Sat, Jan 31, 2026
[Archive]
Volume 22, Issue 5 (May 2024)
IJRM 2024, 22(5): 395-404 |
Back to browse issues page
Ethics code: 0449/UN2.F1/ETIK/2018
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hestiantoro A, Al Maghfira R N, Fathmasari R, Febri R R, Ongko Joyo E, Muharam R, et al . Altered expression of kisspeptin, dynorphin, and related neuropeptides in polycystic ovary syndrome: A cross-sectional study. IJRM 2024; 22 (5) :395-404
URL: http://ijrm.ir/article-1-3085-en.html
URL: http://ijrm.ir/article-1-3085-en.html
Andon Hestiantoro *1 

 , Rachellina Noor Al Maghfira2
, Rachellina Noor Al Maghfira2 

 , Ratna Fathmasari2
, Ratna Fathmasari2 

 , Ririn Rahmala Febri3
, Ririn Rahmala Febri3 

 , Ericko Ongko Joyo4
, Ericko Ongko Joyo4 

 , Raden Muharam5
, Raden Muharam5 

 , Gita Pratama5
, Gita Pratama5 

 , Anom Bowolaksono6
, Anom Bowolaksono6 




 , Rachellina Noor Al Maghfira2
, Rachellina Noor Al Maghfira2 

 , Ratna Fathmasari2
, Ratna Fathmasari2 

 , Ririn Rahmala Febri3
, Ririn Rahmala Febri3 

 , Ericko Ongko Joyo4
, Ericko Ongko Joyo4 

 , Raden Muharam5
, Raden Muharam5 

 , Gita Pratama5
, Gita Pratama5 

 , Anom Bowolaksono6
, Anom Bowolaksono6 


1- Reproductive Immunoendocrinology Division, Department of Obstetrics and Gynecology, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. & Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, University of Indonesia, Jakarta, Indonesia. , andon.hestiantoro@ui.ac.id
2- Department of Biology, Faculty of Mathematics and Natural Sciences, University of Indonesia, Depok, Indonesia.
3- Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, University of Indonesia, Jakarta, Indonesia.
4- Faculty of Medicine University of Indonesia, Jakarta, Indonesia.
5- Reproductive Immunoendocrinology Division, Department of Obstetrics and Gynecology, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. & Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, University of Indonesia, Jakarta, Indonesia.
6- Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, Universitas Indonesia ID & Cellular and Molecular Mechanisms in Biological System Research Group, Department of Biology, Faculty of Mathematics and Natural Sciences, University of Indonesia, Depok, Indonesia.
2- Department of Biology, Faculty of Mathematics and Natural Sciences, University of Indonesia, Depok, Indonesia.
3- Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, University of Indonesia, Jakarta, Indonesia.
4- Faculty of Medicine University of Indonesia, Jakarta, Indonesia.
5- Reproductive Immunoendocrinology Division, Department of Obstetrics and Gynecology, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. & Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, University of Indonesia, Jakarta, Indonesia.
6- Cluster of Human Reproduction, Fertility and Family Planning, Indonesia Medical Education and Research Institute, Universitas Indonesia ID & Cellular and Molecular Mechanisms in Biological System Research Group, Department of Biology, Faculty of Mathematics and Natural Sciences, University of Indonesia, Depok, Indonesia.
Full-Text [PDF 328 kb]
(846 Downloads)
| Abstract (HTML) (1249 Views)
1. Introduction
The identification of the kisspeptin-neurokinin-B-dynorphin (KNDy) neuron network within the hypothalamus has provided valuable insights into the intricate roles played by these neurons in the pathogenesis of polycystic ovary syndrome (PCOS). PCOS is the most common endocrinopathy in women of reproductive age. It is associated with abnormal uterine bleeding and infertility due to ovulation disorders and abnormal androgen production. Diagnostic criteria for PCOS based on the 2003 Rotterdam consensus include hyperandrogenism, oligomenorrhea/anovulation, and polycystic ovarian morphology (1). Increased pulse amplitude and frequency of gonadotropin-releasing hormone (GnRH) in the hypothalamus cause relatively persistent and non-pulsatile luteinizing hormone (LH) secretion, resulting in chronic anovulation and hyperandrogenism, which leads to PCOS (2). The amplitude and frequency of GnRH pulsatile secretion are affected by several neuropeptides, such as kisspeptin (KISS1), neurokinin-B (NKB), and dynorphin, which are expressed by the KISS1, tachykinin-3 (TAC3), and prodynorphin (PDYN) genes, respectively (3). The discovery of the KNDy neuron network in the hypothalamus helps us understand the role of these neurons in regulating GnRH secretion at puberty and during reproductive age.
Numerous studies have investigated the genetic components of PCOS (4-9); for instance, Chaudhary et al. reveal that polymorphism in several genes play a role in the steroidogenesis pathway results in the phenotypic expression of PCOS (4). In another study, Farsimadan et al. found that polymorphisms within KISS1 were associated with an increased risk of PCOS development (5). A recent meta-analysis also reported higher serum KISS1 levels in subjects with PCOS than those without (6). Any disturbance in KISS1 regulation also can lead to a metabolic imbalance, such as obesity (7). Moreover, hormonal profile differences in women with PCOS are still unclear (8, 9). Differences in hormone profiles in PCOS women may be related to differences in leptin (LEP) and neuropeptide-Y (NPY) gene expression levels. These recent studies enhance our understanding of the genetic basis of PCOS and shed light on the potential contributions of these genes to the development of the syndrome.
While some studies have measured the serum levels of neuropeptides in PCOS women (4-9), few studies have focused on the expression of the genes encoding these neuropeptides in peripheral blood (6, 10). Previous studies have stated that the mRNA expressions of these genes were detected in peripheral blood (11-13). Since the secretion of KISS1 is influenced by many factors, such as dynorphin, NKB, LEP, and NPY, it would be interesting to study their role in the context of women with PCOS.
Knowledge of gene expression levels in women with PCOS is required to identify gene variation and its effect on phenotypic hormone variations as a basis for further research. This study aimed to compare KISS1, PDYN, TAC3, LEP, and NPY expression levels in women with and without PCOS.
2. Materials and Methods
2.1. Subjects
In this cross-sectional study, we consecutively included 40 women of reproductive age (15-49 yr), consisting of 20 diagnosed with PCOS and 20 without PCOS, who were referred to Yamin Kencana Clinic, Cipto Mangunkusumo hospital, Jakarta, Indonesia from August to December 2022. The diagnosis of PCOS was determined using the Rotterdam 2003 criteria, which includes the presence of at least 2 of the following: a) oligo-ovulation or anovulation, b) clinical or biochemical signs of hyperandrogenism, and c) polycystic ovaries on ultrasound examination (14). Women diagnosed with PCOS were included in the PCOS group, while women without PCOS served as the control group (n = 20/each). Participants were excluded if they were either pregnant, on medications that could alter metabolic parameters for the past 2 months, or had endocrine abnormalities.
2.2. Sample size
The sample size was calculated using the Lemeshow formula. The prevalence of PCOS in Indonesia is 8-10%, so the proportion value of 10% is used. The percentage or errors used in this study was 5% (α = 5%). The precision used in this was 20% (d = 0.2). Based on these calculations, 10 samples are sufficient for each group. However, we used 20 participants in each group for the power of our study.
2.3. Sample collection and processing
Blood samples were collected at the Yamin Kencana Clinic, Cipto Mangunkusumo hospital, Jakarta, Indonesia following the World Health Organization standard blood collection procedure (15). Sample processing was conducted at the Human Reproductive, Infertility, and Family Planning Laboratory of the Indonesian Medical Education and Research Institute, Faculty of Medicine, University of Indonesia, Jakarta. The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Gene quantification was carried out by the quantitative polymerase chain reaction method using the SensiFAST SYBR Green PCR Master Mix [Bioline] kit and Prime Pro 48 quantitative polymerase chain reaction system from Cole-Parmer Ltd [TechneTM] polymerase chain reaction machine based on the given protocols. The housekeeping gene used in this study was β-actin. The primary base sequence of the LEP, NPY, KISS1, TAC3, PDYN, and β-actin genes was obtained from the National Center for Biotechnology Information database. Relative gene expressions of each gene were obtained by processing the cycle threshold (Ct) value with the Livak method (16).
The Livak method calculates the relative gene expression by comparing the expression of target genes with reference genes. The reference gene Ct normalized target gene Ct and control gene Ct, resulting in ΔCttarget and ΔCtcontrol, respectively. Then ΔCttarget was subtracted against the ΔCtcontrol to obtain the ΔΔCt. The value obtained was a relative comparison to the control using a 2-based exponential (2-ΔΔCt).
In addition to assessing relative gene expression, this study measured hormone levels as demographic variables. Specifically, testosterone, sex hormone-binding globulin (SHBG), and free androgen index were measured to evaluate hormonal profiles in women with and without PCOS. Peripheral venous samples were collected and subjected to centrifugation to obtain serum for the quantitative measurement of testosterone and SHBG concentrations. The enzyme-linked immunosorbent assay method was employed for this purpose. Commercial fluorescence enzyme immunoassay kits were used for the determination of serum testosterone (ST AIA-Pack Testosterone, TOSOH, Japan, Cat. No. 0025204) and SHBG (ST AIA-Pack SHBG, TOSOH Bioscience, Japan), following the manufacturer's instructions. The assay utilized a sandwich-type approach with a pre-coated 96-well plate and enzyme-labeled secondary antibodies. A sample volume of 300 µl was required for analysis. Before measurement, each sample was labeled and prepared in a sample cup and a test cup. The prepared sample and test cups were then inserted into the TOSOH instrument. The free androgen index was calculated as the ratio of total testosterone to SHBG and reported as a percentage. Baseline demographic characteristics and hormonal profiles were compared to see if there were any significant differences between groups that could interfere with the interpretation of the study results. Potential confounding variables, such as age, body mass index, and hormonal level, were identified. Potential confounding variables due to disease and metabolic abnormalities were also controlled using the exclusion criteria.
2.4. Ethical considerations
All research participants gave their permission to be part of this study in the form of written informed consent. The Ethics Committee of the Indonesia and Cipto Mangunkusumo hospital, Jakarta, Indonesia has approved the study (Code: 0449/UN2.F1/ETIK/2018).
2.5. Statistical analysis
Data analysis was performed using SPSS, version 24.0 (IBM Corp., Armonk, New York, USA). Continuous data were presented as mean ± standard deviation (SD) or median and interquartile ranges (IQR) as appropriate. The Student’s t test was used to compare the 2 groups' means if the data distribution was normal. In cases in which the data distribution was not normal, the Mann-Whitney test was used for the comparative analysis. Pearson's correlation analysis was performed to analyze the correlation of the relative expression of KISS1 to the KISS1/PDYN and KISS1/TAC3 ratio with a significant level of < 0.05.
3. Results
3.1. Demographic and hormonal profiles
For this study, a total of 40 women were consecutively screened for eligibility based on the inclusion and exclusion criteria, and none of them were found to be ineligible or refused to participate. Finally, the analysis included 20 women in the PCOS and 20 women in the control group.
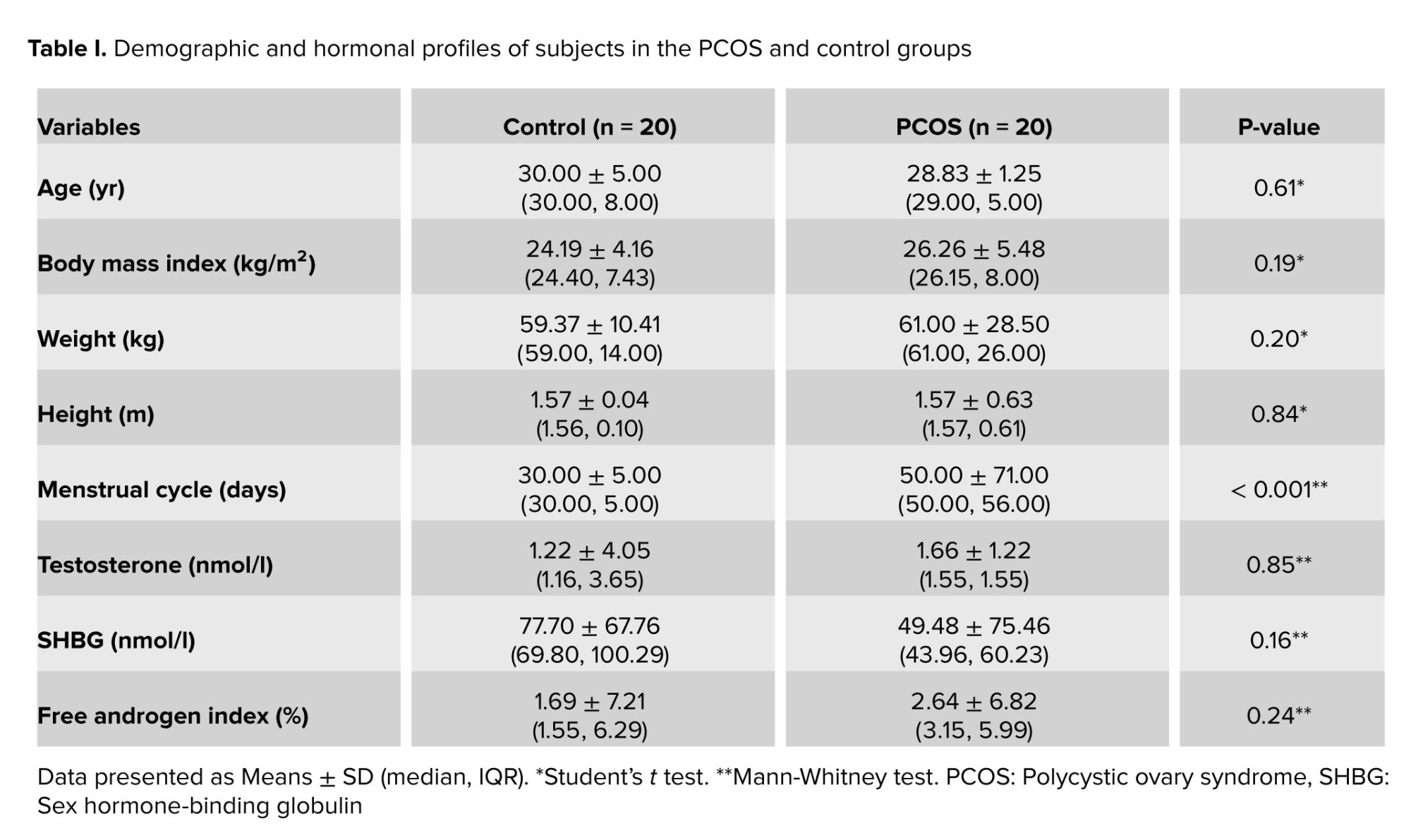
The mean age of the participants was 30.00 ± 5.00 yr in the PCOS and 28.83 ± 1.25 yr in the control group. The 2 groups had no significant differences in age, body mass index, and hormonal profiles, except for a longer menstrual cycle in the PCOS group. Moreover, no significant difference was observed in the total testosterone levels, SHBG levels, and free androgen index in the 2 groups (Table I).
3.2. Comparison of KISS1, PDYN, TAC3, LEP, and NPY mRNA levels
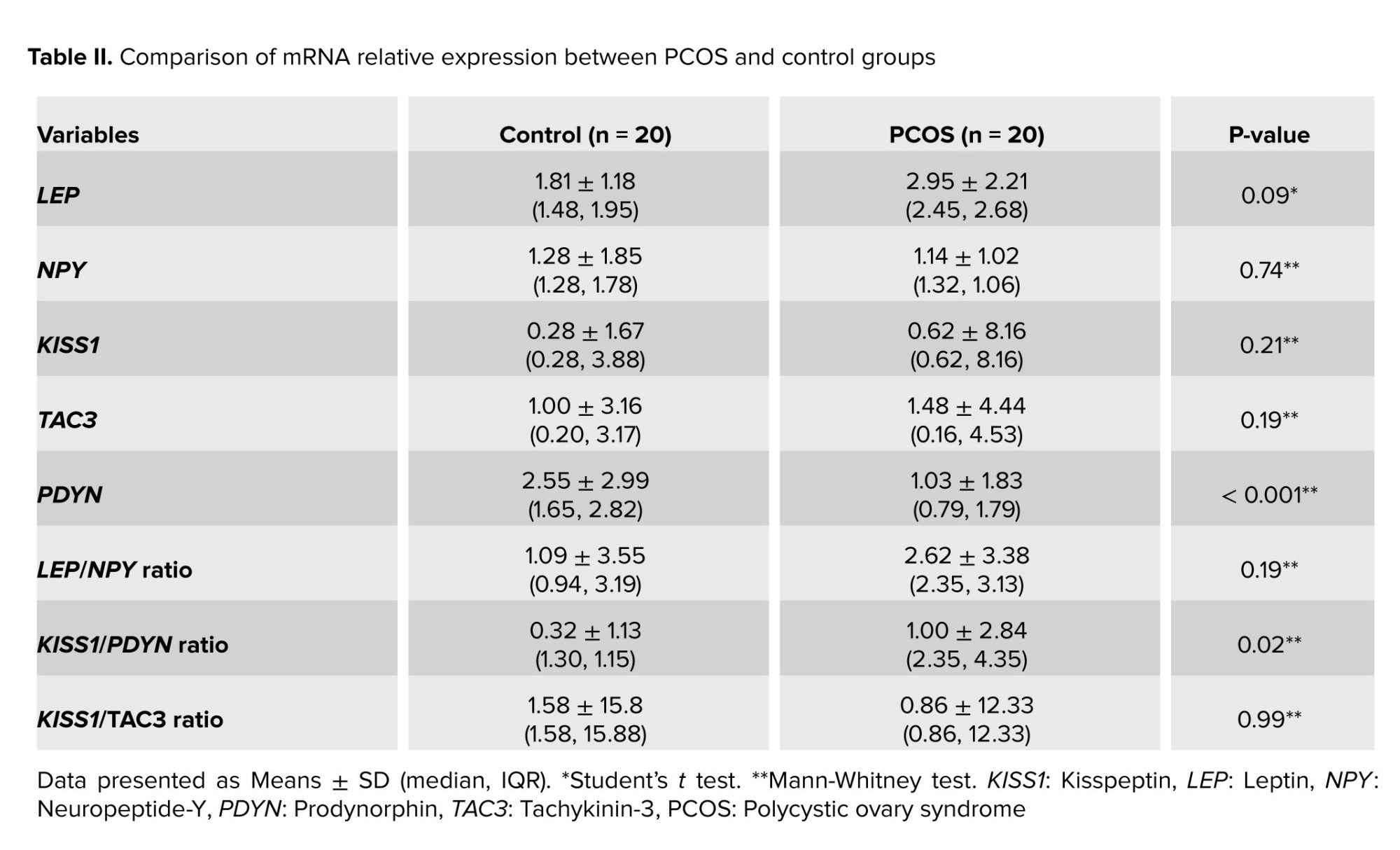
No significant differences were revealed in the relative expression of the KISS1, TAC3, LEP, NPY, kisspeptin-to-tachykinin-3 ratio (KISS/TAC3), and LEP-to-NPY ratio (LEP/NPY) between the PCOS and control groups (Table II). However, PDYN relative expression was significantly lower in the PCOS group than in the control group (0.79 [1.79] vs. 1.65 [2.82]; p < 0.001). This was followed by a significant difference in the kisspeptin-to-dynorphin (KISS1/PDYN) ratio (2.35 [4.35] vs. 1.30 [1.15]; p = 0.02).
3.3. Correlation between KISS1 and its ratio to dynorphin and NKB
We sought the correlation of the relative expression of KISS1 to the KISS1/PDYN and KISS1/TAC3 ratio to reveal the controlled balance of these neuropeptides since KISS1 in the hypothalamus is affected by the inhibitory effect of dynorphin and the stimulatory effect of NKB. By comparing the relative expression of KISS1 against dynorphin and NKB, we get a ratio that reflects the controlled balance of these 2 neuropeptides in the hypothalamus.
Correlation analysis was performed separately for the control and PCOS groups (Figure 1). Pearson's correlation test found a significant positive correlation between KISS1 mRNA expression and the KISS1/PDYN ratio. The correlation of KISS1 and KISS1/PDYN ratio was revealed to be stronger in the PCOS group (Pearson R = 0.93, p < 0.001) than in the control group (Pearson R = 0.66, p < 0.001). On the other hand, a positive and significant correlation was observed between KISS1 and the KISS1/TAC3 ratio in the control group (Pearson R = 0.56, p = 0.01) but was not found in the PCOS group (Pearson R = 0.22, p = 0.35).

4. Discussion
In this study, we could not establish a higher KISS1 expression in the PCOS group than in the control group. The level of protein or peptide contained in the body significantly and positively correlated with the mRNA expression of its genes (17). Despite the absence of studies that have determined the KISS1 mRNA expression in PCOS women, the results of our study are in line with the studies on KISS1 serum levels. Several recent publications reported higher serum levels of KISS1 in subjects with PCOS than in controls (6, 18-22), while 2 reported the contrary (10, 23). Ibrahim et al. report that serum levels of KISS1 were significantly higher in PCOS women than in the control group (22).
Previous research suggests that KISS1 is secreted in a pulsatile manner from the hypothalamus and peripheral organs such as the pancreas, ovaries, adipose tissue, and adrenal glands (24). In contrast to KISS1's intrahypothalamic functions, little is known about KISS1's physiological importance in extrahypothalamic tissues. KISS1 appears to function in the peripheral systems that control reproduction in mammals, but more research is needed (25). The current knowledge does not rule out the potential that extrahypothalamic KISS1 acts as an autocrine/paracrine factor in the peripheral tissues to fulfill its physiological function. Furthermore, KISS1 neurons' development and function can be influenced by metabolic factors at various stages of development. The metabolic changes that KISS1 neurons undergo can be temporary or permanent (for example, epigenetic modifications) (26). This makes it evident that peripheral changes can affect the increase in hypothalamic KISS1 production in PCOS.
This study's foremost, and novel finding was that dynorphin expression was lower in typical PCOS women than in controls. These results demonstrated a significantly higher KISS1/PDYN ratio in PCOS women than in controls. To our knowledge, a similar finding regarding lower dynorphin levels in PCOS women has not been published before. Research on these genes in the pathophysiology of PCOS has not been widely carried out. Although no studies have examined dynorphin levels or the KISS1/PDYN ratio in PCOS women, our findings align with the basic theory of regulatory systems in which KNDy neurons regulate GnRH pulsatility. It is known that KISS1, while the inhibitory function of dynorphin on KISS1, works to control KISS1 secretion with NKB as a balance regulator. Thus, reduced dynorphin production will undoubtedly have an impact on increasing KISS1 secretion, which in turn causes an increase in the frequency and amplitude of the pulsatility of GnRH secretion (27).
Furthermore, we also found a significant correlation between the KISS1 and KISS1/PDYN ratio, which was stronger in the PCOS groups than in the control groups. This is thought to be related to the reduction of dynorphin expression, which then affects the increase in GnRH pulsatility as the pathophysiology of PCOS. Meanwhile, the correlation between KISS1 and KISS1/TAC3 was weaker and non-significant in the PCOS groups. These different correlation findings indicate that increased KISS1 in PCOS women is not caused by increased NKB expression. However, it is more strongly associated with diminished dynorphin expression. Therefore, the diminished dynorphin expression in PCOS women seems to have a significant effect on KISS1, proven by the finding of a significant correlation, thus masking the effect of NKB, indicated by the correlation of KISS1 and KISS1/TAC3 ratio, which was not significant in the PCOS groups but in the control groups.
Another critical pathological feature of PCOS is the disruption of progesterone-negative feedback to the GnRH neuronal network that inhibits LH and GnRH pulsation frequency (28). Progesterone receptors are not generally found on GnRH neurons, so it is suspected that another system transmits this action. Arcuate neurons express dynorphin, which exerts an inhibitory effect on GnRH and LH expression and is implied to mediate progesterone-negative feedback (29). The mechanism of disruption of progesterone-negative feedback occurs due to exposure to prenatal androgens in sheep, where there is a reduction in dynorphin expression without a change in KISS1 expression, which causes a decrease in inhibition of KNDy neurons to progesterone, even though progesterone sensitivity is not impaired (30). Nevertheless, these findings do not necessarily apply to humans because the study was done on sheep. Our findings support the hypothesis that decreased dynorphin expression may disrupt negative progesterone feedback. This is indicated by the significant finding of lower PDYN expression in PCOS women in this study.
The main limitation of this study is that there is no evidence to clarify that alterations in mRNA levels of KNDy peptides in the blood accurately reflect the levels of gene expression in the hypothalamus. To obtain a more meaningful understanding of PCOS, the findings of this study must be supported by future studies that directly tie blood levels of KNDy peptides to those in the hypothalamus. Moreover, this study only observed a small number of subjects. Increasing the sample size in future studies would allow better intergroup analyses. Furthermore, peripheral blood testing is expected to be a potential non-invasive alternative diagnosis for PCOS.
5. Conclusion
The low expression level of the mRNA gene encoding dynorphin is highly specific for PCOS. These findings were followed by a high KISS1/PDYN ratio specific to the PCOS group. Furthermore, a significant positive correlation was established between KISS1 and the KISS1/PDYN ratio. This study indicates that the expression of these neuropeptide genes in peripheral blood is associated with PCOS.
Data availability
Data supporting the findings of this study is available at RIN Dataverse at https://data.brin.go.id, reference number hdl: 20.500.12690/RIN/BK50G5.
Author contributions
Andon Hestiantoro contributed substantially to the conception and design of the study, subject recruitment, interpretation of data, drafting the manuscript, and final approval of the version to be published. Rachellina Noor Al Maghfira and Ratna Fathmasari contributed substantially to laboratory workup, acquisition of data, and data completion. Ririn Rahmala Febri contributed substantially to laboratory preparation, laboratory workup, sampling management, laboratory management, and data analysis. Ericko Ongko Joyo contributed substantially to data analysis, drafting the article, and finalization of the article. Raden Muharam and Gita Pratama contributed substantially in revising it critically for important intellectual content. Anom Bowolaksono contributed substantially in supervising laboratory workup, interpretation of data, design of laboratory workup, and revising the article. Each author agrees to be personally accountable for the entire work.
Acknowledgments
The authors would like to thank Kresna Mutia, Pritta Ameilia Iffanolida, Naylah Muna, and Oki Riayati for the laboratory preparation and data analysis. University of Indonesia supported this work (Grant number: NKB-1590/UN2.RST/HKP.05.00/2020).
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (180 Views)
1. Introduction
The identification of the kisspeptin-neurokinin-B-dynorphin (KNDy) neuron network within the hypothalamus has provided valuable insights into the intricate roles played by these neurons in the pathogenesis of polycystic ovary syndrome (PCOS). PCOS is the most common endocrinopathy in women of reproductive age. It is associated with abnormal uterine bleeding and infertility due to ovulation disorders and abnormal androgen production. Diagnostic criteria for PCOS based on the 2003 Rotterdam consensus include hyperandrogenism, oligomenorrhea/anovulation, and polycystic ovarian morphology (1). Increased pulse amplitude and frequency of gonadotropin-releasing hormone (GnRH) in the hypothalamus cause relatively persistent and non-pulsatile luteinizing hormone (LH) secretion, resulting in chronic anovulation and hyperandrogenism, which leads to PCOS (2). The amplitude and frequency of GnRH pulsatile secretion are affected by several neuropeptides, such as kisspeptin (KISS1), neurokinin-B (NKB), and dynorphin, which are expressed by the KISS1, tachykinin-3 (TAC3), and prodynorphin (PDYN) genes, respectively (3). The discovery of the KNDy neuron network in the hypothalamus helps us understand the role of these neurons in regulating GnRH secretion at puberty and during reproductive age.
Numerous studies have investigated the genetic components of PCOS (4-9); for instance, Chaudhary et al. reveal that polymorphism in several genes play a role in the steroidogenesis pathway results in the phenotypic expression of PCOS (4). In another study, Farsimadan et al. found that polymorphisms within KISS1 were associated with an increased risk of PCOS development (5). A recent meta-analysis also reported higher serum KISS1 levels in subjects with PCOS than those without (6). Any disturbance in KISS1 regulation also can lead to a metabolic imbalance, such as obesity (7). Moreover, hormonal profile differences in women with PCOS are still unclear (8, 9). Differences in hormone profiles in PCOS women may be related to differences in leptin (LEP) and neuropeptide-Y (NPY) gene expression levels. These recent studies enhance our understanding of the genetic basis of PCOS and shed light on the potential contributions of these genes to the development of the syndrome.
While some studies have measured the serum levels of neuropeptides in PCOS women (4-9), few studies have focused on the expression of the genes encoding these neuropeptides in peripheral blood (6, 10). Previous studies have stated that the mRNA expressions of these genes were detected in peripheral blood (11-13). Since the secretion of KISS1 is influenced by many factors, such as dynorphin, NKB, LEP, and NPY, it would be interesting to study their role in the context of women with PCOS.
Knowledge of gene expression levels in women with PCOS is required to identify gene variation and its effect on phenotypic hormone variations as a basis for further research. This study aimed to compare KISS1, PDYN, TAC3, LEP, and NPY expression levels in women with and without PCOS.
2. Materials and Methods
2.1. Subjects
In this cross-sectional study, we consecutively included 40 women of reproductive age (15-49 yr), consisting of 20 diagnosed with PCOS and 20 without PCOS, who were referred to Yamin Kencana Clinic, Cipto Mangunkusumo hospital, Jakarta, Indonesia from August to December 2022. The diagnosis of PCOS was determined using the Rotterdam 2003 criteria, which includes the presence of at least 2 of the following: a) oligo-ovulation or anovulation, b) clinical or biochemical signs of hyperandrogenism, and c) polycystic ovaries on ultrasound examination (14). Women diagnosed with PCOS were included in the PCOS group, while women without PCOS served as the control group (n = 20/each). Participants were excluded if they were either pregnant, on medications that could alter metabolic parameters for the past 2 months, or had endocrine abnormalities.
2.2. Sample size
The sample size was calculated using the Lemeshow formula. The prevalence of PCOS in Indonesia is 8-10%, so the proportion value of 10% is used. The percentage or errors used in this study was 5% (α = 5%). The precision used in this was 20% (d = 0.2). Based on these calculations, 10 samples are sufficient for each group. However, we used 20 participants in each group for the power of our study.
2.3. Sample collection and processing
Blood samples were collected at the Yamin Kencana Clinic, Cipto Mangunkusumo hospital, Jakarta, Indonesia following the World Health Organization standard blood collection procedure (15). Sample processing was conducted at the Human Reproductive, Infertility, and Family Planning Laboratory of the Indonesian Medical Education and Research Institute, Faculty of Medicine, University of Indonesia, Jakarta. The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Gene quantification was carried out by the quantitative polymerase chain reaction method using the SensiFAST SYBR Green PCR Master Mix [Bioline] kit and Prime Pro 48 quantitative polymerase chain reaction system from Cole-Parmer Ltd [TechneTM] polymerase chain reaction machine based on the given protocols. The housekeeping gene used in this study was β-actin. The primary base sequence of the LEP, NPY, KISS1, TAC3, PDYN, and β-actin genes was obtained from the National Center for Biotechnology Information database. Relative gene expressions of each gene were obtained by processing the cycle threshold (Ct) value with the Livak method (16).
The Livak method calculates the relative gene expression by comparing the expression of target genes with reference genes. The reference gene Ct normalized target gene Ct and control gene Ct, resulting in ΔCttarget and ΔCtcontrol, respectively. Then ΔCttarget was subtracted against the ΔCtcontrol to obtain the ΔΔCt. The value obtained was a relative comparison to the control using a 2-based exponential (2-ΔΔCt).
In addition to assessing relative gene expression, this study measured hormone levels as demographic variables. Specifically, testosterone, sex hormone-binding globulin (SHBG), and free androgen index were measured to evaluate hormonal profiles in women with and without PCOS. Peripheral venous samples were collected and subjected to centrifugation to obtain serum for the quantitative measurement of testosterone and SHBG concentrations. The enzyme-linked immunosorbent assay method was employed for this purpose. Commercial fluorescence enzyme immunoassay kits were used for the determination of serum testosterone (ST AIA-Pack Testosterone, TOSOH, Japan, Cat. No. 0025204) and SHBG (ST AIA-Pack SHBG, TOSOH Bioscience, Japan), following the manufacturer's instructions. The assay utilized a sandwich-type approach with a pre-coated 96-well plate and enzyme-labeled secondary antibodies. A sample volume of 300 µl was required for analysis. Before measurement, each sample was labeled and prepared in a sample cup and a test cup. The prepared sample and test cups were then inserted into the TOSOH instrument. The free androgen index was calculated as the ratio of total testosterone to SHBG and reported as a percentage. Baseline demographic characteristics and hormonal profiles were compared to see if there were any significant differences between groups that could interfere with the interpretation of the study results. Potential confounding variables, such as age, body mass index, and hormonal level, were identified. Potential confounding variables due to disease and metabolic abnormalities were also controlled using the exclusion criteria.
2.4. Ethical considerations
All research participants gave their permission to be part of this study in the form of written informed consent. The Ethics Committee of the Indonesia and Cipto Mangunkusumo hospital, Jakarta, Indonesia has approved the study (Code: 0449/UN2.F1/ETIK/2018).
2.5. Statistical analysis
Data analysis was performed using SPSS, version 24.0 (IBM Corp., Armonk, New York, USA). Continuous data were presented as mean ± standard deviation (SD) or median and interquartile ranges (IQR) as appropriate. The Student’s t test was used to compare the 2 groups' means if the data distribution was normal. In cases in which the data distribution was not normal, the Mann-Whitney test was used for the comparative analysis. Pearson's correlation analysis was performed to analyze the correlation of the relative expression of KISS1 to the KISS1/PDYN and KISS1/TAC3 ratio with a significant level of < 0.05.
3. Results
3.1. Demographic and hormonal profiles
For this study, a total of 40 women were consecutively screened for eligibility based on the inclusion and exclusion criteria, and none of them were found to be ineligible or refused to participate. Finally, the analysis included 20 women in the PCOS and 20 women in the control group.
The mean age of the participants was 30.00 ± 5.00 yr in the PCOS and 28.83 ± 1.25 yr in the control group. The 2 groups had no significant differences in age, body mass index, and hormonal profiles, except for a longer menstrual cycle in the PCOS group. Moreover, no significant difference was observed in the total testosterone levels, SHBG levels, and free androgen index in the 2 groups (Table I).
3.2. Comparison of KISS1, PDYN, TAC3, LEP, and NPY mRNA levels
No significant differences were revealed in the relative expression of the KISS1, TAC3, LEP, NPY, kisspeptin-to-tachykinin-3 ratio (KISS/TAC3), and LEP-to-NPY ratio (LEP/NPY) between the PCOS and control groups (Table II). However, PDYN relative expression was significantly lower in the PCOS group than in the control group (0.79 [1.79] vs. 1.65 [2.82]; p < 0.001). This was followed by a significant difference in the kisspeptin-to-dynorphin (KISS1/PDYN) ratio (2.35 [4.35] vs. 1.30 [1.15]; p = 0.02).
3.3. Correlation between KISS1 and its ratio to dynorphin and NKB
We sought the correlation of the relative expression of KISS1 to the KISS1/PDYN and KISS1/TAC3 ratio to reveal the controlled balance of these neuropeptides since KISS1 in the hypothalamus is affected by the inhibitory effect of dynorphin and the stimulatory effect of NKB. By comparing the relative expression of KISS1 against dynorphin and NKB, we get a ratio that reflects the controlled balance of these 2 neuropeptides in the hypothalamus.
Correlation analysis was performed separately for the control and PCOS groups (Figure 1). Pearson's correlation test found a significant positive correlation between KISS1 mRNA expression and the KISS1/PDYN ratio. The correlation of KISS1 and KISS1/PDYN ratio was revealed to be stronger in the PCOS group (Pearson R = 0.93, p < 0.001) than in the control group (Pearson R = 0.66, p < 0.001). On the other hand, a positive and significant correlation was observed between KISS1 and the KISS1/TAC3 ratio in the control group (Pearson R = 0.56, p = 0.01) but was not found in the PCOS group (Pearson R = 0.22, p = 0.35).



4. Discussion
In this study, we could not establish a higher KISS1 expression in the PCOS group than in the control group. The level of protein or peptide contained in the body significantly and positively correlated with the mRNA expression of its genes (17). Despite the absence of studies that have determined the KISS1 mRNA expression in PCOS women, the results of our study are in line with the studies on KISS1 serum levels. Several recent publications reported higher serum levels of KISS1 in subjects with PCOS than in controls (6, 18-22), while 2 reported the contrary (10, 23). Ibrahim et al. report that serum levels of KISS1 were significantly higher in PCOS women than in the control group (22).
Previous research suggests that KISS1 is secreted in a pulsatile manner from the hypothalamus and peripheral organs such as the pancreas, ovaries, adipose tissue, and adrenal glands (24). In contrast to KISS1's intrahypothalamic functions, little is known about KISS1's physiological importance in extrahypothalamic tissues. KISS1 appears to function in the peripheral systems that control reproduction in mammals, but more research is needed (25). The current knowledge does not rule out the potential that extrahypothalamic KISS1 acts as an autocrine/paracrine factor in the peripheral tissues to fulfill its physiological function. Furthermore, KISS1 neurons' development and function can be influenced by metabolic factors at various stages of development. The metabolic changes that KISS1 neurons undergo can be temporary or permanent (for example, epigenetic modifications) (26). This makes it evident that peripheral changes can affect the increase in hypothalamic KISS1 production in PCOS.
This study's foremost, and novel finding was that dynorphin expression was lower in typical PCOS women than in controls. These results demonstrated a significantly higher KISS1/PDYN ratio in PCOS women than in controls. To our knowledge, a similar finding regarding lower dynorphin levels in PCOS women has not been published before. Research on these genes in the pathophysiology of PCOS has not been widely carried out. Although no studies have examined dynorphin levels or the KISS1/PDYN ratio in PCOS women, our findings align with the basic theory of regulatory systems in which KNDy neurons regulate GnRH pulsatility. It is known that KISS1, while the inhibitory function of dynorphin on KISS1, works to control KISS1 secretion with NKB as a balance regulator. Thus, reduced dynorphin production will undoubtedly have an impact on increasing KISS1 secretion, which in turn causes an increase in the frequency and amplitude of the pulsatility of GnRH secretion (27).
Furthermore, we also found a significant correlation between the KISS1 and KISS1/PDYN ratio, which was stronger in the PCOS groups than in the control groups. This is thought to be related to the reduction of dynorphin expression, which then affects the increase in GnRH pulsatility as the pathophysiology of PCOS. Meanwhile, the correlation between KISS1 and KISS1/TAC3 was weaker and non-significant in the PCOS groups. These different correlation findings indicate that increased KISS1 in PCOS women is not caused by increased NKB expression. However, it is more strongly associated with diminished dynorphin expression. Therefore, the diminished dynorphin expression in PCOS women seems to have a significant effect on KISS1, proven by the finding of a significant correlation, thus masking the effect of NKB, indicated by the correlation of KISS1 and KISS1/TAC3 ratio, which was not significant in the PCOS groups but in the control groups.
Another critical pathological feature of PCOS is the disruption of progesterone-negative feedback to the GnRH neuronal network that inhibits LH and GnRH pulsation frequency (28). Progesterone receptors are not generally found on GnRH neurons, so it is suspected that another system transmits this action. Arcuate neurons express dynorphin, which exerts an inhibitory effect on GnRH and LH expression and is implied to mediate progesterone-negative feedback (29). The mechanism of disruption of progesterone-negative feedback occurs due to exposure to prenatal androgens in sheep, where there is a reduction in dynorphin expression without a change in KISS1 expression, which causes a decrease in inhibition of KNDy neurons to progesterone, even though progesterone sensitivity is not impaired (30). Nevertheless, these findings do not necessarily apply to humans because the study was done on sheep. Our findings support the hypothesis that decreased dynorphin expression may disrupt negative progesterone feedback. This is indicated by the significant finding of lower PDYN expression in PCOS women in this study.
The main limitation of this study is that there is no evidence to clarify that alterations in mRNA levels of KNDy peptides in the blood accurately reflect the levels of gene expression in the hypothalamus. To obtain a more meaningful understanding of PCOS, the findings of this study must be supported by future studies that directly tie blood levels of KNDy peptides to those in the hypothalamus. Moreover, this study only observed a small number of subjects. Increasing the sample size in future studies would allow better intergroup analyses. Furthermore, peripheral blood testing is expected to be a potential non-invasive alternative diagnosis for PCOS.
5. Conclusion
The low expression level of the mRNA gene encoding dynorphin is highly specific for PCOS. These findings were followed by a high KISS1/PDYN ratio specific to the PCOS group. Furthermore, a significant positive correlation was established between KISS1 and the KISS1/PDYN ratio. This study indicates that the expression of these neuropeptide genes in peripheral blood is associated with PCOS.
Data availability
Data supporting the findings of this study is available at RIN Dataverse at https://data.brin.go.id, reference number hdl: 20.500.12690/RIN/BK50G5.
Author contributions
Andon Hestiantoro contributed substantially to the conception and design of the study, subject recruitment, interpretation of data, drafting the manuscript, and final approval of the version to be published. Rachellina Noor Al Maghfira and Ratna Fathmasari contributed substantially to laboratory workup, acquisition of data, and data completion. Ririn Rahmala Febri contributed substantially to laboratory preparation, laboratory workup, sampling management, laboratory management, and data analysis. Ericko Ongko Joyo contributed substantially to data analysis, drafting the article, and finalization of the article. Raden Muharam and Gita Pratama contributed substantially in revising it critically for important intellectual content. Anom Bowolaksono contributed substantially in supervising laboratory workup, interpretation of data, design of laboratory workup, and revising the article. Each author agrees to be personally accountable for the entire work.
Acknowledgments
The authors would like to thank Kresna Mutia, Pritta Ameilia Iffanolida, Naylah Muna, and Oki Riayati for the laboratory preparation and data analysis. University of Indonesia supported this work (Grant number: NKB-1590/UN2.RST/HKP.05.00/2020).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Neven ACH, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: Diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med 2018; 36: 5-12. [DOI:10.1055/s-0038-1668085] [PMID]
2. McCartney ChR, Campbell RE. Abnormal GnRH pulsatility in polycystic ovary syndrome: Recent insights. Curr Opin Endocr Metab Res 2020; 12: 78-84. [DOI:10.1016/j.coemr.2020.04.005] [PMID] [PMCID]
3. Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A 2021; 118: e2009156118. [DOI:10.1073/pnas.2009156118] [PMID] [PMCID]
4. Chaudhary H, Patel J, Jain NK, Joshi R. The role of polymorphism in various potential genes on polycystic ovary syndrome susceptibility and pathogenesis. J Ovarian Res 2021; 14: 125. [DOI:10.1186/s13048-021-00879-w] [PMID] [PMCID]
5. Farsimadan M, Moammadzadeh Ghosi F, Takamoli S, Vaziri H. Association analysis of KISS1 polymorphisms and haplotypes with polycystic ovary syndrome. Br J Biomed Sci 2021; 78: 201-205. [DOI:10.1080/09674845.2020.1864109] [PMID]
6. Liu J, Qu T, Li Z, Yu L, Zhang S, Yuan D, et al. Serum kisspeptin levels in polycystic ovary syndrome: A meta-analysis. J Obstet Gynaecol Res 2021; 47: 2157-2165. [DOI:10.1111/jog.14767] [PMID]
7. Dudek M, Ziarniak K, Sliwowska JH. Kisspeptin and metabolism: The brain and beyond. Front Endocrinol 2018; 9: 145. [DOI:10.3389/fendo.2018.00145] [PMID] [PMCID]
8. Koseci T, Kaya O, Haksoyler V, Yildirim DD, Sezer K. Investigation of the relationship between insulin resistance and neuropeptide Y levels in polycystic ovary syndrome. Marmara Med J 2019; 32: 1-6. [DOI:10.5472/marumj.500032]
9. Rodriguez Paris V, Bertoldo M. The mechanism of androgen actions in PCOS etiology. Med Sci 2019; 7: 89. [DOI:10.3390/medsci7090089] [PMID] [PMCID]
10. Emekci Ozay O, Ozay AC, Acar B, Cagliyan E, Seçil M, Küme T. Role of kisspeptin in polycystic ovary syndrome (PCOS). Gynecol Endocrinol 2016; 32: 718-722. [DOI:10.3109/09513590.2016.1161019] [PMID]
11. Chen L, Liu M, Ji J, Lin W, Shan F, Liu H. Diagnostic accuracy of peripheral blood Kisspeptin mRNA and plasma CA125 protein for detection of epithelial ovarian cancer in patients who have ever been pregnant. Neoplasma 2016; 63: 999-1006. [DOI:10.4149/neo_2016_620] [PMID]
12. Shahkarami K, Vousooghi N, Golab F, Mohsenzadeh A, Baharvand P, Sadat-Shirazi MS, et al. Evaluation of dynorphin and kappa-opioid receptor level in the human blood lymphocytes and plasma: Possible role as a biomarker in severe opioid use disorder. Drug Alcohol Depend 2019; 205: 107638. [DOI:10.1016/j.drugalcdep.2019.107638] [PMID]
13. Kolić I, Stojković L, Dinčić E, Jovanović I, Stanković A, Živković M. Expression of LEP, LEPR and PGC1A genes is altered in peripheral blood mononuclear cells of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol 2020; 338: 577090. [DOI:10.1016/j.jneuroim.2019.577090] [PMID]
14. Chang S, Dunaif A. Diagnosis of polycystic ovary syndrome: Which criteria to use and when? Endocrinol Metab Clin North Am 2021; 50: 11-23. [DOI:10.1016/j.ecl.2020.10.002] [PMID] [PMCID]
15. Lima-Oliveira G, Lippi G, Salvagno GL, Picheth G, Guidi GC. Laboratory diagnostics and quality of blood collection. J Med Biochem 2015; 34: 288-294. [DOI:10.2478/jomb-2014-0043] [PMID] [PMCID]
16. Ganger MT, Dietz GD, Ewing SJ. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinformatics 2017; 18: 534. [DOI:10.1186/s12859-017-1949-5] [PMID] [PMCID]
17. Payne SH. The utility of protein and mRNA correlation. Trends Biochem Sci 2015; 40: 1-3. [DOI:10.1016/j.tibs.2014.10.010] [PMID] [PMCID]
18. Tang R, Ding X, Zhu J. Kisspeptin and polycystic ovary syndrome. Front Endocrinol 2019; 10: 298. [DOI:10.3389/fendo.2019.00298] [PMID] [PMCID]
19. Gorkem U, Togrul C, Arslan E, Sargin Oruc A, Buyukkayaci Duman N. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol 2018; 34: 157-160. [DOI:10.1080/09513590.2017.1379499] [PMID]
20. Umayal B, Jayakody SN, Chandrasekharan NV, Wijesundera WS, Wijeyaratne CN. Polycystic ovary syndrome (PCOS) and kisspeptin: A Sri Lankan study. J Postgrad Med 2019; 65: 18-23. [DOI:10.4103/jpgm.JPGM_683_17] [PMID] [PMCID]
21. Wang T, Han S, Tian W, Zhao M, Zhang H. Effects of kisspeptin on pathogenesis and energy metabolism in polycystic ovarian syndrome (PCOS). Gynecol Endocrinol 2019; 35: 807-810. [DOI:10.1080/09513590.2019.1597343] [PMID]
22. Ibrahim RO, Omer SH, Fattah CN. The correlation between hormonal disturbance in PCOS women and serum level of kisspeptin. Int J Endocrinol 2020; 2020: 6237141. [DOI:10.1155/2020/6237141] [PMID] [PMCID]
23. Daghestani MH. Evaluation of biochemical, endocrine, and metabolic biomarkers for the early diagnosis of polycystic ovary syndrome among non-obese Saudi women. Int J Gynaecol Obstet 2018; 142: 162-169. [DOI:10.1002/ijgo.12527] [PMID]
24. Wahab F, Atika B, Shahab M, Behr R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat Rev Urol 2016; 13: 21-32. [DOI:10.1038/nrurol.2015.277] [PMID]
25. Uenoyama Y, Pheng V, Tsukamura H, Maeda KI. The roles of kisspeptin revisited: Inside and outside the hypothalamus. J Reprod Dev 2016; 62: 537-545. [DOI:10.1262/jrd.2016-083] [PMID] [PMCID]
26. Navarro VM. Metabolic regulation of kisspeptin- the link between energy balance and reproduction. Nat Rev Endocrinol 2020; 16: 407-420. [DOI:10.1038/s41574-020-0363-7] [PMID] [PMCID]
27. Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology 2020; 161: 164. [DOI:10.1210/endocr/bqaa018] [PMID] [PMCID]
28. Ruddenklau A, Campbell RE. Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology 2019; 160: 2230-2242. [DOI:10.1210/en.2019-00428] [PMID]
29. Matsuzaki T, Tungalagsuvd A, Iwasa T, Munkhzaya M, Yanagihara R, Tokui T, et al. Kisspeptin mRNA expression is increased in the posterior hypothalamus in the rat model of polycystic ovary syndrome. Endocr J 2017; 64: 7-14. [DOI:10.1507/endocrj.EJ16-0282] [PMID]
30. Cernea M, Phillips R, Padmanabhan V, Coolen LM, Lehman MN. Prenatal testosterone exposure decreases colocalization of insulin receptors in kisspeptin/neurokinin b/dynorphin and agouti-related peptide neurons of the adult ewe. Eur J Neurosci 2016; 44: 2557-2568. [DOI:10.1111/ejn.13373] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




