Sat, Jan 31, 2026
[Archive]
Volume 21, Issue 8 (August 2023)
IJRM 2023, 21(8): 629-638 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abedi H, Nemati M, Ebrahimi B, Dehghani M, Mikaeiliagah E, Abdollahzadeh P, et al . The protective effect of hydroalcoholic extract of Ephedra pachyclada leaves on ovarian damage induced by cyclophosphamide in rat: An experimental study. IJRM 2023; 21 (8) :629-638
URL: http://ijrm.ir/article-1-3101-en.html
URL: http://ijrm.ir/article-1-3101-en.html
Hassanali Abedi1 

 , Mahnaz Nemati2
, Mahnaz Nemati2 

 , Bahareh Ebrahimi3
, Bahareh Ebrahimi3 

 , Maryam Dehghani1
, Maryam Dehghani1 

 , Elmira Mikaeiliagah4
, Elmira Mikaeiliagah4 

 , Pegah Abdollahzadeh1
, Pegah Abdollahzadeh1 

 , Aref Ghanaatpisheh5
, Aref Ghanaatpisheh5 

 , Nazanin Shafiei Jahromi6
, Nazanin Shafiei Jahromi6 

 , Hossein Kargar Jahromi *7
, Hossein Kargar Jahromi *7 




 , Mahnaz Nemati2
, Mahnaz Nemati2 

 , Bahareh Ebrahimi3
, Bahareh Ebrahimi3 

 , Maryam Dehghani1
, Maryam Dehghani1 

 , Elmira Mikaeiliagah4
, Elmira Mikaeiliagah4 

 , Pegah Abdollahzadeh1
, Pegah Abdollahzadeh1 

 , Aref Ghanaatpisheh5
, Aref Ghanaatpisheh5 

 , Nazanin Shafiei Jahromi6
, Nazanin Shafiei Jahromi6 

 , Hossein Kargar Jahromi *7
, Hossein Kargar Jahromi *7 


1- Research Center for Noncommunicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran.
2- Amir Oncology Hospital, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Shiraz Geriatric Research Center, Shiraz University of Medical Science, Shiraz, Iran.
4- Department of Biology, Ardabil Branch, Islamic Azad University, Ardabil, Iran. Stem Cell and Tissue Engineering Laboratory, Department of Orthopaedics, West Virginia University, Morgantown, USA.
5- Student Research Committee, Jahrom University of Medical Sciences, Jahrom, Iran.
6- Department of Microbiology, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
7- Research Center for Noncommunicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran. ,h.kargar@jums.ac.ir
2- Amir Oncology Hospital, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Shiraz Geriatric Research Center, Shiraz University of Medical Science, Shiraz, Iran.
4- Department of Biology, Ardabil Branch, Islamic Azad University, Ardabil, Iran. Stem Cell and Tissue Engineering Laboratory, Department of Orthopaedics, West Virginia University, Morgantown, USA.
5- Student Research Committee, Jahrom University of Medical Sciences, Jahrom, Iran.
6- Department of Microbiology, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
7- Research Center for Noncommunicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran. ,
Full-Text [PDF 593 kb]
(846 Downloads)
| Abstract (HTML) (1177 Views)
Full-Text: (271 Views)
1. Introduction
Cyclophosphamide (CP) is an anticancer drug, which acts as an alkylation agent and inhibits DNA replication by alkylating. This drug is widely used in clinics to treat autoimmune disorders and ovarian cancer. The chemotherapy process by CP in the reproductive system is highly toxic, especially in the sexual organs. One of its most important complications is the changes in the function of the reproductive system and ultimately infertility, which can affect the fertility rate in men and women. It also has detrimental effects on folliculogenesis, leading to irreversible ovarian damage or inflammation (1). CP metabolism occurs in the liver. CP undergoes the effects of microsomal enzymes in the liver, then decomposes into its active metabolites such as Acrolein and phosphoramide mustard. Overdose of CP leads to inhibiting immune responses and stopping tumor growth by weakening the body's defense mechanism (2).
CP probably inhibits cancerous cell proliferation. Studies have also shown that it can cause apoptosis in cancer cells in rat fetuses, lung cells, and, rat thymus (3). Ephedra pachyclada is a plant of the Ephedraceae family. The plants of this family are famous because of many ephedrine alkaloids, the most important of them are ephedrine and pseudoephedrine (4), they have many healing properties, and have been used for thousands of years in treating respiratory diseases such as asthma, bronchitis, and allergies (4, 5). Ephedra also has anti-inflammatory, antioxidant, and blood sugar-lowering effects, as well as imitating the sympathetic nervous system (6, 7). Among the alkaloids, ephedrine is an active ingredient in this plant, and pseudoephedrine is also found in some species of Ephedra. Ephedrine and pseudoephedrine make up more than 80% of the alkaloids in the Ephedra plant. In addition to ephedrine, other secondary metabolites, such as flavonoids, tannins, etc., are also present in the Ephedra plant as alkaloids (8). A study on one of the species of Ephedra found that hydroalcoholic extract had a better effect than aqueous extract, and it has stronger antioxidant, anti-inflammatory, and antiproliferative capacities than aqueous extract (9).
Oxidative stress and inflammation has been shown to have a close relationship and free radicals can cause inflammation (10). Although the effectiveness of different species of Ephedra on ovarian tissue and the destructive effects of CP has been confirmed in several studies, there are few reports of the plant’s effect on ovarian-related hormones and its tissue changes. Therefore, the effect of Ephedra pachyclada hydroalcoholic extract (EPHE) on ovarian tissue in rats treated with CP and its modifications has been investigated in this study.
2. Materials and Methods
2.1. Collection and extraction of Ephedra
This plant was collected from the areas around Shiraz and then the extraction was done by the Kelvinger device. The stems of the Ephedra pachyclada plant were dried (30°C) and powdered. Crude plant powder was soaked with an ethanol/water mixture (70/30) for 7 days at 35 ± 5°C. The mixture was then filtered using a sterile cloth sheet and dried under reduced pressure at < 45°C with a rotary evaporator to obtain a dark red residue with a yield of 7%. Before use, the extract was stored in universal bottles and refrigerated at 4°C (11).
2.2. Experiment design
48 healthy female Wistar rats (9-10 wk, 180-200 gr) were used in this experimental study, and the rats were randomly (random numbers table) divided into 6 groups (n = 8/each). The rats were kept in the animal laboratory of Jahrom University of Medical Science, Jahrom, Iran for a week to adapt (4 rats per cage). 12 hr light/dark cycle, with the humidity of about 50-55% were considered. According to previous studies, EPHE was prescribed with concentrations of 250, 500, and 1000 mg per kg body weight. The EPHE was fed to animals by gavage. The prescribed concentration of CP was determined as 5 mg/kg body weight (11). Therefore, the Sham and experimental groups in this study include the following groups. The control group (no treatment), Sham group (distilled water, by gavage) and experimental groups; experimental group 1 (5 mg/kg CP monohydrate, Sigma-Aldrich, St, Louis, MO), and experimental 2, 3, and 4 received 5 mg/kg CP and EPHE with concentrations of 250, 500, and 1000 mg per kg body weight respectively, like the rest of groups for 28 consecutive days at 8-10 AM.
2.3. Biochemical analysis
At the end of the study (day 29), after weighing the animals, blood was drawn directly from the animal's heart with a 5 cc syringe (under anesthesia using Ketamine and Xylazine). Then, their serum was collected by the centrifugal device (for 15 min and 3000 rpm) and kept at -20°C until the experiment’s time. To measure the gonadotropin-releasing hormone, follicle stimulating hormone (FSH), luteinizing hormone (LH), estrogen, and progesterone hormones, ELIZA kits (Shanghai crystal day biotech Co., China) for rats made were used.
2.4. Histological examination
For histological examination, left and right ovarian tissue were removed, placed informalin 10%, and after 48 hr, paraffin sections (5, and 20 um thickness) were prepared. 12 sections of each ovary were selected and stained by the Haemotoxylin and Eosin (H&E( method. Finally, primordial, primary, secondary, Graafian, and atretic follicles were examined with light microscopy. To count the follicles, the optical dissector method, mikrokator, and special counting frame were used. The frames were placed on the microscopic fields (100× objective), up to a depth of 5 µm each slice. The desired depths were obtained with a mikrokator (MT12, Germany) (12).
2.5. Analysis of oxidative stress markers
2.5.1. Lipid peroxidation and glutathione peroxidase (GSH-Px) assay
Plasma Mmalondialdehyde (nM/mL) and GSH-Px activity were measured using commercial kits.
2.5.2. Total antioxidant capacity (TAC) and total oxidant status (TOS) measurements
TAC and TOS were measured with commercially available kits (Rel Assay Diagnostics, Turkey). Measurements were performed according to the manufacturer's instructions.
2.6. Ethical considerations
In this research, all ethical issues regarding how to work with laboratory animals have been considered by Jahrom University of Medical Science Ethics committee, Jahrom, Iran (Code: IR.JUMS.REC.1396.019).
2.7. Statistical analysis
At first, the normality of the data was measured using the Shapiro-Wilk normality test. The data were analyzed by a one-way ANOVA test and Duncan Post Hoc using SPSS version 21 software (SPSS, Chicago, IL, USA). P ≤ 0.05 were considered statistically significant. All data were reported as mean ± SD.
3. Results
3.1. Biochemical examinations
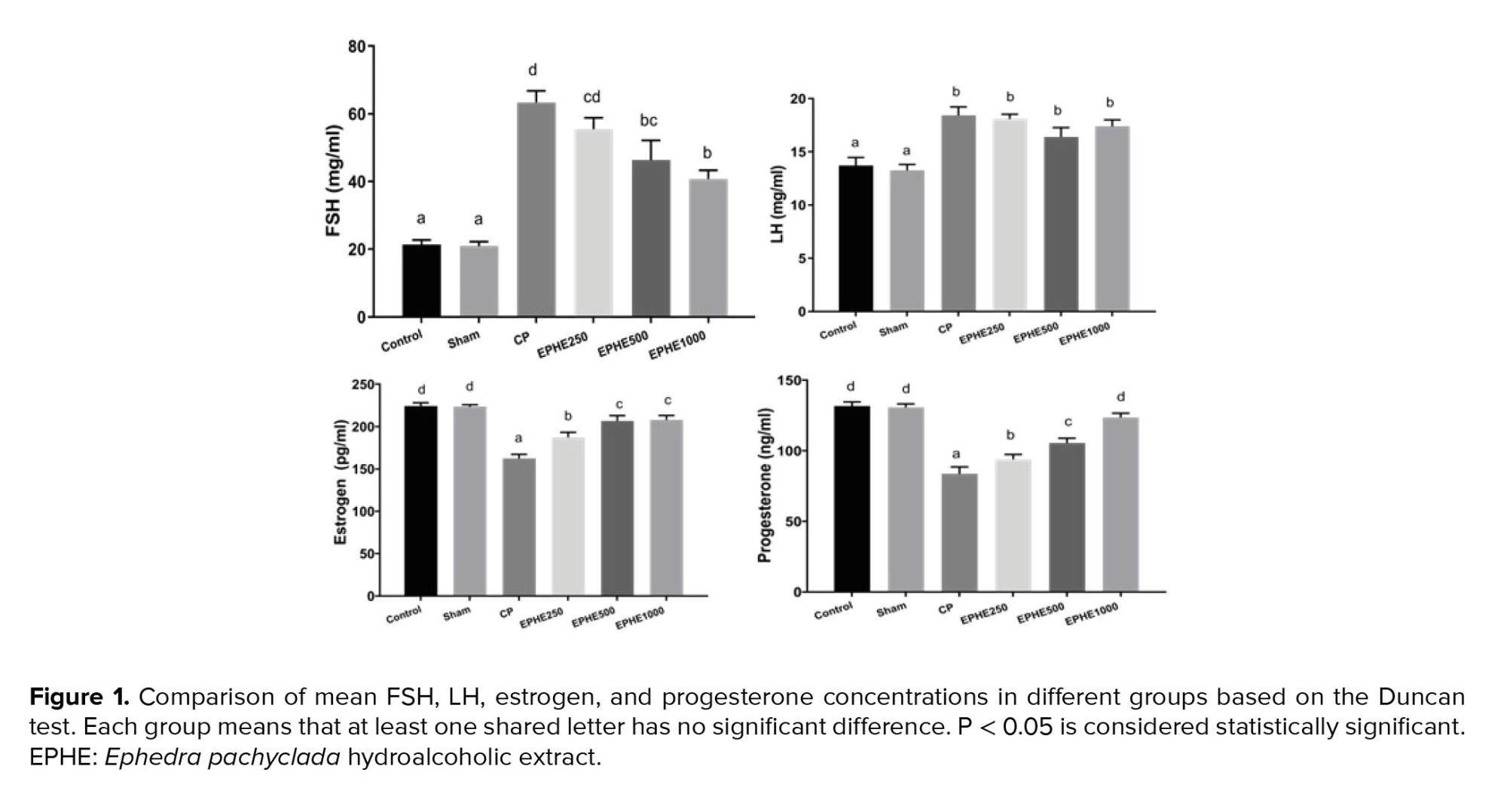
No significant difference in FSH, LH, estrogen, and progesterone concentrations were observed between groups. Serum concentrations of FSH and LH were significantly increased in all experimental groups compared to the control, and serum concentrations of estrogen and progesterone significantly decreased. Also, mean serum concentrations of FSH and LH in experimental groups CP + 250 mg/kg EPHE, CP + 500 mg/kg EPHE, and CP + 1000 mg/kg EPHE showed a significant decrease compared to the CP group, and estrogen and progesterone showed a significant increase. A comparison of groups that received both CP and EPHE showed that the concentration of 1000 mg/kg had more effect than other concentrations on reducing FSH and increasing progesterone and estrogen, and the concentration of 500 mg/kg had more effect on reducing LH and increasing estrogen than other concentrations but the decrease in LH was not significant. Generally, the concentration of 1000 mg/kg of EPHE showed the greatest effect compared to other concentrations in most variables (Figure 1).
3.2. Histopathological examinations
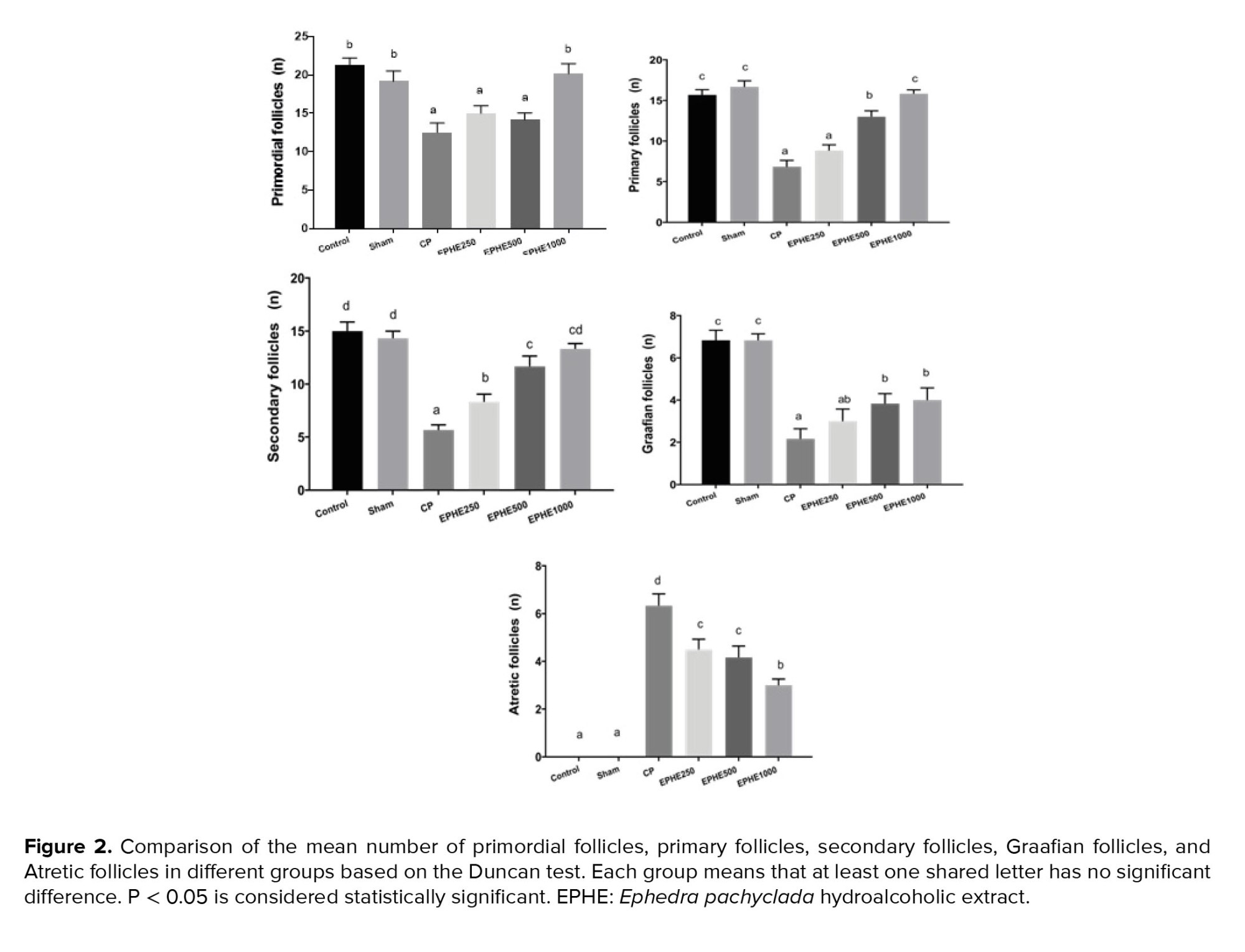
No significant difference was observed between Sham and control groups regarding the mean number of primordial, primary, secondary, Graafian, and atretic follicles. In experimental groups, 1, 2, and 3, primordial, primary, and secondary follicles were significantly reduced compared to the control group. Graafian follicles were significantly reduced in all experimental groups, and a significant increase was observed in atretic follicles compared to the control group. Also, the mean number of primary, secondary, and Graafian follicles in experimental groups 3 and 4 showed a significant increase compared to the CP group (experimental 1). The mean number of atretic follicles in all experimental groups compared to the CP group showed a significant decrease at the level of p < 0.05. A comparison of groups receiving both CP and EPHE showed that the concentration of 1000 mg/kg had more effect than other concentrations in increasing or decreasing the number of follicles (Figure 2).
3.3. Evaluation of oxidant/antioxidant status
As shown in figure 3, CP significantly decreased the TAC (p < 0.01) and increased TOS (p < 0.05) at the end of the study (day 29). EPHE (250, 500, and 1000 mg/kg) treatment elevated the (p < 0.01) and attenuated the TOS (p < 0.05) when compared with the CP group. After 29 days in the CP group, the Malondialdehyde levels showed a significant increased (p < 0.01), while the GSH-Px activity level was reduced (p < 0.05) in comparison with the control and sham groups. Using EPHE (250, 500, and 1000 mg/kg) could reduce the Malondialdehyde levels and increase GSH-P activity significantly in comparison with CP. Interestingly, EPHE (1000 mg/kg) group brought these factors to the control and Sham level.

4. Discussion
The process of chemotherapy with CP in the reproductive system is highly toxic and has adverse effects on folliculogenesis, which lead to irreversible damage to the ovaries (13). The results of one study showed that CP consumption in female rats significantly increased the mean serum concentrations of LH and FSH.
The number of atretic follicles significantly decreased the serum concentration of estrogen and the number of primordial, primary, secondary, and Graafian follicles. This destruction of the follicle is one of the main causes of ovarian failure and infertility caused by chemotherapy, confirming the destructive effects of CP on these parameters and is consistent with other studies (14). In other studies that checked out the effect of CP on male mice, the levels of LH, FSH, progesterone, testicular weight, and the count and motility of sperm decreased significantly compared to the control group (15). The gonadotropin hormone regulates folliculogenesis, so the decrease in the number of follicles and the increase in the number of atretic follicles can be caused by a decrease in gonadotropin hormones following the toxic effects of CP.
Many plants have antioxidant properties and are involved in antimutagenic cellular activities. Since the progression of cancer and chemotherapy has a close relationship with oxidative stress, compounds with antioxidant properties can be helpful to treat and prevent this (16). In this study, it is possible that different concentrations of EPHE due to its antioxidant properties, which are mostly due to the presence of ephedrine and pseudoephedrine, can modify the changes made by CP and play a role in the treatment of induced reproductive disorders in female rats. This property can also be caused by herbacetin glycosides, an active biological compound in the Ephedra plant (5). In addition to ephedrine and pseudoephedrine alkaloids, other studies had reported that Ephedra had other metabolits, such as flavones, carboxylic acid and volatile terpenes.
Various studies have shown the antimetastatic and anticancer effects of this plant, its mechanism was inhibition and suppression of cancer cells motility, as well as the inhibition of the hepatocyte growth factor due to the movement of cancer cells (5). The results of the present study showed that its effect was related to the maximum concentration of Ephedra, that is 1000 mg/kg, which indicates that hydroalcoholic extract is dose-dependent, as it significantly performs better.
Acrolein, a metabolite derived from CP, is responsible for toxic effects such as cell death and apoptosis by disrupting the body's antioxidant pathways and producing free radicals (17). There are several theories about the mechanism of action of CP: CP affects cell division and induces intra and inter-standard cross-link, and also because of its cytotoxic and mutagenic properties, it is considered an alkylating agent. CP connects to twe molecular strands of DNA, break it down and finally inhibits protein synthesis. CP induced reactive molecules affect nucleophilic groups of DNA, especially 7-n-guanine (18). CP especially affects the ovary; it causes severe ovarian damage, altered folliculogenesis, steroid production, and a reduced number of follicles. CP causes ovarian dysfunction by increasing apoptosis, altering folliculogenesis, and affecting steroids (19). CP is converted to 4-hydroxy CP by cytochrome P-450 enzymes in the liver, which accelerates the pathway of apoptosis (20).
Acrolein interferes in the antioxidant system and produces oxygen-free radicals. This metabolite also inhibits the GSH‐Px system. Following inhibition, the cell nucleus in the ovary is destroyed, and the cells become apoptotic. CP can increase apoptotic cells in the ovary and disrupt the function of the oxidative stress system in the ovaries. The most important oxidative system in the ovaries is the GSH‐Px system, where its activity is reduced by CP, and increased cell damage due to increase in the ROS levels. Jeelani et al., reported that exposure to reactive oxygen species produced after exposure to CP reduces ovum quality (21). Some studies show that CP toxicity causes ovarian damage through direct or indirect effects. The direct effect leads to the death of the ovum, and the indirect effect targets the somatic cells, in which both induce apoptosis (22).
CP activating PI3K/PTEN/Akt pathway can affects oocytes, pregranulosa cells, and primordial follicles (23).
The expression of B-cell lymphoma 2 family genes, which play an important role in the apoptosis pathway, is disrupted in ovarian cells exposed to CP. In addition, CP activates the caspase 3 enzyme, which is one of the main and effective enzymes of the downstream apoptotic pathway (24, 25). In another study, in CP-treated mice, the anti-apoptotic factor B-cell lymphoma-extra-large decreased, and the proapoptotic factor BAX increased.
Another theory about CP is that it induces diminished ovarian reserve due to the rapid absorption of primordial follicles (26). Another cause of increased apoptosis following CP intake is an increase in the body's autoimmune activity, which causes the ovaries to invade and destroy the rest of the oocytes and, eventually the remaining follicles (27). Most follicles become atretic during the menstruation cycle and cause antigen production. These antigens do not normally trigger an immune attack. It is thought that, as a result of stimulation, regulatory T cells suppress and control autoreactive T cells induced by their antigens (28).
Therefore, it is assumed that CP stimulates the production of autoreactive T cells by causing extensive apoptosis, which results in the loss of the remaining oocytes. CP is also known as an immune system suppressor, and studies show that it also destroys the regulatory T cells (29). Ephedra can inhibit prostaglandin estradiol biosynthesis through pseudoephedrine, in this way it can induce analgesic and anti-inflammatory effects (30).
All studies on the effects of CP on ovarian tissue confirm the results of this study. Since CP is a widely used drug in chemotherapy and treatment of refractory diseases, it is also an alkylating agent, which has mutagenic and cytotoxic properties and can damage the body tissues through oxidative stress. Hence, taking antioxidants during chemotherapy or other procedures along with this drug effectively reduces oxidative stress and tissue detoxification. According to this study, it can be said that CP has harmful effects on ovarian tissue, secretion of sex hormones, and ovarian follicles in female rats.
In addition, EPHE improves the damages caused by CP, which can be effective in preventing the destructive effects of this drug, preserves the ovaries and follicles, and modifies sex hormones using its antioxidant effects on ovarian tissue. As more effects were observed in female rats, therefore it is said to be dose dependent. These doses may be different in humans. Therefore, this should be considered to generalize the results in humans.
5. Conclusion
In this study, CP as an alkylating agent caused destructive effects on ovarian tissue of rats, by disrupting the function of cells and sex hormones, and production of toxic metabolites and free radicals. However, EPHE, with antioxidant properties, reduced these side effects and improved it.
Acknowledgments
The authors are grateful to the Deputy of Research of Jahrom University of Medical Sciences for their financial support. This experimental laboratory research has been approved by the Student Research Committee of the Jahrom University of Medical Sciences, Jahrom, Iran (grant no. 96019).
Conflicts of Interest
The authors declare that there is no conflict of interest.
Cyclophosphamide (CP) is an anticancer drug, which acts as an alkylation agent and inhibits DNA replication by alkylating. This drug is widely used in clinics to treat autoimmune disorders and ovarian cancer. The chemotherapy process by CP in the reproductive system is highly toxic, especially in the sexual organs. One of its most important complications is the changes in the function of the reproductive system and ultimately infertility, which can affect the fertility rate in men and women. It also has detrimental effects on folliculogenesis, leading to irreversible ovarian damage or inflammation (1). CP metabolism occurs in the liver. CP undergoes the effects of microsomal enzymes in the liver, then decomposes into its active metabolites such as Acrolein and phosphoramide mustard. Overdose of CP leads to inhibiting immune responses and stopping tumor growth by weakening the body's defense mechanism (2).
CP probably inhibits cancerous cell proliferation. Studies have also shown that it can cause apoptosis in cancer cells in rat fetuses, lung cells, and, rat thymus (3). Ephedra pachyclada is a plant of the Ephedraceae family. The plants of this family are famous because of many ephedrine alkaloids, the most important of them are ephedrine and pseudoephedrine (4), they have many healing properties, and have been used for thousands of years in treating respiratory diseases such as asthma, bronchitis, and allergies (4, 5). Ephedra also has anti-inflammatory, antioxidant, and blood sugar-lowering effects, as well as imitating the sympathetic nervous system (6, 7). Among the alkaloids, ephedrine is an active ingredient in this plant, and pseudoephedrine is also found in some species of Ephedra. Ephedrine and pseudoephedrine make up more than 80% of the alkaloids in the Ephedra plant. In addition to ephedrine, other secondary metabolites, such as flavonoids, tannins, etc., are also present in the Ephedra plant as alkaloids (8). A study on one of the species of Ephedra found that hydroalcoholic extract had a better effect than aqueous extract, and it has stronger antioxidant, anti-inflammatory, and antiproliferative capacities than aqueous extract (9).
Oxidative stress and inflammation has been shown to have a close relationship and free radicals can cause inflammation (10). Although the effectiveness of different species of Ephedra on ovarian tissue and the destructive effects of CP has been confirmed in several studies, there are few reports of the plant’s effect on ovarian-related hormones and its tissue changes. Therefore, the effect of Ephedra pachyclada hydroalcoholic extract (EPHE) on ovarian tissue in rats treated with CP and its modifications has been investigated in this study.
2. Materials and Methods
2.1. Collection and extraction of Ephedra
This plant was collected from the areas around Shiraz and then the extraction was done by the Kelvinger device. The stems of the Ephedra pachyclada plant were dried (30°C) and powdered. Crude plant powder was soaked with an ethanol/water mixture (70/30) for 7 days at 35 ± 5°C. The mixture was then filtered using a sterile cloth sheet and dried under reduced pressure at < 45°C with a rotary evaporator to obtain a dark red residue with a yield of 7%. Before use, the extract was stored in universal bottles and refrigerated at 4°C (11).
2.2. Experiment design
48 healthy female Wistar rats (9-10 wk, 180-200 gr) were used in this experimental study, and the rats were randomly (random numbers table) divided into 6 groups (n = 8/each). The rats were kept in the animal laboratory of Jahrom University of Medical Science, Jahrom, Iran for a week to adapt (4 rats per cage). 12 hr light/dark cycle, with the humidity of about 50-55% were considered. According to previous studies, EPHE was prescribed with concentrations of 250, 500, and 1000 mg per kg body weight. The EPHE was fed to animals by gavage. The prescribed concentration of CP was determined as 5 mg/kg body weight (11). Therefore, the Sham and experimental groups in this study include the following groups. The control group (no treatment), Sham group (distilled water, by gavage) and experimental groups; experimental group 1 (5 mg/kg CP monohydrate, Sigma-Aldrich, St, Louis, MO), and experimental 2, 3, and 4 received 5 mg/kg CP and EPHE with concentrations of 250, 500, and 1000 mg per kg body weight respectively, like the rest of groups for 28 consecutive days at 8-10 AM.
2.3. Biochemical analysis
At the end of the study (day 29), after weighing the animals, blood was drawn directly from the animal's heart with a 5 cc syringe (under anesthesia using Ketamine and Xylazine). Then, their serum was collected by the centrifugal device (for 15 min and 3000 rpm) and kept at -20°C until the experiment’s time. To measure the gonadotropin-releasing hormone, follicle stimulating hormone (FSH), luteinizing hormone (LH), estrogen, and progesterone hormones, ELIZA kits (Shanghai crystal day biotech Co., China) for rats made were used.
2.4. Histological examination
For histological examination, left and right ovarian tissue were removed, placed informalin 10%, and after 48 hr, paraffin sections (5, and 20 um thickness) were prepared. 12 sections of each ovary were selected and stained by the Haemotoxylin and Eosin (H&E( method. Finally, primordial, primary, secondary, Graafian, and atretic follicles were examined with light microscopy. To count the follicles, the optical dissector method, mikrokator, and special counting frame were used. The frames were placed on the microscopic fields (100× objective), up to a depth of 5 µm each slice. The desired depths were obtained with a mikrokator (MT12, Germany) (12).
2.5. Analysis of oxidative stress markers
2.5.1. Lipid peroxidation and glutathione peroxidase (GSH-Px) assay
Plasma Mmalondialdehyde (nM/mL) and GSH-Px activity were measured using commercial kits.
2.5.2. Total antioxidant capacity (TAC) and total oxidant status (TOS) measurements
TAC and TOS were measured with commercially available kits (Rel Assay Diagnostics, Turkey). Measurements were performed according to the manufacturer's instructions.
2.6. Ethical considerations
In this research, all ethical issues regarding how to work with laboratory animals have been considered by Jahrom University of Medical Science Ethics committee, Jahrom, Iran (Code: IR.JUMS.REC.1396.019).
2.7. Statistical analysis
At first, the normality of the data was measured using the Shapiro-Wilk normality test. The data were analyzed by a one-way ANOVA test and Duncan Post Hoc using SPSS version 21 software (SPSS, Chicago, IL, USA). P ≤ 0.05 were considered statistically significant. All data were reported as mean ± SD.
3. Results
3.1. Biochemical examinations
No significant difference in FSH, LH, estrogen, and progesterone concentrations were observed between groups. Serum concentrations of FSH and LH were significantly increased in all experimental groups compared to the control, and serum concentrations of estrogen and progesterone significantly decreased. Also, mean serum concentrations of FSH and LH in experimental groups CP + 250 mg/kg EPHE, CP + 500 mg/kg EPHE, and CP + 1000 mg/kg EPHE showed a significant decrease compared to the CP group, and estrogen and progesterone showed a significant increase. A comparison of groups that received both CP and EPHE showed that the concentration of 1000 mg/kg had more effect than other concentrations on reducing FSH and increasing progesterone and estrogen, and the concentration of 500 mg/kg had more effect on reducing LH and increasing estrogen than other concentrations but the decrease in LH was not significant. Generally, the concentration of 1000 mg/kg of EPHE showed the greatest effect compared to other concentrations in most variables (Figure 1).
3.2. Histopathological examinations
No significant difference was observed between Sham and control groups regarding the mean number of primordial, primary, secondary, Graafian, and atretic follicles. In experimental groups, 1, 2, and 3, primordial, primary, and secondary follicles were significantly reduced compared to the control group. Graafian follicles were significantly reduced in all experimental groups, and a significant increase was observed in atretic follicles compared to the control group. Also, the mean number of primary, secondary, and Graafian follicles in experimental groups 3 and 4 showed a significant increase compared to the CP group (experimental 1). The mean number of atretic follicles in all experimental groups compared to the CP group showed a significant decrease at the level of p < 0.05. A comparison of groups receiving both CP and EPHE showed that the concentration of 1000 mg/kg had more effect than other concentrations in increasing or decreasing the number of follicles (Figure 2).
3.3. Evaluation of oxidant/antioxidant status
As shown in figure 3, CP significantly decreased the TAC (p < 0.01) and increased TOS (p < 0.05) at the end of the study (day 29). EPHE (250, 500, and 1000 mg/kg) treatment elevated the (p < 0.01) and attenuated the TOS (p < 0.05) when compared with the CP group. After 29 days in the CP group, the Malondialdehyde levels showed a significant increased (p < 0.01), while the GSH-Px activity level was reduced (p < 0.05) in comparison with the control and sham groups. Using EPHE (250, 500, and 1000 mg/kg) could reduce the Malondialdehyde levels and increase GSH-P activity significantly in comparison with CP. Interestingly, EPHE (1000 mg/kg) group brought these factors to the control and Sham level.



4. Discussion
The process of chemotherapy with CP in the reproductive system is highly toxic and has adverse effects on folliculogenesis, which lead to irreversible damage to the ovaries (13). The results of one study showed that CP consumption in female rats significantly increased the mean serum concentrations of LH and FSH.
The number of atretic follicles significantly decreased the serum concentration of estrogen and the number of primordial, primary, secondary, and Graafian follicles. This destruction of the follicle is one of the main causes of ovarian failure and infertility caused by chemotherapy, confirming the destructive effects of CP on these parameters and is consistent with other studies (14). In other studies that checked out the effect of CP on male mice, the levels of LH, FSH, progesterone, testicular weight, and the count and motility of sperm decreased significantly compared to the control group (15). The gonadotropin hormone regulates folliculogenesis, so the decrease in the number of follicles and the increase in the number of atretic follicles can be caused by a decrease in gonadotropin hormones following the toxic effects of CP.
Many plants have antioxidant properties and are involved in antimutagenic cellular activities. Since the progression of cancer and chemotherapy has a close relationship with oxidative stress, compounds with antioxidant properties can be helpful to treat and prevent this (16). In this study, it is possible that different concentrations of EPHE due to its antioxidant properties, which are mostly due to the presence of ephedrine and pseudoephedrine, can modify the changes made by CP and play a role in the treatment of induced reproductive disorders in female rats. This property can also be caused by herbacetin glycosides, an active biological compound in the Ephedra plant (5). In addition to ephedrine and pseudoephedrine alkaloids, other studies had reported that Ephedra had other metabolits, such as flavones, carboxylic acid and volatile terpenes.
Various studies have shown the antimetastatic and anticancer effects of this plant, its mechanism was inhibition and suppression of cancer cells motility, as well as the inhibition of the hepatocyte growth factor due to the movement of cancer cells (5). The results of the present study showed that its effect was related to the maximum concentration of Ephedra, that is 1000 mg/kg, which indicates that hydroalcoholic extract is dose-dependent, as it significantly performs better.
Acrolein, a metabolite derived from CP, is responsible for toxic effects such as cell death and apoptosis by disrupting the body's antioxidant pathways and producing free radicals (17). There are several theories about the mechanism of action of CP: CP affects cell division and induces intra and inter-standard cross-link, and also because of its cytotoxic and mutagenic properties, it is considered an alkylating agent. CP connects to twe molecular strands of DNA, break it down and finally inhibits protein synthesis. CP induced reactive molecules affect nucleophilic groups of DNA, especially 7-n-guanine (18). CP especially affects the ovary; it causes severe ovarian damage, altered folliculogenesis, steroid production, and a reduced number of follicles. CP causes ovarian dysfunction by increasing apoptosis, altering folliculogenesis, and affecting steroids (19). CP is converted to 4-hydroxy CP by cytochrome P-450 enzymes in the liver, which accelerates the pathway of apoptosis (20).
Acrolein interferes in the antioxidant system and produces oxygen-free radicals. This metabolite also inhibits the GSH‐Px system. Following inhibition, the cell nucleus in the ovary is destroyed, and the cells become apoptotic. CP can increase apoptotic cells in the ovary and disrupt the function of the oxidative stress system in the ovaries. The most important oxidative system in the ovaries is the GSH‐Px system, where its activity is reduced by CP, and increased cell damage due to increase in the ROS levels. Jeelani et al., reported that exposure to reactive oxygen species produced after exposure to CP reduces ovum quality (21). Some studies show that CP toxicity causes ovarian damage through direct or indirect effects. The direct effect leads to the death of the ovum, and the indirect effect targets the somatic cells, in which both induce apoptosis (22).
CP activating PI3K/PTEN/Akt pathway can affects oocytes, pregranulosa cells, and primordial follicles (23).
The expression of B-cell lymphoma 2 family genes, which play an important role in the apoptosis pathway, is disrupted in ovarian cells exposed to CP. In addition, CP activates the caspase 3 enzyme, which is one of the main and effective enzymes of the downstream apoptotic pathway (24, 25). In another study, in CP-treated mice, the anti-apoptotic factor B-cell lymphoma-extra-large decreased, and the proapoptotic factor BAX increased.
Another theory about CP is that it induces diminished ovarian reserve due to the rapid absorption of primordial follicles (26). Another cause of increased apoptosis following CP intake is an increase in the body's autoimmune activity, which causes the ovaries to invade and destroy the rest of the oocytes and, eventually the remaining follicles (27). Most follicles become atretic during the menstruation cycle and cause antigen production. These antigens do not normally trigger an immune attack. It is thought that, as a result of stimulation, regulatory T cells suppress and control autoreactive T cells induced by their antigens (28).
Therefore, it is assumed that CP stimulates the production of autoreactive T cells by causing extensive apoptosis, which results in the loss of the remaining oocytes. CP is also known as an immune system suppressor, and studies show that it also destroys the regulatory T cells (29). Ephedra can inhibit prostaglandin estradiol biosynthesis through pseudoephedrine, in this way it can induce analgesic and anti-inflammatory effects (30).
All studies on the effects of CP on ovarian tissue confirm the results of this study. Since CP is a widely used drug in chemotherapy and treatment of refractory diseases, it is also an alkylating agent, which has mutagenic and cytotoxic properties and can damage the body tissues through oxidative stress. Hence, taking antioxidants during chemotherapy or other procedures along with this drug effectively reduces oxidative stress and tissue detoxification. According to this study, it can be said that CP has harmful effects on ovarian tissue, secretion of sex hormones, and ovarian follicles in female rats.
In addition, EPHE improves the damages caused by CP, which can be effective in preventing the destructive effects of this drug, preserves the ovaries and follicles, and modifies sex hormones using its antioxidant effects on ovarian tissue. As more effects were observed in female rats, therefore it is said to be dose dependent. These doses may be different in humans. Therefore, this should be considered to generalize the results in humans.
5. Conclusion
In this study, CP as an alkylating agent caused destructive effects on ovarian tissue of rats, by disrupting the function of cells and sex hormones, and production of toxic metabolites and free radicals. However, EPHE, with antioxidant properties, reduced these side effects and improved it.
Acknowledgments
The authors are grateful to the Deputy of Research of Jahrom University of Medical Sciences for their financial support. This experimental laboratory research has been approved by the Student Research Committee of the Jahrom University of Medical Sciences, Jahrom, Iran (grant no. 96019).
Conflicts of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Physiology
References
1. More GS, Thomas AB, Chitlange SS, Nanda RK, Gajbhiye RL. Nitrogen mustards as alkylating agents: A review on chemistry, mechanism of action and current USFDA status of drugs. Anti-Cancer Agents Med Chem 2019; 19: 1080-1102. [DOI:10.2174/1871520619666190305141458] [PMID]
2. Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical stem cell transplantation with posttransplant cyclophosphamide therapy vs other donor transplantations in adults with hematologic cancers: A systematic review and meta-analysis. JAMA Oncol 2019; 5: 1739-1748. [DOI:10.1001/jamaoncol.2019.3541] [PMID] [PMCID]
3. Shrief AI, Hamed WHE, Mazroa SA, Moustafa AM. Histological study of the role of CD34+ stem cells and mast cells in cyclophosphamide-induced thymic injury in rats and the possible attenuating role of melatonin. Histochem Cell Biol 2023; 59: 501-512. [DOI:10.1007/s00418-023-02185-6] [PMID] [PMCID]
4. Sadeghi Dousari A, Satarzadeh N, Amirheidari B, Forootanfar H. Medicinal and therapeutic properties of ephedra. Revista Brasileira de Farmacognosia 2022; 32: 883-899. [DOI:10.1007/s43450-022-00304-3]
5. González-Juárez DE, Escobedo-Moratilla A, Flores J, Hidalgo-Figueroa S, Martínez-Tagüeña N, Morales-Jiménez J, et al. A review of the Ephedra genus: Distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules 2020; 25: 3283. [DOI:10.3390/molecules25143283] [PMID] [PMCID]
6. Bouafia W, Hamdi A, Mouffouk S, Arcos RR, Araujo AJ, Bejarano RG, et al. Phenolic composition, in vitro alpha-amylase and pancreatic lipase inhibitory effects, anti-inflammatory and antioxidant activities of ephedra altissima. Indian J Pharm Sci 2022; 84: 890-901. [DOI:10.36468/pharmaceutical-sciences.984]
7. Costa VM, Rossato Grando LG, Milandri E, Nardi J, Teixeira P, Mladenka P, et al. Natural sympathomimetic drugs: From pharmacology to toxicology. Biomolecules 2022; 12: 1793. [DOI:10.3390/biom12121793] [PMID] [PMCID]
8. Zhang BM, Wang ZB, Xin P, Wang QH, Bu H, Kuang HX. Phytochemistry and pharmacology of genus Ephedra. Chin J Nat Med 2018; 16: 811-828. [DOI:10.1016/S1875-5364(18)30123-7] [PMID]
9. Kallassy H, Fayyad-Kazan M, Makki R, El-Makhour Y, Rammal H, Leger DY, et al. Chemical composition and antioxidant, anti-inflammatory, and antiproliferative activities of lebanese ephedra campylopoda plant. Med Sci Monit Basic Res 2017; 23: 313-325.
https://doi.org/10.12659/MSMBR.905066 [DOI:10.12659/MSMBR.905056]
10. Biswas S, Das R, Banerjee ER. Role of free radicals in human inflammatory diseases. AIMS Biophysics 2017; 4: 596-614. [DOI:10.3934/biophy.2017.4.596]
11. Ghasemi Pirbalouti A, Amirmohammadi M, Azizi Sh, Craker L. Healing effect of hydro-alcoholic extract of Ephedra pachyclada Boiss in experimental gastric ulcer in rat. Acta Pol Pharm 2013; 70: 1003-1009.
12. Kalhori Z, Soleimani Mehranjani M, Azadbakht M, Shariaatzadeh MA. Ovary stereological features and serum biochemical factors following induction of polycystic ovary syndrome with testosterone enanthate in mice: An experimental study. Int J Reprod BioMed 2018; 16: 267-274. [DOI:10.29252/ijrm.16.4.267] [PMID] [PMCID]
13. Delkhosh A, Delashoub M, Tehrani AA, Bahrami AM, Niazi V, Shoorei H, et al. Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J Biochem Mol Toxicol 2019; 33: e22398. [DOI:10.1002/jbt.22398] [PMID]
14. Hamzeh M, Hosseinimehr SJ, Mohammadi HR, Yaghubi Beklar S, Dashti A, Talebpour Amiri F. Atorvastatin attenuates the ovarian damage induced by cyclophosphamide in rat: An experimental study. Int J Reprod BioMed 2018; 16: 323-334. [DOI:10.29252/ijrm.16.5.323] [PMID] [PMCID]
15. Rostamzadeh A, Amini-Khoei H, Mardani Korani MJ, Rahimi-Madiseh M. Comparison effects of olive leaf extract and oleuropein compounds on male reproductive function in cyclophosphamide exposed mice. Heliyon 2020; 6: e03785. [DOI:10.1016/j.heliyon.2020.e03785] [PMID] [PMCID]
16. Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M, Sohraby M, Shahlari N, et al. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J Tradit Complement Med 2016; 7: 24-29. [DOI:10.1016/j.jtcme.2015.09.004] [PMID] [PMCID]
17. Khamis T, Hegazy AA, El-Fatah SSA, Abdelfattah ER, Abdelfattah MMM, Fericean LM, et al. Hesperidin mitigates cyclophosphamide-induced testicular dysfunction via altering the hypothalamic pituitary gonadal axis and testicular steroidogenesis, inflammation, and apoptosis in male rats. Pharmaceuticals 2023; 16: 301. [DOI:10.3390/ph16020301] [PMID] [PMCID]
18. Sun Y, Liu Y, Ma X, Hu H. The influence of cell cycle regulation on chemotherapy. Int J Mol Sci 2021; 22: 6923. [DOI:10.3390/ijms22136923] [PMID] [PMCID]
19. Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019; 25: 673-693. [DOI:10.1093/humupd/dmz027] [PMID] [PMCID]
20. Steinbrecht S, Kiebist J, Konig R, Thiessen M, Schmidtke K-U, Kammerer S, et al. Synthesis of cyclophosphamide metabolites by a peroxygenase from Marasmius rotula for toxicological studies on human cancer cells. AMB Express 2020; 10: 128. [DOI:10.1186/s13568-020-01064-w] [PMID] [PMCID]
21. Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr HR, Aldhaheri SR, Najafi T, et al. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med 2017; 110: 11-18. [DOI:10.1016/j.freeradbiomed.2017.05.006] [PMID] [PMCID]
22. Chi YN, Yang JM, Liu N, Cui YH, Ma L, Lan XB, et al. Development of protective agents against ovarian injury caused by chemotherapeutic drugs. Biomed Pharmacother 2022; 155: 113731. [DOI:10.1016/j.biopha.2022.113731] [PMID]
23. Barberino RS, Lins TLB, Monte APO, Gouveia BB, Campinho DSP, Palheta Jr RC, et al. Melatonin attenuates cyclophosphamide-induced primordial follicle loss by interaction with MT1 receptor and modulation of PTEN/Akt/FOXO3a proteins in the mouse ovary. Reprod Sci 2022; 29: 2505-2514. [DOI:10.1007/s43032-021-00768-z] [PMID]
24. Mobasher MA, Hassen MT, Ebiya RA, Alturki NA, Alzamami A, Mohamed HK, et al. Ameliorative effect of citrus lemon peel extract and resveratrol on premature ovarian failure rat model: Role of iNOS/Caspase-3 pathway. Molecules 2022; 28: 122. [DOI:10.3390/molecules28010122] [PMID] [PMCID]
25. Yılmaz TE, Taşdemir M, Kaya M, Arıcan N, Ahıshalı B. The effects of magnesium sulfate on cyclophosphamide-induced ovarian damage: Folliculogenesis. Acta Histochem 2020; 122: 151470. [DOI:10.1016/j.acthis.2019.151470] [PMID]
26. Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and "burnout"; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 2013; 5: 185ra62. [DOI:10.1126/scitranslmed.3005402] [PMID]
27. Huang C, Song K, Ma W, Ding J, Chen Z, Zhang M. Immunomodulatory mechanism of Bushen Huoxue Recipe alleviates cyclophosphamide-induced diminished ovarian reserve in mouse model. J Ethnopharmacol 2017; 208: 44-56. [DOI:10.1016/j.jep.2017.06.022] [PMID]
28. Rocamora-Reverte L, Melzer FL, Wurzner R, Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol 2021; 11: 616949. [DOI:10.3389/fimmu.2020.616949] [PMID] [PMCID]
29. Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004; 34: 336-344. [DOI:10.1002/eji.200324181] [PMID]
30. Tang S, Ren J, Kong L, Yan G, Liu C, Han Y, et al. Ephedrae herba: A review of its phytochemistry, pharmacology, clinical application, and alkaloid toxicity. Molecules 2023; 28: 663. [DOI:10.3390/molecules28020663] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




