Front Cell Dev Biol 2021; 9: 648057. [DOI:10.3389/fcell.2021.648057] [PMID] [PMCID]
5. Linder D, McCaw BK, Hecht F. Parthenogenic origin of benign ovarian teratomas. N Engl J Med 1975; 292: 63-66. [DOI:10.1056/NEJM197501092920202] [PMID]
6. Oliveira FG, Dozortsev D, Diamond MP, Fracasso A, Abdelmassih S, Abdelmassih V, et al. Evidence of parthenogenetic origin of ovarian teratoma: Case report. Hum Reprod 2004; 19: 1867-1870. [DOI:10.1093/humrep/deh345] [PMID]
7. Miura K, Kurabayashi T, Satoh C, Sasaki K, Ishiguro T, Yoshiura KI, et al. Fetiform teratoma was a parthenogenetic tumor arising from a mature ovum. J Hum Genet 2017; 62: 803-808. [DOI:10.1038/jhg.2017.45] [PMID]
8. Strain L, Warner JP, Johnston T, Bonthron DT. A human parthenogenetic chimaera. Nat Genet 1995; 11: 164-169. [DOI:10.1038/ng1095-164] [PMID]
9. Kaufman MH, Barton SC, Surani MAH. Normal postimplantation development of mouse parthenogenetic embryos to the forelimb bud stag. Nature 1977; 265: 53-55. [DOI:10.1038/265053a0] [PMID]
10. Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, et al. Birth of parthenogenetic mice that can develop to adulthood. Nature 2004; 428: 860-864. [DOI:10.1038/nature02402] [PMID]
11. Paffoni A, Brevini TA, Somigliana E, Restelli L, Gandolfi F, Ragni G. In vitro development of human oocytes after parthenogenetic activation or intracytoplasmic sperm injection. Fertil Steril 2007; 87: 77-82. [DOI:10.1016/j.fertnstert.2006.05.063] [PMID]
12. Jiang Y, Song G, Yuan JC, Zhang XH, Wu XH. Genetic analysis of recurrent parthenogenesis: A case report and literature review. Exp Ther Med 2022; 24: 530. [DOI:10.3892/etm.2022.11457] [PMID] [PMCID]
13. Combelles CMH, Kearns WG, Fox JH, Racowsky C. Cellular and genetic analysis of oocytes and embryos in a human case of spontaneous oocyte activation. Hum Reprod 2011; 26: 545-552. [DOI:10.1093/humrep/deq363] [PMID]
14. Socolov R, Ebner T, Gorduza V, Martiniuc V, Angioni S, Socolov D. Self-oocyte activation and parthenogenesis: An unusual outcome of a misconducted IVF cycle. Gynecol Endocrinol 2015; 31: 529-530. [DOI:10.3109/09513590.2015.1062861] [PMID]
15. Ye Y, Li N, Yan X, Wu R, Zhou W, Cheng L, et al. Genetic analysis of embryo in a human case of spontaneous oocyte activation: A case report. Gynecol Endocrinol 2020; 36: 294-296. [DOI:10.1080/09513590.2019.1687671] [PMID]
16. Lampert KP. Facultative parthenogenesis in vertebrates: Reproductive error or chance? Sex Dev 2008; 2: 290-301. [DOI:10.1159/000195678] [PMID]
17. Engelstädter J. Asexual but not clonal: Evolutionary processes in automictic populations. Genetics 2017; 206: 993-1009. [DOI:10.1534/genetics.116.196873] [PMID] [PMCID]
18. Pazhayam NM, Turcotte CA, Sekelsky J. Meiotic crossover patterning. Front Cell Dev Biol 2021; 9: 681123. [DOI:10.3389/fcell.2021.681123] [PMID] [PMCID]
19. Li X, Zou C, Li M, Fang C, Li K, Liu Z, et al. Transcriptome analysis of in vitro fertilization and parthenogenesis activation during early embryonic development in pigs. Genes (Basel) 2021; 12: 1461. [DOI:10.3390/genes12101461] [PMID] [PMCID]
20. Sagi I, Chia G, Golan-Lev T, Peretz M, Weissbein U, Sui L, et al. Derivation and differentiation of haploid human embryonic stem cells. Nature 2016; 532: 107-111. [DOI:10.1038/nature17408] [PMID]
21. Lakhoo K. Neonatal teratomas. Early Hum Dev 2010; 86: 643-647. [DOI:10.1016/j.earlhumdev.2010.08.016] [PMID]
22. Isaacs Jr H. Perinatal (fetal and neonatal) germ cell tumors. J Pediatr Surg 2004; 39: 1003-1013. [DOI:10.1016/j.jpedsurg.2004.03.045] [PMID]
23. Comerci Jr JT, Licciardi F, Bregh PA, Gregorgi C, Breen JL. Mature cystic teratoma: A clinicopathologic evaluation of 517 cases and review of literature. Obstet Gynecol 1994; 84: 22-28.
24. Coskun S, Maddirevula S, Awartani K, Aldeery M, Qubbaj W, Kashir J, et al. Recurrent spontaneous oocyte activation causes female infertility. J Assist Reprod Genet 2022; 39: 675-680. [DOI:10.1007/s10815-022-02435-x] [PMID] [PMCID]
25. Ahmed A, Lotfollahzadeh S. Cystic Teratoma. [Updated 2022 Dec 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2022 Jan. Available at: https://www.ncbi.nlm.nih.gov/books/NBK564325/
26. Hegazy AA. Is there any mean to postpone the menopausal ovarian senescence? Int J Fertil Steril 2020; 13: 346-347.
27. Horner VL, Wolfner MF. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev Dyn 2008; 237: 527-544. [DOI:10.1002/dvdy.21454] [PMID]
28. Nazarian H, Azad N, Nazari L, Piryaei A, Heidari MH, Masteri-Farahani R, et al. Effect of artificial oocyte activation on intra-cytoplasmic sperm injection outcomes in patients with lower percentage of sperm containing phospholipase Cζ: A randomized clinical trial. J Reprod Infertil 2019; 20: 3-9.
29. Rahbaran M, Razeghian E, Maashi MS, Jalil AT, Widjaja G, Thangavelu L, et al. Cloning and embryo splitting in mammalians: Brief history, methods, and achievements. Stem Cells Int 2021; 2021: 2347506. [DOI:10.1155/2021/2347506] [PMID] [PMCID]
30. Meerschaut FV, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online 2014; 28: 560-571. [DOI:10.1016/j.rbmo.2014.01.008] [PMID]
31. Kashir J, Ganesh D, Jones C, Coward K. Oocyte activation deficiency and assisted oocyte activation: Mechanisms, obstacles and prospects for clinical application. Hum Reprod Open 2022; 2022: hoac003. [DOI:10.1093/hropen/hoac003] [PMID] [PMCID]
32. Mitalipov SM, Wolf DP. Mammalian cloning: Possibilities and threats. Ann Med 2000; 32: 462-468. [DOI:10.3109/07853890009002021] [PMID]
33. Bonzi M, Fiorelli EM, Tobaldini E, Milani O, Bozzano V. A giant ovary teratoma with malignant transformation. Intern Emerg Med 2020; 15: 147-148. [DOI:10.1007/s11739-019-02220-2] [PMID]
34. Zhao Y, Xu T, Bu X, Yuan D, Wu Y, Qian H. Immature teratoma arising from uterine corpus in an 11-year-old girl: Case report and review of the literature. J Obstet Gynaecol Res 2021; 47: 452-455. [DOI:10.1111/jog.14547] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








