Thu, Feb 19, 2026
[Archive]
Volume 22, Issue 4 (April 2024)
IJRM 2024, 22(4): 317-322 |
Back to browse issues page
Ethics code: IR.ZAUMS.REC.1398.217
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khazaei H A, Farzaneh F, Sarhadi S, Dehghan Haghighi J, Forghani F, Sheikhi V, et al . Comparison of serum levels of interleukin 33 in combination with serum levels of C-reactive protein, Immunoglobulin G, Immunoglobulin A, and Immunoglobulin M in recurrent pregnancy loss: A case-control study. IJRM 2024; 22 (4) :317-322
URL: http://ijrm.ir/article-1-3081-en.html
URL: http://ijrm.ir/article-1-3081-en.html
Hossein Ali Khazaei1 

 , Farahnaz Farzaneh2
, Farahnaz Farzaneh2 

 , Saeedeh Sarhadi *3
, Saeedeh Sarhadi *3 

 , Javid Dehghan Haghighi4
, Javid Dehghan Haghighi4 

 , Forough Forghani5
, Forough Forghani5 

 , Vahid Sheikhi6
, Vahid Sheikhi6 

 , Bahman Khazaei7
, Bahman Khazaei7 

 , Lida Asaollahi7
, Lida Asaollahi7 




 , Farahnaz Farzaneh2
, Farahnaz Farzaneh2 

 , Saeedeh Sarhadi *3
, Saeedeh Sarhadi *3 

 , Javid Dehghan Haghighi4
, Javid Dehghan Haghighi4 

 , Forough Forghani5
, Forough Forghani5 

 , Vahid Sheikhi6
, Vahid Sheikhi6 

 , Bahman Khazaei7
, Bahman Khazaei7 

 , Lida Asaollahi7
, Lida Asaollahi7 


1- Clinical Immunology Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran. Department of Community Medicine, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran. ,Dr.sarhadi93@gmail.com
4- Department of Community Medicine, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
6- Department of Pediatrics, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
7- School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran. Department of Community Medicine, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran. ,
4- Department of Community Medicine, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
6- Department of Pediatrics, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
7- School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
Full-Text [PDF 265 kb]
(967 Downloads)
| Abstract (HTML) (1169 Views)
Full-Text: (226 Views)
1. Introduction
About 1-2% of women trying to get pregnant experience recurrent pregnancy loss (RPL) (1, 2). For the medical and scientific communities, RPL is challenging. In reality, only about 50% of RPL cases can be defined (3). The causes of RPL are chromosomal defects of the fetus, uterine disorders, infections, hormonal or endocrine causes, disorders of immunological factors, hereditary and acquired thrombophilia, or environmental and nutritional factors. However, in more than 50% of cases, the reasons for abortion are etiologically unknown (2). Studies have shown that chromosomal abnormalities in miscarriage in the first trimester is well established and that about 5% of couples with 2 or more miscarriages carry chromosomal structural abnormalities or may have multiple problems simultaneously (4-6). The innate and adaptive immune system plays a significant role in endometrial remodeling and maternal tolerance towards the embryo (7).
On the other hand, a successful pregnancy depends on the role of inflammatory markers, as mentioned above, which can be protective or harmful to fertilization. It has been suggested that a balance between pro-inflammatory and anti-inflammatory factors such as C-reactive protein (CRP), Immunoglobulin G (IgG), Immunoglobulin A (IgA), Immunoglobulin M (IgM), and cytokines is essential for successful pregnancy (8, 9). Interleukin (IL) 33, which is a member of the IL-1, plays an important role in host defense, immune regulation, neuronal damage, and inflammation (10, 11). Most of the markers reported in previous studies showed that the role of regulatory T cells in RPL in Iranian women is highlighted by their association with them. Endothelial, epithelial, T helper 2-activated, and mast cells are the main cells that express IL-33 (9, 12). Endothelial cells and smooth muscle cells within the placenta, chorioamniotic membranes, and umbilical cord are where the expression of IL-33 occurs. Also, some studies have shown that IL-33 serum levels in recurrent miscarriages are different compared to normal pregnancies (10, 13). Another study reported that IL-33 serum levels were significantly lower in women with recurrent miscarriages than in control group (14). It also shows what cellular and molecular mechanisms may be involved in the dysregulation of IL-33 signaling and poor pregnancy outcomes in women (11). This complexity further fuels the ongoing controversy about which immunological factors play a role in the pathogenesis of RPL (15).
Due to no information available and the role of immune function, we aimed to evaluate and compare the IL-33 serum level some related immunological factors in patients with RPL and normal individuals referred to the gynecology and obstetrics clinic of Ali Ibn Abi Taleb hospital in Zahedan, Iran.
This study aimed to determine the association of the serum levels of IL-33, CRP, IgG, IgM, IgA with RPL.
2. Materials and Methods
This case-control study was conducted on 66 women aged between 18 and 35 yr, who referred to the Clinic of Gynecology and Obstetrics, Ali Ibn Abi Taleb hospital, Zahedan, Iran from August-December 2019. Participants were selected by an easy and accessible method and divided into 2 groups of 33 as a case (women with a history of RPL) and 33 as the control group (including healthy women).
Inclusion criteria were having a history of recurrent miscarriage (3 consecutive miscarriages before 20th wk), at least 4-6 months after the last miscarriage.
The control group included 33 healthy women aged between 18 and 35 yr without a history of abortion and who have at least one child (at least 4-6 months have passed since the last delivery).
Those women having uterine anatomical disorders, such as double uterus, arcuate uterus, unicornuate uterus, bicornuate uterus, septate uterus, thyroid disorders, systemic diseases such as antiphospholipid syndrome, systemic lupus erythematosus, rheumatoid arthritis, hyperprolactinemia, chromosomal problems of parents, and allergic diseases were excluded from the study. 6 women were excluded according to exclusion criteria )autoimmune diseases, History of allergic reactions, hypothyroidism).
In this study, repeated abortions meant losing pregnancy products before the 20th wk of pregnancy on at least 3 consecutive or non-consecutive occasions. 5 cc of peripheral blood was taken from each participant to determine the serum levels of IL-33, CRP, IgG, IgA, and IgM and sent to the laboratory of Ali Ibn Abi Taleb hospital, Zahedan, Iran. The blood samples were centrifuged according to the kit instructions. The serum was separated and stored in the laboratory freezer until the end of the study. Finally, after completing the number of samples, the IL-33 serum level in 2 groups was measured by enzyme-linked immunosorbent assay method (EASTBIOPHARM CO., China).
2.1. Sample size
A preliminary estimate of the study’s sample size was calculated from a previous study (16). Mean IL-33 serum levels were used, with a type I error of 0.05 and a type II error of 0.2. According to the comparison of the 2 mean formulas, each group necessarily had at least 12 participants. During the study, this number increased to 33 patients in each group.
2.2. Ethical considerations
This study was approved by the Ethical Committee of Zahedan University of Medical Sciences, Zahedan, Iran (Code: IR.ZAUMS.REC.1398.217). Participants were enrolled after obtaining informed written consent.
2.3. Statistical analysis
The Kolmogorov-Smirnov test was used to evaluate the normal distribution of data. Due to the existence of abnormal distribution in quantitative variables, Mann-Whitney non-parametric test was used to compare the level of CRP and antibodies between case and control groups. P < 0.05 was considered significant. IBM SPSS Statistics for Windows, version 22.0 (IBM Corp), was used for the analysis, 2013, IBM Corp., Armonk, New York.
3. Results
In this study, 66 women were divided into 2 groups of 33, a case group (women with a history of RPL) and 33 as a control group (including healthy women) referred to the clinic of gynecology and obstetrics, the mean age of the participants was 30.8 ± 3.80 with a maximum and minimum value of 19.35 respectively. The mean age of participants in the group with recurrent abortion (case) was 30.9 ± 3.1 yr, and for women without recurrent abortion (control) was 30.7 ± 4.4 yr (p = 0.73). Those who met the study inclusion and exclusion criteria were investigated. It should be noted that due to the lack of normal distribution of 2 variables, age (p < 0.001) and IL-33 (p = 0.01), the nonparametric test was used to compare the 2 groups. It is also indicated that the mean age of participants in this study was 30.8, with a standard deviation of 3.8. No significant difference was observed in age distribution between the case and control groups (p = 0.73).
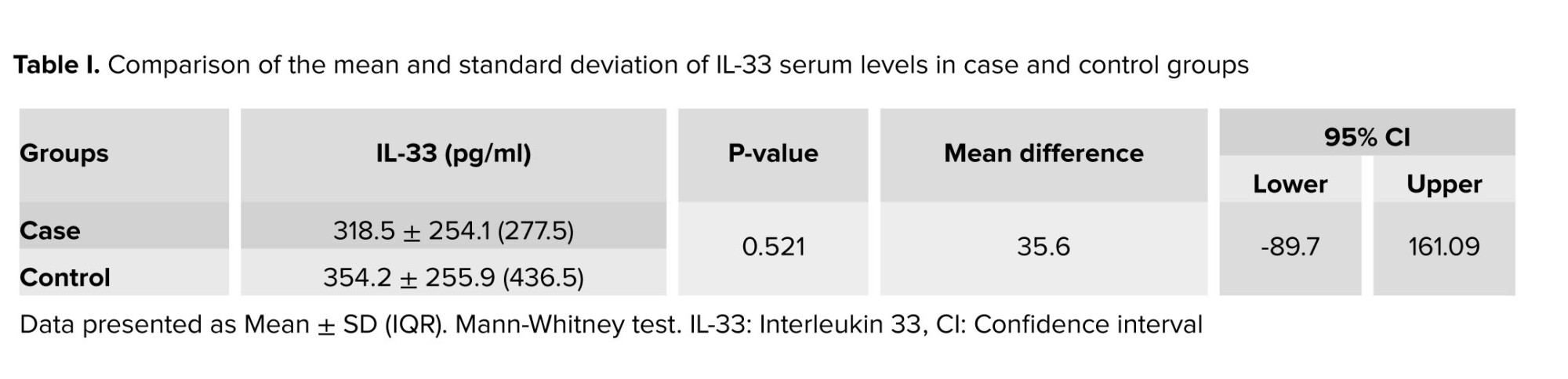
The mean and standard deviation of IL-33 serum level in the case and control groups are shown in table I. According to the results of this study, the mean in the group of patients with recurrent miscarriage was 318.5 pg/ml with a standard deviation of 254.1, and the mean serum level of IL-33 in the group of healthy women without recurrent miscarriage was obtained as 354.2 pg/ml with standard deviation 9/255. According to table I, the mean serum level of IL-33 in the case group (318.5 pg/ml) was lower than the mean in the control group (354.22 pg/ml). Still, this difference was not statistically significant (p = 0.52, Table I).
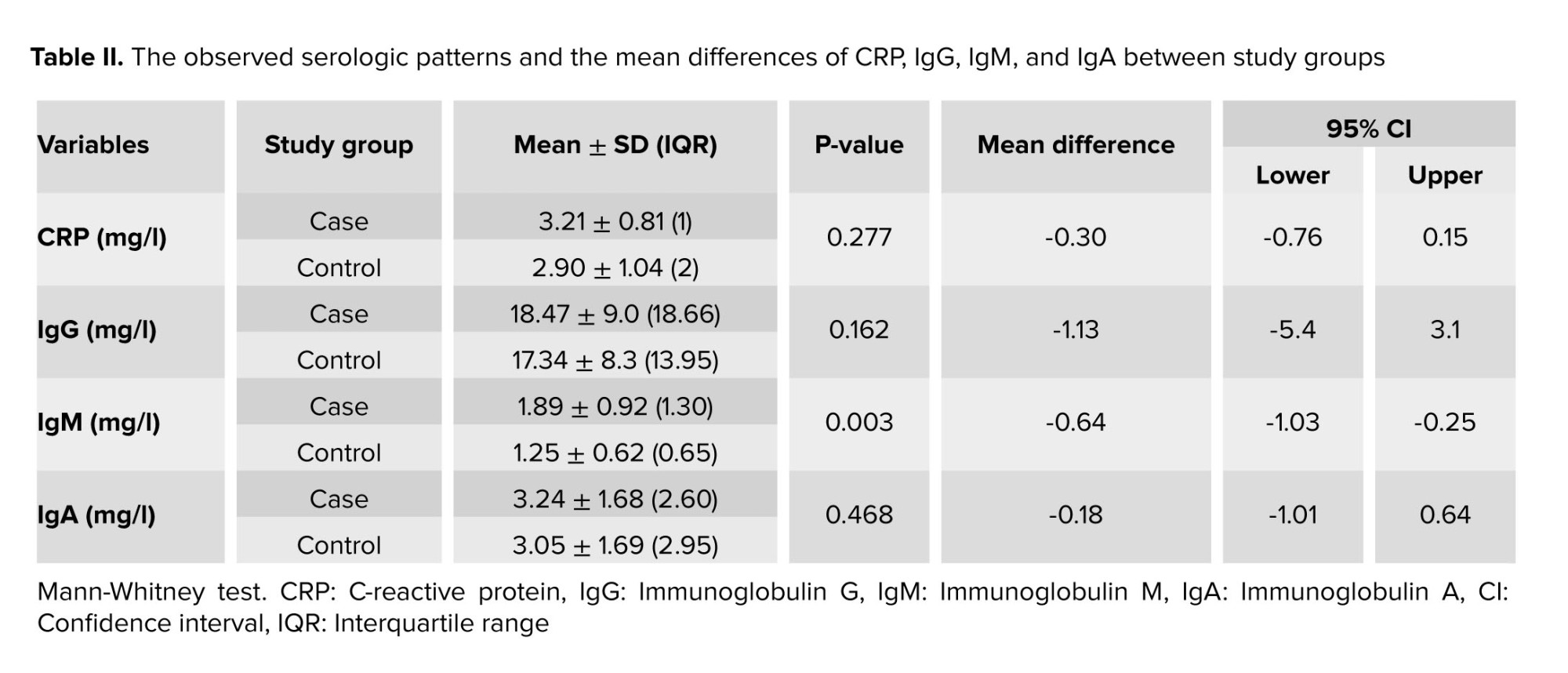
Furthermore, the results of this study showed that CRP levels were not significantly different in case and control groups (p = 0.27). Regarding IgA and IgG, no significant difference was observed in the levels of these antibodies in the 2 groups with and without a history of recurrent miscarriage (p = 0.16, p = 0.46). While the difference in IgM level between the 2 groups was statistically significant (p < 0.001). The observed serologic patterns are detailed in table II.
4. Discussion
This study compared IL-33, CRP, IgM, IgG, and IgA in RPL and control group. As a result of this study, only IgM levels showed a statistically significant difference between the case and control groups. Recent research has shown that IL-33 is expressed in endothelial and smooth muscle cells in the placenta, chorioamniotic membranes, and umbilical cord. Also, some studies have shown that polymorphism of IL-33 was correlated with RPL risk comparing control group, and it gave further details that rs1929992 (IL-33) polymorphisms could be associated with RPL in the Iranian population (13). Yue notes that IL-33 serum levels are significantly lower in idiopathic recurrent miscarriage cases than in the control group. Activation of the IL-33 suppression of tumorigenicity 2 signaling pathway in human endometrial stromal cells has been reported to be critical for a successful pregnancy. At 6 wk gestation, a significant increase in IL-33 serum levels were observed in patients who are to be at risk for abortions compared to healthy control pregnancies (14). It seems that when interpreting IL-33 levels, consideration of the week of pregnancy is essential and should be considered. On the other hand, the larger sample size and differences in geographic area in Yue’s study should be noted in justifying the difference between the results of our study and the aforementioned study.
Finally, it can be summarized that the IL-33 serum level, at least 4-6 months after the last abortion (in the case group) and the previous live delivery (in the control group), did not show a significant difference between the 2 groups. However, due to the different results of earlier studies in this field, there is still a need for further studies. The results of the present study showed no significant difference in the IL-33 serum level between normal women without recurrent miscarriages and women with a history of recurrent miscarriages. However, the mean IL-33 serum level was slightly lower in women with a history of recurrent miscarriage, which could be a significant difference in larger samples due to the limited sample size in this study.
A protein phenotype may be related to the ethnicity and racial status of the patient; hence more extensive studies in this field are essential. Other suggestions include repetition of studies similar to the follow-up period of most patients to more accurately identify the IL-33 serum level in different conditions and times, designing new studies to identify pathways affecting changes in IL-33 serum levels outside of pregnancy. Moreover, determination and comparison of changes in IL-33 serum levels up to the 20th wk of pregnancy, determining the cytokine phenotype in patients according to their demographic conditions, determining the genotype of this cytokine in patients according to their demographic conditions, they could be suitable research fields in the future.
5. Conclusion
In conclusion, in this study, IgM was found to be significantly increased in patients with recurrent miscarriage. This relationship was not observed in IL-33 and other immunological factors. Therefore, the role of IgM as an acute inflammatory marker in recurrent miscarriage has been confirmed. It seems that different aspects of the effects of IL-33 as a predictive biomarker in PRL, should be evaluated in more comprehensive studies.
Data availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author contributions
Hossein Ali Khazaei and Farahnaz Farzaneh designed the study and conducted the research. Saeedeh Sarhadi, Javid Dehghan Haghighi, Forough Forghani, Vahid Sheikhi, Bahman Khazaei, and Lida Asadollahi, monitored, evaluated, and analyzed the results of the study. Further Saeedeh Sarhadi and Hossein Ali Khazaei reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The authors wish to express their thanks for the financial support of Zahedan University of Medical Sciences, Zahedan, Iran.
Conflict of Interest
All authors declare that there is no conflict of interest.
About 1-2% of women trying to get pregnant experience recurrent pregnancy loss (RPL) (1, 2). For the medical and scientific communities, RPL is challenging. In reality, only about 50% of RPL cases can be defined (3). The causes of RPL are chromosomal defects of the fetus, uterine disorders, infections, hormonal or endocrine causes, disorders of immunological factors, hereditary and acquired thrombophilia, or environmental and nutritional factors. However, in more than 50% of cases, the reasons for abortion are etiologically unknown (2). Studies have shown that chromosomal abnormalities in miscarriage in the first trimester is well established and that about 5% of couples with 2 or more miscarriages carry chromosomal structural abnormalities or may have multiple problems simultaneously (4-6). The innate and adaptive immune system plays a significant role in endometrial remodeling and maternal tolerance towards the embryo (7).
On the other hand, a successful pregnancy depends on the role of inflammatory markers, as mentioned above, which can be protective or harmful to fertilization. It has been suggested that a balance between pro-inflammatory and anti-inflammatory factors such as C-reactive protein (CRP), Immunoglobulin G (IgG), Immunoglobulin A (IgA), Immunoglobulin M (IgM), and cytokines is essential for successful pregnancy (8, 9). Interleukin (IL) 33, which is a member of the IL-1, plays an important role in host defense, immune regulation, neuronal damage, and inflammation (10, 11). Most of the markers reported in previous studies showed that the role of regulatory T cells in RPL in Iranian women is highlighted by their association with them. Endothelial, epithelial, T helper 2-activated, and mast cells are the main cells that express IL-33 (9, 12). Endothelial cells and smooth muscle cells within the placenta, chorioamniotic membranes, and umbilical cord are where the expression of IL-33 occurs. Also, some studies have shown that IL-33 serum levels in recurrent miscarriages are different compared to normal pregnancies (10, 13). Another study reported that IL-33 serum levels were significantly lower in women with recurrent miscarriages than in control group (14). It also shows what cellular and molecular mechanisms may be involved in the dysregulation of IL-33 signaling and poor pregnancy outcomes in women (11). This complexity further fuels the ongoing controversy about which immunological factors play a role in the pathogenesis of RPL (15).
Due to no information available and the role of immune function, we aimed to evaluate and compare the IL-33 serum level some related immunological factors in patients with RPL and normal individuals referred to the gynecology and obstetrics clinic of Ali Ibn Abi Taleb hospital in Zahedan, Iran.
This study aimed to determine the association of the serum levels of IL-33, CRP, IgG, IgM, IgA with RPL.
2. Materials and Methods
This case-control study was conducted on 66 women aged between 18 and 35 yr, who referred to the Clinic of Gynecology and Obstetrics, Ali Ibn Abi Taleb hospital, Zahedan, Iran from August-December 2019. Participants were selected by an easy and accessible method and divided into 2 groups of 33 as a case (women with a history of RPL) and 33 as the control group (including healthy women).
Inclusion criteria were having a history of recurrent miscarriage (3 consecutive miscarriages before 20th wk), at least 4-6 months after the last miscarriage.
The control group included 33 healthy women aged between 18 and 35 yr without a history of abortion and who have at least one child (at least 4-6 months have passed since the last delivery).
Those women having uterine anatomical disorders, such as double uterus, arcuate uterus, unicornuate uterus, bicornuate uterus, septate uterus, thyroid disorders, systemic diseases such as antiphospholipid syndrome, systemic lupus erythematosus, rheumatoid arthritis, hyperprolactinemia, chromosomal problems of parents, and allergic diseases were excluded from the study. 6 women were excluded according to exclusion criteria )autoimmune diseases, History of allergic reactions, hypothyroidism).
In this study, repeated abortions meant losing pregnancy products before the 20th wk of pregnancy on at least 3 consecutive or non-consecutive occasions. 5 cc of peripheral blood was taken from each participant to determine the serum levels of IL-33, CRP, IgG, IgA, and IgM and sent to the laboratory of Ali Ibn Abi Taleb hospital, Zahedan, Iran. The blood samples were centrifuged according to the kit instructions. The serum was separated and stored in the laboratory freezer until the end of the study. Finally, after completing the number of samples, the IL-33 serum level in 2 groups was measured by enzyme-linked immunosorbent assay method (EASTBIOPHARM CO., China).
2.1. Sample size
A preliminary estimate of the study’s sample size was calculated from a previous study (16). Mean IL-33 serum levels were used, with a type I error of 0.05 and a type II error of 0.2. According to the comparison of the 2 mean formulas, each group necessarily had at least 12 participants. During the study, this number increased to 33 patients in each group.
2.2. Ethical considerations
This study was approved by the Ethical Committee of Zahedan University of Medical Sciences, Zahedan, Iran (Code: IR.ZAUMS.REC.1398.217). Participants were enrolled after obtaining informed written consent.
2.3. Statistical analysis
The Kolmogorov-Smirnov test was used to evaluate the normal distribution of data. Due to the existence of abnormal distribution in quantitative variables, Mann-Whitney non-parametric test was used to compare the level of CRP and antibodies between case and control groups. P < 0.05 was considered significant. IBM SPSS Statistics for Windows, version 22.0 (IBM Corp), was used for the analysis, 2013, IBM Corp., Armonk, New York.
3. Results
In this study, 66 women were divided into 2 groups of 33, a case group (women with a history of RPL) and 33 as a control group (including healthy women) referred to the clinic of gynecology and obstetrics, the mean age of the participants was 30.8 ± 3.80 with a maximum and minimum value of 19.35 respectively. The mean age of participants in the group with recurrent abortion (case) was 30.9 ± 3.1 yr, and for women without recurrent abortion (control) was 30.7 ± 4.4 yr (p = 0.73). Those who met the study inclusion and exclusion criteria were investigated. It should be noted that due to the lack of normal distribution of 2 variables, age (p < 0.001) and IL-33 (p = 0.01), the nonparametric test was used to compare the 2 groups. It is also indicated that the mean age of participants in this study was 30.8, with a standard deviation of 3.8. No significant difference was observed in age distribution between the case and control groups (p = 0.73).
The mean and standard deviation of IL-33 serum level in the case and control groups are shown in table I. According to the results of this study, the mean in the group of patients with recurrent miscarriage was 318.5 pg/ml with a standard deviation of 254.1, and the mean serum level of IL-33 in the group of healthy women without recurrent miscarriage was obtained as 354.2 pg/ml with standard deviation 9/255. According to table I, the mean serum level of IL-33 in the case group (318.5 pg/ml) was lower than the mean in the control group (354.22 pg/ml). Still, this difference was not statistically significant (p = 0.52, Table I).
Furthermore, the results of this study showed that CRP levels were not significantly different in case and control groups (p = 0.27). Regarding IgA and IgG, no significant difference was observed in the levels of these antibodies in the 2 groups with and without a history of recurrent miscarriage (p = 0.16, p = 0.46). While the difference in IgM level between the 2 groups was statistically significant (p < 0.001). The observed serologic patterns are detailed in table II.


4. Discussion
This study compared IL-33, CRP, IgM, IgG, and IgA in RPL and control group. As a result of this study, only IgM levels showed a statistically significant difference between the case and control groups. Recent research has shown that IL-33 is expressed in endothelial and smooth muscle cells in the placenta, chorioamniotic membranes, and umbilical cord. Also, some studies have shown that polymorphism of IL-33 was correlated with RPL risk comparing control group, and it gave further details that rs1929992 (IL-33) polymorphisms could be associated with RPL in the Iranian population (13). Yue notes that IL-33 serum levels are significantly lower in idiopathic recurrent miscarriage cases than in the control group. Activation of the IL-33 suppression of tumorigenicity 2 signaling pathway in human endometrial stromal cells has been reported to be critical for a successful pregnancy. At 6 wk gestation, a significant increase in IL-33 serum levels were observed in patients who are to be at risk for abortions compared to healthy control pregnancies (14). It seems that when interpreting IL-33 levels, consideration of the week of pregnancy is essential and should be considered. On the other hand, the larger sample size and differences in geographic area in Yue’s study should be noted in justifying the difference between the results of our study and the aforementioned study.
Finally, it can be summarized that the IL-33 serum level, at least 4-6 months after the last abortion (in the case group) and the previous live delivery (in the control group), did not show a significant difference between the 2 groups. However, due to the different results of earlier studies in this field, there is still a need for further studies. The results of the present study showed no significant difference in the IL-33 serum level between normal women without recurrent miscarriages and women with a history of recurrent miscarriages. However, the mean IL-33 serum level was slightly lower in women with a history of recurrent miscarriage, which could be a significant difference in larger samples due to the limited sample size in this study.
A protein phenotype may be related to the ethnicity and racial status of the patient; hence more extensive studies in this field are essential. Other suggestions include repetition of studies similar to the follow-up period of most patients to more accurately identify the IL-33 serum level in different conditions and times, designing new studies to identify pathways affecting changes in IL-33 serum levels outside of pregnancy. Moreover, determination and comparison of changes in IL-33 serum levels up to the 20th wk of pregnancy, determining the cytokine phenotype in patients according to their demographic conditions, determining the genotype of this cytokine in patients according to their demographic conditions, they could be suitable research fields in the future.
5. Conclusion
In conclusion, in this study, IgM was found to be significantly increased in patients with recurrent miscarriage. This relationship was not observed in IL-33 and other immunological factors. Therefore, the role of IgM as an acute inflammatory marker in recurrent miscarriage has been confirmed. It seems that different aspects of the effects of IL-33 as a predictive biomarker in PRL, should be evaluated in more comprehensive studies.
Data availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author contributions
Hossein Ali Khazaei and Farahnaz Farzaneh designed the study and conducted the research. Saeedeh Sarhadi, Javid Dehghan Haghighi, Forough Forghani, Vahid Sheikhi, Bahman Khazaei, and Lida Asadollahi, monitored, evaluated, and analyzed the results of the study. Further Saeedeh Sarhadi and Hossein Ali Khazaei reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The authors wish to express their thanks for the financial support of Zahedan University of Medical Sciences, Zahedan, Iran.
Conflict of Interest
All authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE guideline: Recurrent pregnancy loss. Hum Reprod Open 2018; 2018: hoy004. [DOI:10.1093/hropen/hoy004] [PMID] [PMCID]
2. Yalcintepe SA, Silan F, Hacivelioglu SO, Uludag A, Cosar E, Ozdemir O. Fetal VEGF genotype is more important for abortion risk than mother genotype. Int J Mol Cell Med 2014; 3: 88-94.
3. Deshmukh H, Way SS. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol 2019; 14: 185-210. [DOI:10.1146/annurev-pathmechdis-012418-012743] [PMID] [PMCID]
4. Elkarhat Z, Kindil Z, Zarouf L, Razoki L, Aboulfaraj J, Elbakay C, et al. Chromosomal abnormalities in couples with recurrent spontaneous miscarriage: A 21-year retrospective study, a report of a novel insertion, and a literature review. J Assist Reprod Genet 2019; 36: 499-507. [DOI:10.1007/s10815-018-1373-4] [PMID] [PMCID]
5. Khamees DA, Al-Ouqaili MTS. Cross-sectional study of chromosomal aberrations and immunologic factors in Iraqi couples with recurrent pregnancy loss. PeerJ 2022; 10: e12801. [DOI:10.7717/peerj.12801] [PMID] [PMCID]
6. Asgari A, Ghahremani S, Saeedi S, Kamrani E. The study of chromosomal abnormalities and heteromorphism in couples with 2 or 3 recurrent abortions in Shahid Beheshti Hospital of Hamedan. Iran J Reprod Med 2013; 11: 201-208.
7. Ticconi C, Pietropolli A, Di Simone N, Piccione E, Fazleabas A. Endometrial immune dysfunction in recurrent pregnancy loss. Int J Mol Sci 2019; 20: 5332. [DOI:10.3390/ijms20215332] [PMID] [PMCID]
8. Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol 2014; 5: 253. [DOI:10.3389/fimmu.2014.00253] [PMID] [PMCID]
9. Moghbeli M. Genetics of recurrent pregnancy loss among Iranian population. Mol Genet Genomic Med 2019; 7: e891. [DOI:10.1002/mgg3.891] [PMID] [PMCID]
10. Kamrani A, Rahmani SA, Mosapour P, Chavoshi R. Association of IL-33 gene rs16924159 polymorphism and recurrent pregnancy loss in Iranian Azeri women. Horm Mol Biol Clin Investig 2020; 41: 20200010. [DOI:10.1515/hmbci-2020-0010] [PMID]
11. Valero-Pacheco N, Tang EK, Massri N, Loia R, Chemerinski A, Wu T, et al. Maternal IL-33 critically regulates tissue remodeling and type 2 immune responses in the uterus during early pregnancy in mice. Proc Natl Acad Sci USA 2022; 119: e2123267119. [DOI:10.1073/pnas.2123267119] [PMID] [PMCID]
12. Topping V, Romero R, Than NG, Tarca AL, Xu Z, Kim SY, et al. Interleukin-33 in the human placenta. J Matern Fetal Neonatal Med 2013; 26: 327-338. [DOI:10.3109/14767058.2012.735724] [PMID] [PMCID]
13. Soheilyfar S, Nikyar T, Fathi Maroufi N, Mohebi Chamkhorami F, Amini Z, Ahmadi M, et al. Association of IL-10, IL-18, and IL-33 genetic polymorphisms with recurrent pregnancy loss risk in Iranian women. Gynecol Endocrinol 2019; 35: 342-345. [DOI:10.1080/09513590.2018.1528220] [PMID]
14. Yue J, Tong Y, Xie L, Ma T, Yang J. Genetic variant in IL-33 is associated with idiopathic recurrent miscarriage in Chinese. Sci Rep 2016; 6: 23806. [DOI:10.1038/srep23806] [PMID] [PMCID]
15. Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med 2013; 11: 154. [DOI:10.1186/1741-7015-11-154] [PMID] [PMCID]
16. Kaitu'u-Lino TJ, Tuohey L, Tong S. Maternal serum interleukin-33 and soluble ST2 across early pregnancy, and their association with miscarriage. J Reprod Immunol 2012; 95: 46-49. [DOI:10.1016/j.jri.2012.06.003] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




