Tue, Jul 15, 2025
[Archive]
Volume 21, Issue 6 ( June 2023 2023)
IJRM 2023, 21(6): 481-490 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moini A, Eslami Moayed M, Kashani L, Farid Mojtahedi M, Rezaee T, Tabasizadeh H, et al . Pregnancy outcomes in women with adenomyosis, undergoing artificial endometrial preparation with and without gonadotropin-releasing hormone agonist pretreatment in frozen embryo transfer cycles: An RCT. IJRM 2023; 21 (6) :481-490
URL: http://ijrm.ir/article-1-2668-en.html
URL: http://ijrm.ir/article-1-2668-en.html
Ashraf Moini *1 

 , Marzieh Eslami Moayed2
, Marzieh Eslami Moayed2 

 , Ladan Kashani3
, Ladan Kashani3 

 , Maryam Farid Mojtahedi3
, Maryam Farid Mojtahedi3 

 , Tawoos Rezaee3
, Tawoos Rezaee3 

 , Hamed Tabasizadeh4
, Hamed Tabasizadeh4 

 , Khadije Maajani5
, Khadije Maajani5 

 , Nazila Yamini6
, Nazila Yamini6 




 , Marzieh Eslami Moayed2
, Marzieh Eslami Moayed2 

 , Ladan Kashani3
, Ladan Kashani3 

 , Maryam Farid Mojtahedi3
, Maryam Farid Mojtahedi3 

 , Tawoos Rezaee3
, Tawoos Rezaee3 

 , Hamed Tabasizadeh4
, Hamed Tabasizadeh4 

 , Khadije Maajani5
, Khadije Maajani5 

 , Nazila Yamini6
, Nazila Yamini6 


1- Department of Gynecology and Obstetrics, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran. , a_moini@royaninstitute.org
2- Department of Gynecology and Obstetrics, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran. Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. Breast Disease Research Center (BDRC), Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Gynecology and Obstetrics, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Pediatrics, Clinical Research Development Unit, Bahar Hospital, Shahroud University of Medical Sciences, Shahroud, Iran.
5- Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
6- Department of Embryology, Arash Women’s Hospital, Tehran University of Medical Science, Tehran, Iran.
2- Department of Gynecology and Obstetrics, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran. Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. Breast Disease Research Center (BDRC), Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Gynecology and Obstetrics, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Pediatrics, Clinical Research Development Unit, Bahar Hospital, Shahroud University of Medical Sciences, Shahroud, Iran.
5- Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
6- Department of Embryology, Arash Women’s Hospital, Tehran University of Medical Science, Tehran, Iran.
Keywords: Adenomyosis, Embryo transfer, Hormone replacement therapy, Gonadotropin-releasing hormone.
Full-Text [PDF 303 kb]
(759 Downloads)
| Abstract (HTML) (1050 Views)
1. Introduction
Adenomyosis is a benign disease of the uterus defined by the growth of the basal endometrium into the sub-endometrial myometrium (1). Due to postponing pregnancy until the third decade of a woman’s life, it is much more recognized among infertile cases who underwent assisted reproductive technology (2). Adenomyosis manifests with dysmenorrhea, menorrhagia, pelvic pain, and an enlarged and tender uterus (3). The conceivable etiology is recommended as disturbance of the typical boundary between the endometrium and the myometrium during physiological recovery and mending of the endometrium, driving to myometrial intrusion by endometrium (4). Endometrial healing activates the immune system, resulting in increased estrogen following microtrauma to the endometrial-myometrial junction and increased uterine peristalsis activity (4, 5).
Adenomyosis's effects on fertility remains unclear, even though uterine peristalsis activity negatively affects sperm and embryo transfer (5). Based on the last meta-analysis published on the effect of adenomyosis on pregnancy outcomes following assisted reproductive technology, it is concluded that adenomyosis hurts in vitro fertilization clinical outcomes; therefore, pretreatment with the use of long-term gonadotropin-releasing hormone agonist (GnRHa) or long protocol could be advantageous (6).
Hormonal replacement therapy (HRT) is a common protocol for frozen embryo transfer (FET) cycles because of its more flexible program, enabling physicians to choose the day of embryo transfer suitable for women with or without regular ovulation. This protocol uses estrogen supplementation for endometrial preparation (7, 8). Pretreatment with a GnRHa added to the HRT protocol, results in better pituitary downregulation (9). The GnRHa protocol reduces tissue inflammation and angiogenesis and increases the apoptotic index, which improves endometrial receptivity in adenomyosis (5, 10).
Local hypoestrogenism is effective in myometrial hyperperistalsis activities and benefits implantation (11, 12). There is insufficient evidence to support preferring an endometrial preparation protocol with or without GnRHa for FET in these women. Li and colleagues, in a retrospective study, reported that pretreatment with GnRHa in an HRT cycle did not improve the clinical pregnancy or live birth rates of following FET among infertile women with adenomyosis (13).
Due to the deficiency of clinical trial studies on endometrial preparation using HRT cycles with and without GnRHa pretreatment in adenomyosis cases, we designed a randomized clinical trial to compare the pregnancy outcomes in infertile women with adenomyosis who underwent HRT cycles with and without long-term pituitary downregulation with GnRHa.
2. Materials and Methods
2.1. Subjects
A phase III randomized clinical trial study was conducted on 140 adenomyosis cases, who underwent FET cycles at Arash Women’s hospital (an academic hospital linked with Tehran Medical University), Tehran, Iran from May 2020 to March 2021.
The diagnostic criteria of adenomyosis in vaginal ultrasonography included: the presence of myometrial cysts, echogenic striation, nodules, cystic striation, myometrial thickening, enlarged global uterus, disrupted myometrial-endometrial junction, and posterior diameter of the myometrium that is larger than its anterior diameter (14, 15), which was accomplished by exclusively an expert gynecology sonographer using a PHILIPS EPIQ-7 3D ultrasonography machine (Netherlands). Adenomyosis was classified as diffuse and focal according to the ultrasonographic characteristics defined earlier (16).
The participants aged between 30-45 yr, who had a normal uterine cavity that underwent the 1st or 2nd cycle of FET and adenomyosis diagnosis that was either focal or generalized, with or without a concomitant diagnosis of endometriosis and myoma, were included in the study. The following diagnoses were considered as exclusion criteria: recurrent implantation failure, hydrosalpinx, uterine anomalies, severe male factor infertility, myoma > 5 cm, severe endometriosis, and uterine distortion due to myoma.
The history of menorrhagia, dysmenorrhea, or dyspareunia and other concomitant diseases, such as endometriosis and myoma diagnosis, have been examined and recorded.
2.2. Sample size
The sample size was estimated to be a minimum of 130 (n = 65/ each) by considering the significance level of 5%, the power of 80%, according to the rate of clinical pregnancy between 2 groups (p1 = 51.35%) (p2 = 24.83%), and used the following formula based on the previous study (11).
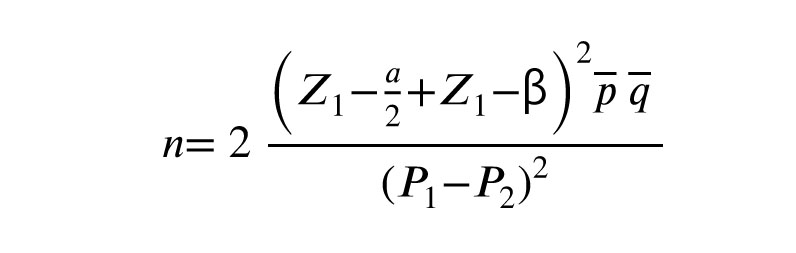
2.3. Randomization and blinding
Participants were allocated randomly into HRT and HRT+GnRHa pretreatment group (HRT+GnRHa) (n = 70/ each). The block randomization method was designed by the epidemiologist using STATA software, version 13. The type of study group was placed in a sealed envelope, and when the physicians approved the participants' eligibility for the study, the midwife then delivered the envelope to them. The researcher who followed up on the pregnancy results and the statistician did not know about the study grouping of the participants.
2.4. Embryos assessment and endometrial preparation protocols
All top-quality embryos were frozen by the vitrification strategy 3 days after ovum pick-up for the research. Briefly, the embryo morphology was defined according to the criteria of previous study by evaluating the number and normality of blastomeres arrangement and the degree of fragmentation on the 3rd day (17).
Due to the need for less hormonal monitoring, fewer women visit for ultrasound monitoring, and greater flexibility is observed in determining the day of embryo transfer according to the physician and individual preferences, HRT cycles were used instead of natural processes for FET embryo transfer.
In the HRT+GnRHa group, 2 doses of leuprolide acetate (Diphereline; Ipsen, France) 3.75 mg were prescribed through the early follicular stage of the menstrual cycle and 28 days afterward, and the HRT protocol was initiated in the following 10-14 days. In both groups, 2 mg estradiol valerate (Abureyhan, Iran) was administered daily, increasing the dose to 6 mg daily. A vaginal ultrasound was performed 10-14 days after estrogen supplementation to monitor endometrial thickness. When endometrial thickness exceeded ≥ 8 mm, progesterone supplement (an injectable progesterone 100 mg/daily) (Abureyhan, Iran) was administered, and frozen embryo replacement was planned.
The number of transferred embryos was chosen on the basis of the maternal age and embryo quality. 2 or a maximum of 3 cleavage-stage embryos were for the most part transferred on the 4th day of progesterone supplementation by exclusively an expert gynecologist using a cook embryo transfer catheter (MODEL: Arbor Medical Kororacija).
Embryo transfer was canceled if the optimal endometrial thickness was not achieved after 20 days. Serum β-human chorionic gonadotropin levels were measured 14-16 days after ET. If the test was positive, daily estradiol valerate (Abureyhan, Iran) and progesterone supplementation (Abureyhan, Iran, 50 mg/twice a day) were continued until the 12th wk of pregnancy.
2.5. Endometrial pattern evaluation
The endometrial pattern was determined 10-14 days after estradiol administration by transvaginal ultrasound, which was classified into 3 patterns; [Pattern A: Triple-line pattern consisting of the central hyperechoic line surrounded by 2 hypoechoic layers; pattern B: an intermediate isoechoic pattern with the same reflectivity as the surrounding myometrium; and pattern C: a poorly defined central echogenic line, resembling homogenous hyper echogenic endometrium] (18).
2.6. Outcomes
The primary outcomes were chemical (positive beta human chorionic gonadotropin test 14 days after ET) and clinical pregnancy (the presence of at least 1 fetus with heart activity at 4-6 wk following ET) rates. Secondary outcomes, including twin pregnancy, miscarriage, and live birth rates, were followed up and reported. A twin pregnancy was detected on observation of 2 gestational sacs and the fetal poles with heartbeat in an ultrasound examination at 6-7 wk following ET. The miscarriage rate is considered as the loss of a clinical pregnancy before 20 wk of gestation. Live birth was defined as the delivery of a fetus that shows evidence of life after being entirely outside of the uterus, regardless of the duration of pregnancy. The pregnancy outcomes were reported per ET cycles, and twin births were considered as one live birth.
2.7. Ethical considerations
The intention of the research was clarified to the samples, and all of them completed the informed agreement form, which was ready according to the Declaration of Helsinki. This study was approved by the Ethics Committee of Arash hospital of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.MEDICINE.REC.1399.034) and is registered on the IRCT website, which has been updated on January 01, 2023.
2.8. Statistical analysis
Mean ± standard deviation (SD), frequency, and percentages were used to report quantitative and qualitative variables. For continuous variables, we first checked the normal distribution using the Kolmogorov-Smirnov test. To analyze normal distribution data, we used an independent sample t test, and if data were skewed Mann-Whitney U test was used. To analyze categorical variables, we used Chi-square or Fisher's exact test as needed. Univariable and multivariable logistic regression analysis were performed to detect significant related factors to clinical pregnancy rate individually and in a group model. All statistical analyses were performed using statistical package for the social sciences (SPSS, version 26, SPSS Inc., Chicago, IL), and statistical significance was assumed at p < 0.05.
3. Results
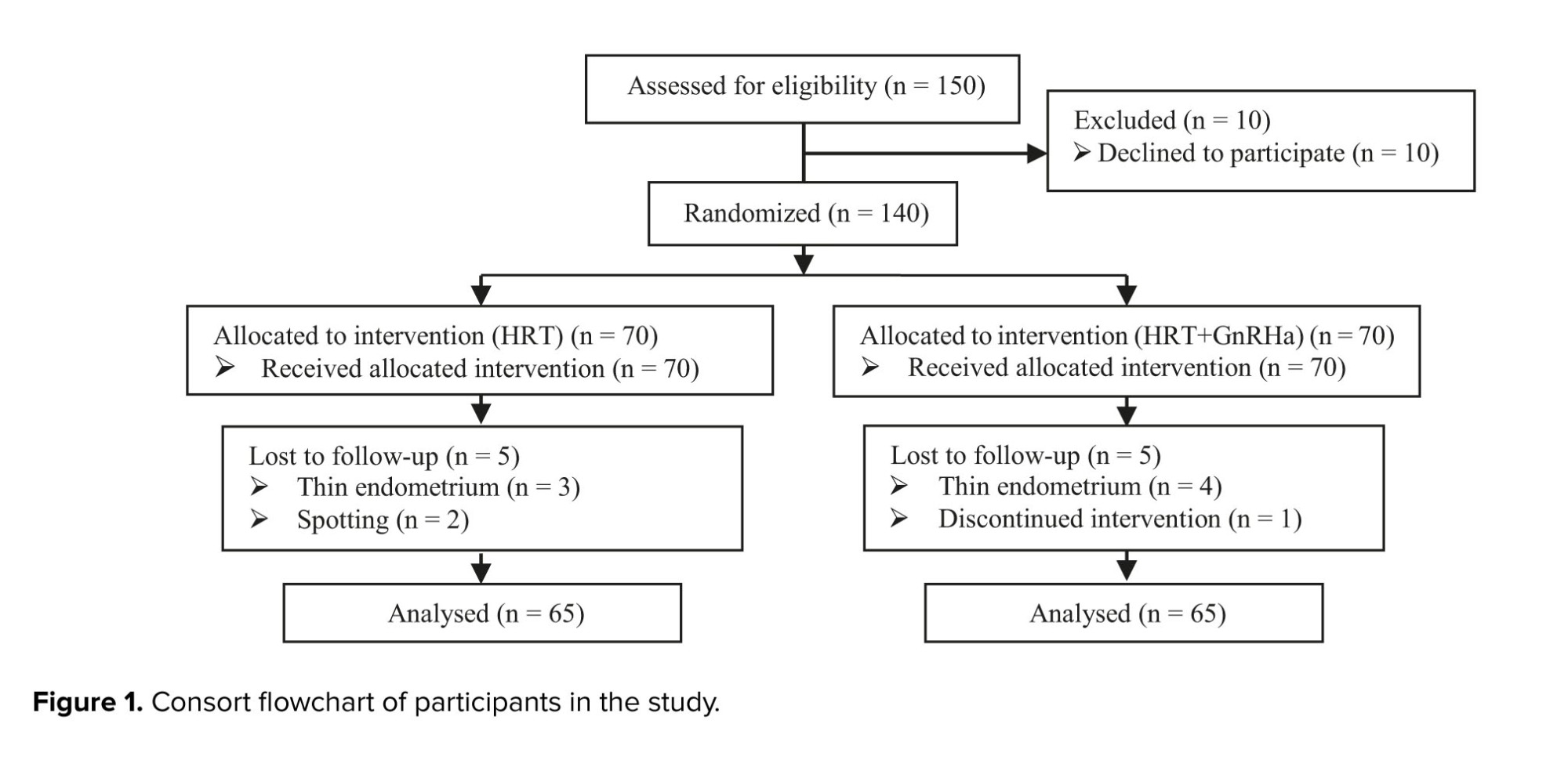
Among 140 participants, 5 and 4 FET cycles were canceled in HRT with and without GnRHa pretreatment groups, respectively, and one woman avoided participating in the study plan. The final analysis included 130 cases with adenomyosis (Figure 1).
The demographic and baseline clinical characteristics of the cases were compared between groups. The means of women’s age at transfer time had no significant difference between groups (p = 0.63). Also, no significant difference was observed between groups in terms of body mass index, type of infertility, cause of infertility, duration of infertility, and ET failure history. The women in pretreatment with the GnRHa group were more likely to have a previous history of FET cycles. In addition, the means of endometrial thickness at starting progesterone administration day were similar in both groups, and the predominant pattern in both groups was the triple line pattern (p = 0.56). No significant differences were observed between groups in the number of transferred embryos and embryo quality. Total estrogen dose and duration of its consumption were significantly higher in HRT with the GnRHa pretreatment group (p < 0.001, p < 0.001, respectively) (Table I).
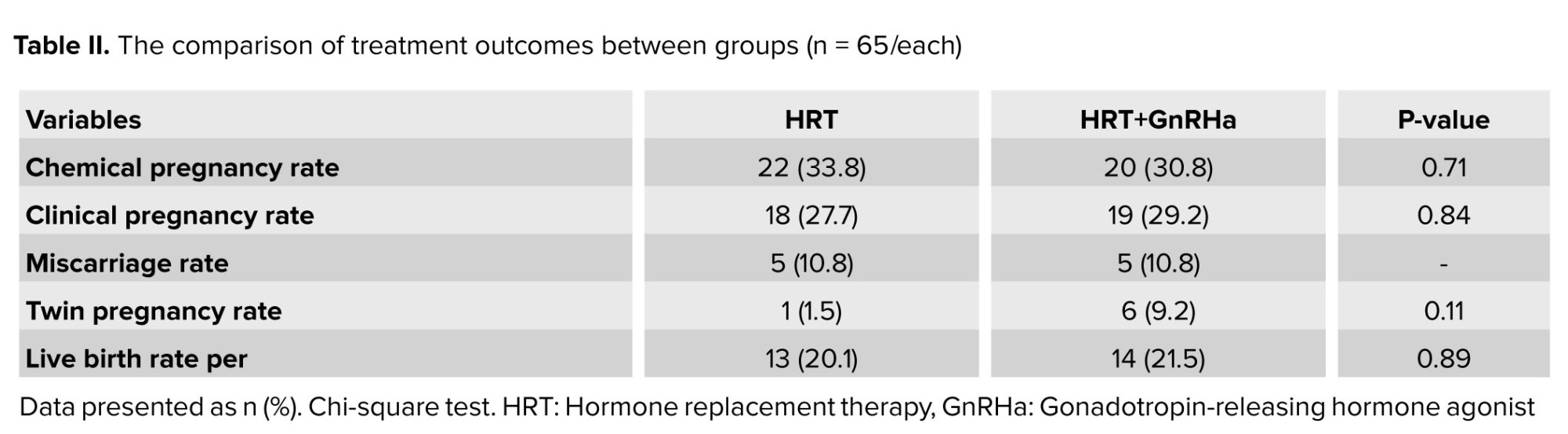
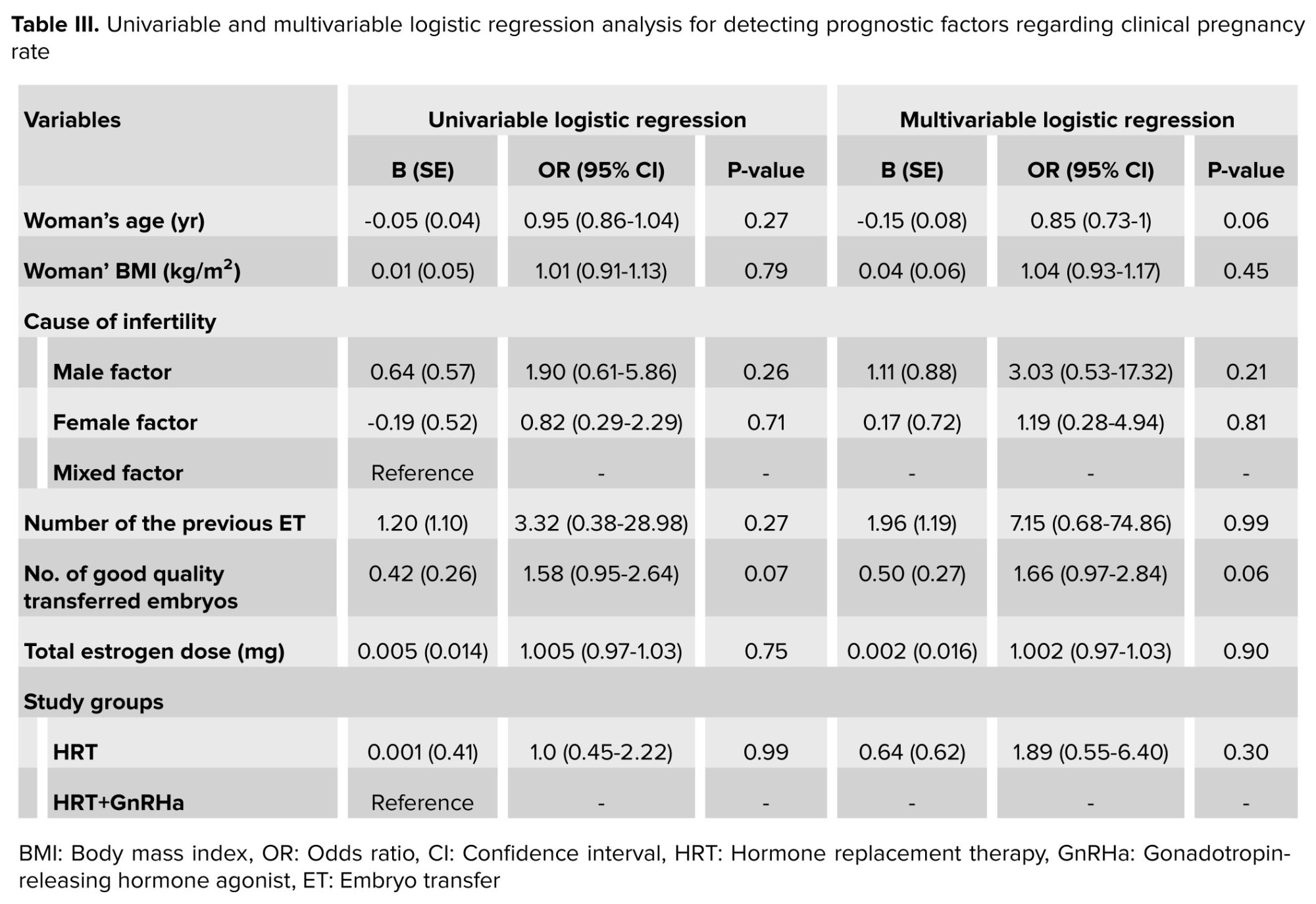
In the follow-up, the chemical pregnancy, clinical pregnancy rates, as well as twin pregnancy, miscarriage, and live birth rates were found to be comparable between groups (p > 0.05) (Table II). All possible related factors were entered in the multivariable logistic regression model to independently identify the significant predictive factors to the clinical pregnancy rate. No significant variables were found (Table III).

4. Discussion
This clinical trial study demonstrated similar clinical and live birth rates in adenomyosis cases who underwent HRT with and without GnRHa pretreatment for FET cycles. Limited retrospective studies have compared the artificial FET cycle following GnRHa pretreatment to HRT, even though most of them suggest improvement of pregnancy outcomes in women with adenomyosis who underwent GnRHa pretreatment. In a retrospective study, it was described that on the day of progesterone administration, endometrial thickness was higher in HRT cycles; however, in the HRT group with pituitary downregulation by GnRHa, clinical pregnancy, implantation, and ongoing pregnancy rates were remarkably higher than that of HRT group, propounding improved pregnancy results in women with adenomyosis who experienced HRT FET cycle with GnRHa pretreatment (11).
The pregnancy outcomes have been compared after fresh ET cycles with and without GnRHa pretreatment and FET cycles following GnRHa pretreatment in a retrospective study. GnRHa pretreatment are associated with higher duration of the stimulation and total dose of gonadotropin, which led to a notably higher number of recovered oocytes in fresh cycles. The clinical pregnancy rate in group C (FET cycles) tended to be higher than those in groups B (fresh with) and A (fresh without), but without a significant difference (19). Recently, the pregnancy outcomes between women who underwent HRT cycles with and without GnRHa pretreatment have been compared in a large retrospective cohort study. They suggested GnRHa pretreatment may improve clinical pregnancy and live birth rate in women with endometriosis (20).
Adenomyosis is an estrogen-responsive disease, and pituitary downregulation with GnRHa for 1-3 months might increase the pregnancy rate (5). GnRHa decrease the inflammatory reaction and angiogenesis in adenomyosis tissues. Also, as GnRHa suppress the hypothalamus–pituitary–ovarian axis and induce a hypoestrogenic situation, leading to adenomyosis cell apoptosis and reducing the uterine size (4, 10, 21). Supraphysiologic estrogen concentrations lead to decreased integrin β3, osteopontin, and leukemia-inhibiting factor as endometrial receptivity markers during the endometrium implantation window in adenomyosis (2, 22).
In the current study, no superiority was observed between HRT+GnRHa and HRT cycles in pregnancy outcomes. On the other hand, we showed that the HRT cycle with GnRHa pretreatment causes significantly prolonged days and doses of estradiol consumption. In agreement with the present finding, in a retrospective study, it was concluded that GnRHa downregulation based on an HRT for the endometrial preparation in the FET cycle among infertile women with adenomyosis did not improve the rates of clinical pregnancy or live birth compared to HRT alone (13).
Due to the shorter time reaching embryo transfer in the HRT group and a lower rate of side effects and costs compared with GnRHa, this protocol is more patient-friendly than the HRT+GnRHa protocol, simultaneously the GnRHa could result in the delayed resumption of spontaneous ovulation after the FET cycle failures (23, 24).
The design of a clinical trial with a significant sample size is the strength of the present study. It was ideal to transfer a single euploid blastocyst to control the embryo quality factor; however, we could not apply this factor in our study due to limitations. Since the 2 groups were homogeneous in terms of baseline characteristics and no significant variable affecting clinical pregnancy was found in logistic regression tests, the findings of the present study are reliable.
5. Conclusion
The present study indicated that endometrial preparation for FET with and without suppression by GnRHa provides similar results in pregnancy outcomes. Moreover, the HRT cycle time interval to embryo transfer is shorter, so this protocol is much simpler, better, and more cost-effective than the HRT cycle with GnRHa pretreatment. Furthermore, prospective RCTs are needed to validate the optimal protocol for FET cycles in cases with adenomyosis.
Acknowledgments
We would like to thank all the participants and coworkers in Arash Women’s hospital, Tehran, Iran for their assistance in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (126 Views)
1. Introduction
Adenomyosis is a benign disease of the uterus defined by the growth of the basal endometrium into the sub-endometrial myometrium (1). Due to postponing pregnancy until the third decade of a woman’s life, it is much more recognized among infertile cases who underwent assisted reproductive technology (2). Adenomyosis manifests with dysmenorrhea, menorrhagia, pelvic pain, and an enlarged and tender uterus (3). The conceivable etiology is recommended as disturbance of the typical boundary between the endometrium and the myometrium during physiological recovery and mending of the endometrium, driving to myometrial intrusion by endometrium (4). Endometrial healing activates the immune system, resulting in increased estrogen following microtrauma to the endometrial-myometrial junction and increased uterine peristalsis activity (4, 5).
Adenomyosis's effects on fertility remains unclear, even though uterine peristalsis activity negatively affects sperm and embryo transfer (5). Based on the last meta-analysis published on the effect of adenomyosis on pregnancy outcomes following assisted reproductive technology, it is concluded that adenomyosis hurts in vitro fertilization clinical outcomes; therefore, pretreatment with the use of long-term gonadotropin-releasing hormone agonist (GnRHa) or long protocol could be advantageous (6).
Hormonal replacement therapy (HRT) is a common protocol for frozen embryo transfer (FET) cycles because of its more flexible program, enabling physicians to choose the day of embryo transfer suitable for women with or without regular ovulation. This protocol uses estrogen supplementation for endometrial preparation (7, 8). Pretreatment with a GnRHa added to the HRT protocol, results in better pituitary downregulation (9). The GnRHa protocol reduces tissue inflammation and angiogenesis and increases the apoptotic index, which improves endometrial receptivity in adenomyosis (5, 10).
Local hypoestrogenism is effective in myometrial hyperperistalsis activities and benefits implantation (11, 12). There is insufficient evidence to support preferring an endometrial preparation protocol with or without GnRHa for FET in these women. Li and colleagues, in a retrospective study, reported that pretreatment with GnRHa in an HRT cycle did not improve the clinical pregnancy or live birth rates of following FET among infertile women with adenomyosis (13).
Due to the deficiency of clinical trial studies on endometrial preparation using HRT cycles with and without GnRHa pretreatment in adenomyosis cases, we designed a randomized clinical trial to compare the pregnancy outcomes in infertile women with adenomyosis who underwent HRT cycles with and without long-term pituitary downregulation with GnRHa.
2. Materials and Methods
2.1. Subjects
A phase III randomized clinical trial study was conducted on 140 adenomyosis cases, who underwent FET cycles at Arash Women’s hospital (an academic hospital linked with Tehran Medical University), Tehran, Iran from May 2020 to March 2021.
The diagnostic criteria of adenomyosis in vaginal ultrasonography included: the presence of myometrial cysts, echogenic striation, nodules, cystic striation, myometrial thickening, enlarged global uterus, disrupted myometrial-endometrial junction, and posterior diameter of the myometrium that is larger than its anterior diameter (14, 15), which was accomplished by exclusively an expert gynecology sonographer using a PHILIPS EPIQ-7 3D ultrasonography machine (Netherlands). Adenomyosis was classified as diffuse and focal according to the ultrasonographic characteristics defined earlier (16).
The participants aged between 30-45 yr, who had a normal uterine cavity that underwent the 1st or 2nd cycle of FET and adenomyosis diagnosis that was either focal or generalized, with or without a concomitant diagnosis of endometriosis and myoma, were included in the study. The following diagnoses were considered as exclusion criteria: recurrent implantation failure, hydrosalpinx, uterine anomalies, severe male factor infertility, myoma > 5 cm, severe endometriosis, and uterine distortion due to myoma.
The history of menorrhagia, dysmenorrhea, or dyspareunia and other concomitant diseases, such as endometriosis and myoma diagnosis, have been examined and recorded.
2.2. Sample size
The sample size was estimated to be a minimum of 130 (n = 65/ each) by considering the significance level of 5%, the power of 80%, according to the rate of clinical pregnancy between 2 groups (p1 = 51.35%) (p2 = 24.83%), and used the following formula based on the previous study (11).
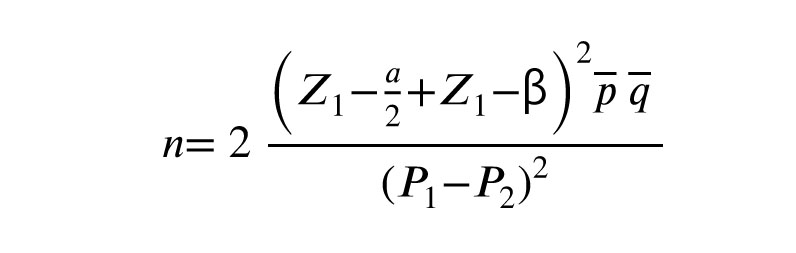
2.3. Randomization and blinding
Participants were allocated randomly into HRT and HRT+GnRHa pretreatment group (HRT+GnRHa) (n = 70/ each). The block randomization method was designed by the epidemiologist using STATA software, version 13. The type of study group was placed in a sealed envelope, and when the physicians approved the participants' eligibility for the study, the midwife then delivered the envelope to them. The researcher who followed up on the pregnancy results and the statistician did not know about the study grouping of the participants.
2.4. Embryos assessment and endometrial preparation protocols
All top-quality embryos were frozen by the vitrification strategy 3 days after ovum pick-up for the research. Briefly, the embryo morphology was defined according to the criteria of previous study by evaluating the number and normality of blastomeres arrangement and the degree of fragmentation on the 3rd day (17).
Due to the need for less hormonal monitoring, fewer women visit for ultrasound monitoring, and greater flexibility is observed in determining the day of embryo transfer according to the physician and individual preferences, HRT cycles were used instead of natural processes for FET embryo transfer.
In the HRT+GnRHa group, 2 doses of leuprolide acetate (Diphereline; Ipsen, France) 3.75 mg were prescribed through the early follicular stage of the menstrual cycle and 28 days afterward, and the HRT protocol was initiated in the following 10-14 days. In both groups, 2 mg estradiol valerate (Abureyhan, Iran) was administered daily, increasing the dose to 6 mg daily. A vaginal ultrasound was performed 10-14 days after estrogen supplementation to monitor endometrial thickness. When endometrial thickness exceeded ≥ 8 mm, progesterone supplement (an injectable progesterone 100 mg/daily) (Abureyhan, Iran) was administered, and frozen embryo replacement was planned.
The number of transferred embryos was chosen on the basis of the maternal age and embryo quality. 2 or a maximum of 3 cleavage-stage embryos were for the most part transferred on the 4th day of progesterone supplementation by exclusively an expert gynecologist using a cook embryo transfer catheter (MODEL: Arbor Medical Kororacija).
Embryo transfer was canceled if the optimal endometrial thickness was not achieved after 20 days. Serum β-human chorionic gonadotropin levels were measured 14-16 days after ET. If the test was positive, daily estradiol valerate (Abureyhan, Iran) and progesterone supplementation (Abureyhan, Iran, 50 mg/twice a day) were continued until the 12th wk of pregnancy.
2.5. Endometrial pattern evaluation
The endometrial pattern was determined 10-14 days after estradiol administration by transvaginal ultrasound, which was classified into 3 patterns; [Pattern A: Triple-line pattern consisting of the central hyperechoic line surrounded by 2 hypoechoic layers; pattern B: an intermediate isoechoic pattern with the same reflectivity as the surrounding myometrium; and pattern C: a poorly defined central echogenic line, resembling homogenous hyper echogenic endometrium] (18).
2.6. Outcomes
The primary outcomes were chemical (positive beta human chorionic gonadotropin test 14 days after ET) and clinical pregnancy (the presence of at least 1 fetus with heart activity at 4-6 wk following ET) rates. Secondary outcomes, including twin pregnancy, miscarriage, and live birth rates, were followed up and reported. A twin pregnancy was detected on observation of 2 gestational sacs and the fetal poles with heartbeat in an ultrasound examination at 6-7 wk following ET. The miscarriage rate is considered as the loss of a clinical pregnancy before 20 wk of gestation. Live birth was defined as the delivery of a fetus that shows evidence of life after being entirely outside of the uterus, regardless of the duration of pregnancy. The pregnancy outcomes were reported per ET cycles, and twin births were considered as one live birth.
2.7. Ethical considerations
The intention of the research was clarified to the samples, and all of them completed the informed agreement form, which was ready according to the Declaration of Helsinki. This study was approved by the Ethics Committee of Arash hospital of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.MEDICINE.REC.1399.034) and is registered on the IRCT website, which has been updated on January 01, 2023.
2.8. Statistical analysis
Mean ± standard deviation (SD), frequency, and percentages were used to report quantitative and qualitative variables. For continuous variables, we first checked the normal distribution using the Kolmogorov-Smirnov test. To analyze normal distribution data, we used an independent sample t test, and if data were skewed Mann-Whitney U test was used. To analyze categorical variables, we used Chi-square or Fisher's exact test as needed. Univariable and multivariable logistic regression analysis were performed to detect significant related factors to clinical pregnancy rate individually and in a group model. All statistical analyses were performed using statistical package for the social sciences (SPSS, version 26, SPSS Inc., Chicago, IL), and statistical significance was assumed at p < 0.05.
3. Results
Among 140 participants, 5 and 4 FET cycles were canceled in HRT with and without GnRHa pretreatment groups, respectively, and one woman avoided participating in the study plan. The final analysis included 130 cases with adenomyosis (Figure 1).
The demographic and baseline clinical characteristics of the cases were compared between groups. The means of women’s age at transfer time had no significant difference between groups (p = 0.63). Also, no significant difference was observed between groups in terms of body mass index, type of infertility, cause of infertility, duration of infertility, and ET failure history. The women in pretreatment with the GnRHa group were more likely to have a previous history of FET cycles. In addition, the means of endometrial thickness at starting progesterone administration day were similar in both groups, and the predominant pattern in both groups was the triple line pattern (p = 0.56). No significant differences were observed between groups in the number of transferred embryos and embryo quality. Total estrogen dose and duration of its consumption were significantly higher in HRT with the GnRHa pretreatment group (p < 0.001, p < 0.001, respectively) (Table I).
In the follow-up, the chemical pregnancy, clinical pregnancy rates, as well as twin pregnancy, miscarriage, and live birth rates were found to be comparable between groups (p > 0.05) (Table II). All possible related factors were entered in the multivariable logistic regression model to independently identify the significant predictive factors to the clinical pregnancy rate. No significant variables were found (Table III).




4. Discussion
This clinical trial study demonstrated similar clinical and live birth rates in adenomyosis cases who underwent HRT with and without GnRHa pretreatment for FET cycles. Limited retrospective studies have compared the artificial FET cycle following GnRHa pretreatment to HRT, even though most of them suggest improvement of pregnancy outcomes in women with adenomyosis who underwent GnRHa pretreatment. In a retrospective study, it was described that on the day of progesterone administration, endometrial thickness was higher in HRT cycles; however, in the HRT group with pituitary downregulation by GnRHa, clinical pregnancy, implantation, and ongoing pregnancy rates were remarkably higher than that of HRT group, propounding improved pregnancy results in women with adenomyosis who experienced HRT FET cycle with GnRHa pretreatment (11).
The pregnancy outcomes have been compared after fresh ET cycles with and without GnRHa pretreatment and FET cycles following GnRHa pretreatment in a retrospective study. GnRHa pretreatment are associated with higher duration of the stimulation and total dose of gonadotropin, which led to a notably higher number of recovered oocytes in fresh cycles. The clinical pregnancy rate in group C (FET cycles) tended to be higher than those in groups B (fresh with) and A (fresh without), but without a significant difference (19). Recently, the pregnancy outcomes between women who underwent HRT cycles with and without GnRHa pretreatment have been compared in a large retrospective cohort study. They suggested GnRHa pretreatment may improve clinical pregnancy and live birth rate in women with endometriosis (20).
Adenomyosis is an estrogen-responsive disease, and pituitary downregulation with GnRHa for 1-3 months might increase the pregnancy rate (5). GnRHa decrease the inflammatory reaction and angiogenesis in adenomyosis tissues. Also, as GnRHa suppress the hypothalamus–pituitary–ovarian axis and induce a hypoestrogenic situation, leading to adenomyosis cell apoptosis and reducing the uterine size (4, 10, 21). Supraphysiologic estrogen concentrations lead to decreased integrin β3, osteopontin, and leukemia-inhibiting factor as endometrial receptivity markers during the endometrium implantation window in adenomyosis (2, 22).
In the current study, no superiority was observed between HRT+GnRHa and HRT cycles in pregnancy outcomes. On the other hand, we showed that the HRT cycle with GnRHa pretreatment causes significantly prolonged days and doses of estradiol consumption. In agreement with the present finding, in a retrospective study, it was concluded that GnRHa downregulation based on an HRT for the endometrial preparation in the FET cycle among infertile women with adenomyosis did not improve the rates of clinical pregnancy or live birth compared to HRT alone (13).
Due to the shorter time reaching embryo transfer in the HRT group and a lower rate of side effects and costs compared with GnRHa, this protocol is more patient-friendly than the HRT+GnRHa protocol, simultaneously the GnRHa could result in the delayed resumption of spontaneous ovulation after the FET cycle failures (23, 24).
The design of a clinical trial with a significant sample size is the strength of the present study. It was ideal to transfer a single euploid blastocyst to control the embryo quality factor; however, we could not apply this factor in our study due to limitations. Since the 2 groups were homogeneous in terms of baseline characteristics and no significant variable affecting clinical pregnancy was found in logistic regression tests, the findings of the present study are reliable.
5. Conclusion
The present study indicated that endometrial preparation for FET with and without suppression by GnRHa provides similar results in pregnancy outcomes. Moreover, the HRT cycle time interval to embryo transfer is shorter, so this protocol is much simpler, better, and more cost-effective than the HRT cycle with GnRHa pretreatment. Furthermore, prospective RCTs are needed to validate the optimal protocol for FET cycles in cases with adenomyosis.
Acknowledgments
We would like to thank all the participants and coworkers in Arash Women’s hospital, Tehran, Iran for their assistance in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Assisted Reproductive Technologies
References
1. Bourdon M, Santulli P, Marcellin L, Maignien C, Maitrot-Mantelet L, Bordonne C, et al. Adenomyosis: An update regarding its diagnosis and clinical features. J Gynecol Obstet Hum Reprod 2021; 50: 102228. [DOI:10.1016/j.jogoh.2021.102228] [PMID]
2. Xiao Y, Li T, Xia E, Yang X, Sun X, Zhou Y. Expression of integrin β3 and osteopontin in the eutopic endometrium of adenomyosis during the implantation window. Eur J Obstet Gynecol Reprod Biol 2013; 170: 419-422. [DOI:10.1016/j.ejogrb.2013.05.007] [PMID]
3. Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril 2018; 109: 380-388. [DOI:10.1016/j.fertnstert.2018.01.006] [PMID]
4. Lacheta J. Uterine adenomyosis: Pathogenesis, diagnostics, symptomatology and treatment. Ceska Gynekol 2019; 84: 240-246.
5. Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril 2019; 111: 629-640. [DOI:10.1016/j.fertnstert.2019.02.008] [PMID]
6. Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: A meta-analysis. Fertil Steril 2017; 108: 483-490. [DOI:10.1016/j.fertnstert.2017.06.025] [PMID]
7. Mackens S, Santos-Ribeiro S, Van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: A review on the optimal endometrial preparation and timing. Hum Reprod 2017; 32: 2234-2242. [DOI:10.1093/humrep/dex285] [PMID]
8. Zheng Q, Zhang H, Xu S, Xiao S, Wang X, Mo M, et al. Optimal endometrial preparation protocols for frozen-thawed embryo transfer cycles by maternal age. Reprod Sci 2021; 28: 2847-2854. [DOI:10.1007/s43032-021-00538-x] [PMID]
9. Chen M, Luo L, Wang Q, Gao J, Chen Y, Zhang Y, et al. Impact of gonadotropin-releasing hormone agonist pre-treatment on the cumulative live birth rate in infertile women with adenomyosis treated with IVF/ICSI: A retrospective cohort study. Front Endocrinol 2020; 11: 318. [DOI:10.3389/fendo.2020.00318] [PMID] [PMCID]
10. Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod 2010; 25: 642-653. [DOI:10.1093/humrep/dep437] [PMID]
11. Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol 2013; 29: 1026-1030. [DOI:10.3109/09513590.2013.824960] [PMID]
12. Kuijsters NPM, Methorst WG, Kortenhorst MSQ, Rabotti C, Mischi M, Schoot BC. Uterine peristalsis and fertility: Current knowledge and future perspectives: A review and meta-analysis. Reprod Biomed Online 2017; 35: 50-71. [DOI:10.1016/j.rbmo.2017.03.019] [PMID]
13. Li M, Xu L, Zhao H, Du Y, Yan L. Effects of artificial cycles with and without gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes in patients with adenomyosis. Sci Rep 2021; 11: 19326. [DOI:10.1038/s41598-021-98918-5] [PMID] [PMCID]
14. Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum Reprod Update 2020; 26: 392-411. [DOI:10.1093/humupd/dmz049] [PMID]
15. Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res 2019; 8: 283. [DOI:10.12688/f1000research.17242.1] [PMID] [PMCID]
16. Lazzeri L, Morosetti G, Centini G, Monti G, Zupi E, Piccione E, et al. A sonographic classification of adenomyosis: Interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril 2018; 110: 1154-1161. [DOI:10.1016/j.fertnstert.2018.06.031] [PMID]
17. Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf 1986; 3: 284-295. [DOI:10.1007/BF01133388] [PMID]
18. Bu Z, Yang X, Song L, Kang B, Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol 2019; 17: 99. [DOI:10.1186/s12958-019-0545-0] [PMID] [PMCID]
19. Park CW, Choi MH, Yang KM, Song IO. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin Exp Reprod Med 2016; 43: 169-173. [DOI:10.5653/cerm.2016.43.3.169] [PMID] [PMCID]
20. Qi Q, Luo J, Wang Y, Xie Q. Effects of artificial cycles with and without gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes. J Int Med Res 2020; 48: 1-8. [DOI:10.1177/0300060520918474] [PMID] [PMCID]
21. Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Ishimaru T, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod 2010; 25: 2878-2890. [DOI:10.1093/humrep/deq240] [PMID]
22. Xiao Y, Sun X, Yang X, Zhang J, Xue Q, Cai B, et al. Leukemia inhibitory factor is dysregulated in the endometrium and uterine flushing fluid of patients with adenomyosis during implantation window. Fertil Steril 2010; 94: 85-89. [DOI:10.1016/j.fertnstert.2009.03.012] [PMID]
23. Chen Q, Yu F, Li Y, Zhang A-J, Zhu X-B. Comparative proteomics reveal negative effects of gonadotropin-releasing hormone agonist and antagonist on human endometrium. Drug Des Dev Ther 2019; 13: 1855-1863. [DOI:10.2147/DDDT.S201871] [PMID] [PMCID]
24. Xu J, Li S-Z, Yin M-N, Liang P-L, Li P, Sun L. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with GnRH agonist: A randomized controlled trial at two centers. Front Endocrinol 2021; 12: 722253. [DOI:10.3389/fendo.2021.722253] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




