Thu, Feb 19, 2026
[Archive]
Volume 22, Issue 7 (July 2024)
IJRM 2024, 22(7): 567-578 |
Back to browse issues page
Ethics code: IR.SUMS.REC.1398.1395
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Poordast T, Alborzi S, Kiani Z, Omidfar N, Askary E, Chamanara K, et al . The role of progesterone and estrogen receptors in treatment choice after endometriosis surgery: A cross-sectional study. IJRM 2024; 22 (7) :567-578
URL: http://ijrm.ir/article-1-3251-en.html
URL: http://ijrm.ir/article-1-3251-en.html
Tahereh Poordast1 

 , Saeed Alborzi2
, Saeed Alborzi2 

 , Ziba Kiani3
, Ziba Kiani3 

 , Navid Omidfar4
, Navid Omidfar4 

 , Elham Askary *5
, Elham Askary *5 

 , Kefayat Chamanara1
, Kefayat Chamanara1 

 , Mansoureh Shokripour4
, Mansoureh Shokripour4 

 , Alimohammad Keshtvarz Hesam Abadi6
, Alimohammad Keshtvarz Hesam Abadi6 




 , Saeed Alborzi2
, Saeed Alborzi2 

 , Ziba Kiani3
, Ziba Kiani3 

 , Navid Omidfar4
, Navid Omidfar4 

 , Elham Askary *5
, Elham Askary *5 

 , Kefayat Chamanara1
, Kefayat Chamanara1 

 , Mansoureh Shokripour4
, Mansoureh Shokripour4 

 , Alimohammad Keshtvarz Hesam Abadi6
, Alimohammad Keshtvarz Hesam Abadi6 


1- Department of Obstetrics and Gynecology, School of Medicine, Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Laparoscopy Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Pathology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,elliaskary_md@yahoo.com
6- Department of Biostatistics, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Laparoscopy Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Pathology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,
6- Department of Biostatistics, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 841 kb]
(1711 Downloads)
| Abstract (HTML) (1314 Views)
Full-Text: (238 Views)
1. Introduction
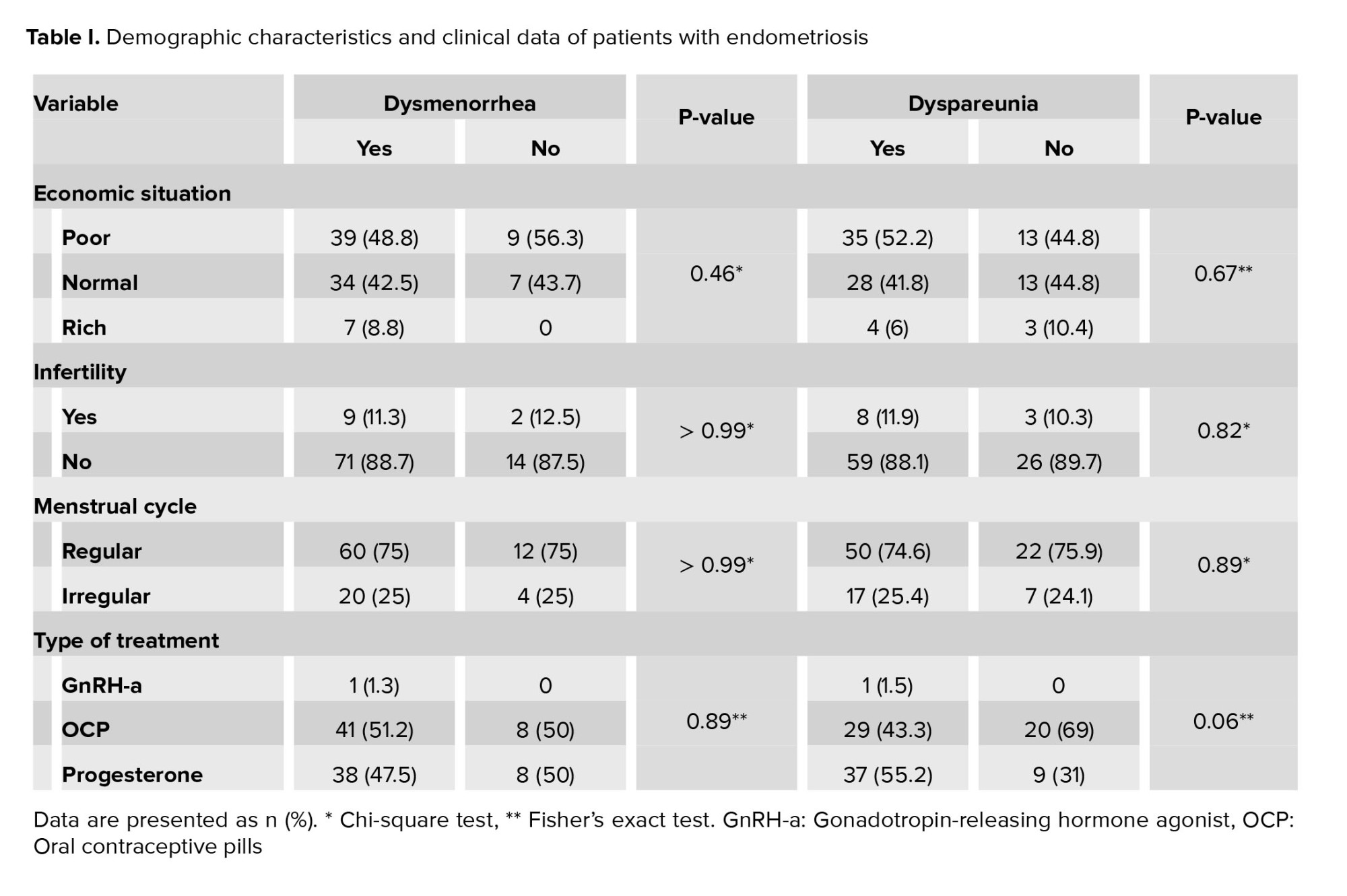
Endometriosis is a complex disease commonly seen in about 10% of women of reproductive age with presence of endometrial glands and stroma outside the uterine cavity (1). Symptoms like dysmenorrhea, dyschezia, dyspareunia, as well as infertility are associated with endometriosis, all of which can affect a person's quality of life significantly (2, 3).
The objective of endometriosis treatment is to reduce inflammation, disease activity, and alleviate the pain. For this purpose, several treatments have been suggested, including hormonal and nonhormonal therapies, (such as gonadotropin-releasing hormone, oral contraceptive pill [OCP], progestin, and nonsteroidal anti-inflammatory drugs) (4). Several hypotheses justify the onset and recurrence of endometriotic lesions; however, estrogen dependence and progesterone resistance have been shown to play a role in the recurrence of endometriotic lesions after surgery (5). In the endometrial implants, estradiol typically stimulates cell proliferation and progesterone stimulates cell differentiation. The function of ovarian hormones is mediated by estrogen (α and β) or (1 and 2) and progesterone receptors (PRs) (A and B). Previous studies have shown that the overexpression of hormone receptors, such as high estrogen receptor (ER)2/ER1 ratio and reduced PR expression play a role in endometriotic lesions. This receptor-mediated signal disorder affects cellular behavior and causes different responses to hormonal therapies (6-8).
One of the most common hormonal treatments for endometriosis is the use of progesterone or the compounds containing it. Progesterone (preg-4-ene-3, 20-dione) is a natural cholesterol catabolite of cyclopentanephydrofentanthrene (cyclopentanoperhydrophenanthrene) that is naturally produced in the corpus luteum (7). Progesterone functions by regulating endometrial decidualization and inhibiting estrogen-derived endometrial proliferation (7, 8). However, in some patients it has been observed that despite the similarity of serum progesterone levels in healthy and endometriotic women, endometriotic lesions do not respond adequately to progesterone (9). In endometriotic lesions, PR expression seems to undergo some changes (10-13).
On the other hand, available sources strongly support the benefits associated with the long term use of hormone therapy after surgery to prevent the recurrence of endometriosis and related symptoms, particularly dysmenorrhea. The lack of improvement in some patients may suggest an inappropriate response to the routine hormonal therapies in these cases. This is, especially true for those who relapse despite receiving hormone therapy after surgery (7, 10-15).
In addition, the production of estradiol (intracrine and paracrine) in endometriotic lesions increases the concentration of steroid hormones and enhances the estrogenic effect. Endometriotic lesions exert a lower level of estradiol inactivation compared to eutopic endometrium, which may further enhance the local effects. A few studies have investigated the role of PR in patients with endometriosis and its effect on treatment failure and disease recurrence (7, 13, 15-17). These studies have mainly focused on dysmenorrhea and endometrioma recurrence rather than dyspareunia and deep infiltrating endometriosis (DIE) lesions (16).
Therefore, the present research aimed to measure the levels of PR and ER in endometriotic lesions and determine a cut-off point for selecting the appropriate treatment based on the hormone receptors of these lesions to help improve the quality of life in patients with endometriosis.
The current article titled "Evaluation of progesterone and estrogen receptor (PR & ER) levels and their role in medical treatment of endometriosis patients" was published on Research Square's preprint site in October 2021 with DOI: 10.21203/rs.3.rs991753/v1.
2. Materials and Methods
In this cross-sectional study, from March 2017-2019, 1500 endometriotic women were referred to Shahid Faghihi and Hazrate Zeinab hospitals, Shiraz, Iran for endometriosis surgery based on the inclusion and exclusion criteria. By reviewing the medical records of patients and testing their archive samples and phone interview if needed, only 86 people were ultimately included in the study.
All women were divided into 2 groups: responsiveness to medical treatment and surgery, and unresponsiveness to surgery and medical treatment. Nonresponse criteria consisted of individuals whose endometriosis pain did not show improvement before the surgery, or experienced pain recurrence with a visual analogue scale (VAS) score > 5 during the 6- and 12-month follow-up visits. They showed no signs of recurrence in the subsequent ultrasound. All individuals were examined and analyzed separately regarding 2 common symptoms of endometriosis, that is, dysmenorrhea and dyspareunia. The relationship between the response to pain of each group was investigated and reported based on the amount of tissue hormone receptors as a result.
The inclusion criteria were: Women with a definitive diagnosis of endometriosis based on a pathology report, complete demographic, and follow-up information retrieved over a phone call (every 6 months, for at least 1 yr after the surgery), those whose VAS score in all the pain symptoms of endometriosis (dysmenorrhea or dyspareunia) were moderate to severe before the surgery (18), those with data about pain response to progesterone-based therapies after surgical treatment, unwillingness to conceive until at least 2 yr after the surgery, a tendency to continue the treatment, despite knowing that they will not have a menstruation period during this time, and women with a regular follow-up after the surgery, and no contraindication for hormonal treatment.
The exclusion criteria were: Incomplete records and follow-up, women with gastrointestinal or urinary tract diseases, or pelvic inflammatory disease, were on hormonal or infertility drugs up to 6 months before the surgery, had previous endometriosis surgery, follicle-stimulating hormone > 10 before the operation, age > 45 at the time of operation, subjects who for some reason stopped taking medications following the operation or used them irregularly, the existence of concomitant malignancy, and unavailability of pathology slides.
2.1. Sample size
Out of 1500 women who underwent surgery, 96 of them met the inclusion criteria, whose medical records were analyzed to assess their response to medical treatment. 100% of them complained of dysmenorrhea and 80% complained of simultaneous preoperative dyspareunia. Meanwhile, in only 86 individuals, the tissue samples were sufficient for pathological examination.
2.2. Data collection
Data were extracted from participants’ medical records. Demographic information, such as age, body mass index, pain symptoms, and the stage of endometriosis disease according to the American Society for Reproductive Medicine classification, the affected area, the type of hormone therapies, treatment duration, and response to the treatment according to VAS were recorded in a checklist.
After all, adhesions were lysed and excised by sharp dissection to fully mobilize the ovaries and ovarian cystectomy; all the areas of superficial active endometriosis involving the other ovary or the pelvic peritoneum were fulgurated. Deep infiltrative endometriotic lesions located in the uterosacral, retrocervical, and rectovaginal area, Douglas pouch, rectum, and bladder were separated and resected from the surrounding normal tissue; while preserving important structures such as the ureter, uterine vessels, and pelvic nerves.
The participants were aware of the 2 treatment methods before their operation and were informed that none had been proven to be superior yet. All the participants were either at stage 3 or 4 of the disease according to the American Society for Reproductive Medicine classification. Medical treatment was initiated on the day of discharge based on the patient’s preference, which included 30 mg daily medroxyprogesterone (Medrofem tablet 5 mg, Iran Hormone Co., Iran) or contraceptive pills with 0.03 mg ethinyl estradiol and 15 ug desogestrel (Desoceptive tablet, Iran Hormone Co., Iran). Medication was administered on a seasonal basis. The women were screened for the recurrence of the disease every 6 months (by ultrasound imaging). During the follow-up period, pain symptoms were checked and recorded at each visit. All the patients were followed-up for at least 12 month after the surgery (8-25 month). Failure in response to medical treatment was defined as new onset or persistence of endometriosis-related pain symptoms in the absence of recurrence of disease in 6 and 12 month of follow-up visits as a VAS score > 5. No recurrence was reported during the follow-up period.
After selecting the subjects based on the inclusion and exclusion criteria and extracting the required data from the patients' records, patients' surgical pathological slides were re-examined and stained for PR and ER for immunohistochemistry.
Primarily, the samples in paraffin were cut down to a size of 5 µm and placed on a slide (19, 20). The slides were deparaffinized and hydrated by a series of washes with xylene and ethanol. After rinsing in distilled water for 5 min, the slides were immersed in 0.01 M sodium citrate buffer for 15 min and then cooled down for 45 min.
The slides were then rinsed in phosphate-buffered saline (PBS) 1%, phosphate-buffered saline tween 20 (PBST) for 5 min (Thermo Scientific Pierce 20X PBS tween-20 was a space-saving stock solution ideal for preparing PBS-tween [PBS-T] wash buffers for the enzyme-linked immunosorbent assay, Western, and other immunoassays as well as a blocking buffer for plate-based assays) and cut with a hydrophobic pen. Endogenous peroxidase was quenched for 5 min with 3% hydrogen peroxide and then rinsed with PBST for 5 min. Nonspecific binding with 5% natural goat serum in PBST was blocked for 1 hr at room temperature.
The primarily used antibodies (PR H-190) (sc_7208; 1: 800) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The slides were incubated with the initial antibody at 4°C overnight. Normal goat IgG (Biotechnology Santa Cruz, CA) was used as a negative control.
Natural endometrium on day 14 was also considered a positive control. Goat-antirabbit biotinylated-secondary antibody was utilized for PR (Vector Laboratories, Burlingame, CA) for 1 hr at room temperature. The slides were washed in 1% PBS and incubated at ABC Elite (Vector Laboratories) for 30 min at room temperature, then washed again with 1% PBS, and incubated with diaminobenzidine (Vector Laboratories) for 41 sec.
They were subsequently exposed to hematoxylin as a counterstain for 30 sec, and finally, rinsed with ethanol and xylene for 5 min and washed and mounted with permount (Thermo Fisher Scientific, Waltham, MA) (7, 21, 22). For each slide, the histopathologic score (H-score) for immunohistochemical staining was determined based on the receptor staining percentage. Each slide was scored separately by 2 pathologists unaware of the patients, and the H-scores were average. The H-score was calculated using the modified version, expressed as negative (score 0), weakly positive (score 1), positive (score 2), and strongly positive (score 3) (8, 22, 23).
2.3. Ethical considerations
The Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran approved this research (Code: IR.SUMS.REC.1398.1395). All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the participants for using their medical information on the first visit after surgery. This was done so that their information files and histological samples archived in the pathology department at the first visit in both clinics could be used.
2.4. Statistical analysis
The collected data were entered in Statistical Package for the Social Sciences, version 20.0, Statistical Package for the Social Sciences (SPSS) Inc., Chicago, Illinois, USA. For the final analysis, the participants were divided into 2 groups based on their response to the treatment, the data of the 2 groups were then compared. Qualitative data were compared using the Chi-square test and if necessary, Fisher’s exact test, the quantitative data were compared using the t test. P < 0.05 were considered statistically significant.
3. Results
In this study, 96 women met the inclusion criteria, whose medical records were analyzed to assess their response to medical treatment. 100% of them complained of dysmenorrhea and 80% complained of simultaneous preoperative dyspareunia. Meanwhile, in only 86 individuals, the tissue samples were sufficient for pathological examination.
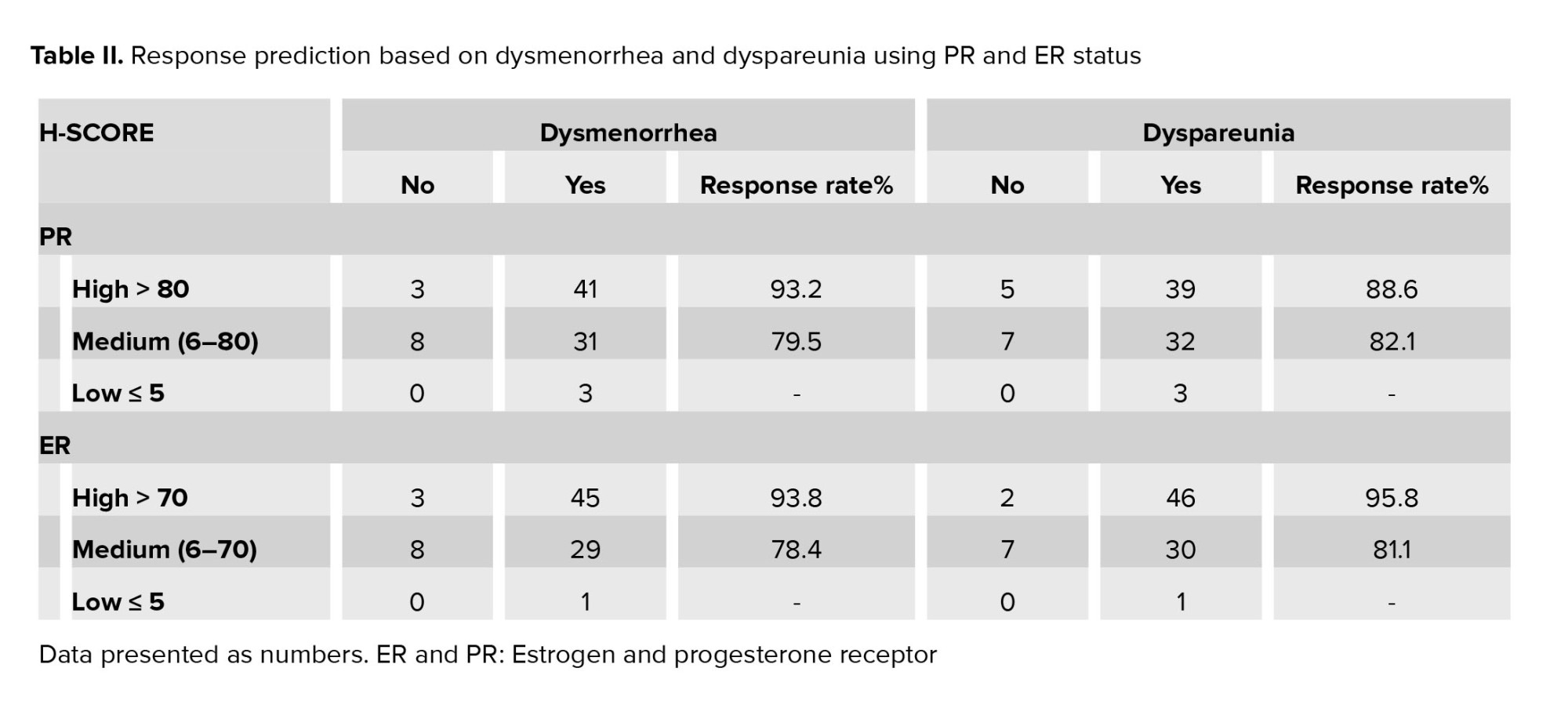
Demographic characteristics based on the improvement of dysmenorrhea or dyspareunia were the same for the responsive and nonresponsive groups (Table I). The women’s mean age and mean body mass index were 34.71 ± 6.01 and 24.04 ± 4.16, respectively. Based on receiver operating characteristic (ROC) curve analysis with 2 threshold strategies, all individuals were categorized into 3 groups based on estrogen and PR density: low, moderate, and high receptor density as shown in the table II.
According to the data analysis, an H-score ≤ 5 was selected owing to high sensitivity (100%), and an H-score ≥ 80 for the PR and ≥ 70 for the ER were selected because of high specificity (100%).
Response to treatment of dysmenorrhea and dyspareunia (VAS < 3) was directly related to the increase of H-score, which is shown in table II. By elevating the PR and estrogen H-score from medium to high, the treatment response rises. While this enhanced response to treatment with increased tissue receptor was statistically significant solely in the high ER group (p < 0.05).
Due to a small sample size in the group with a low H-score, the results obtained from this group could not be considered valid.
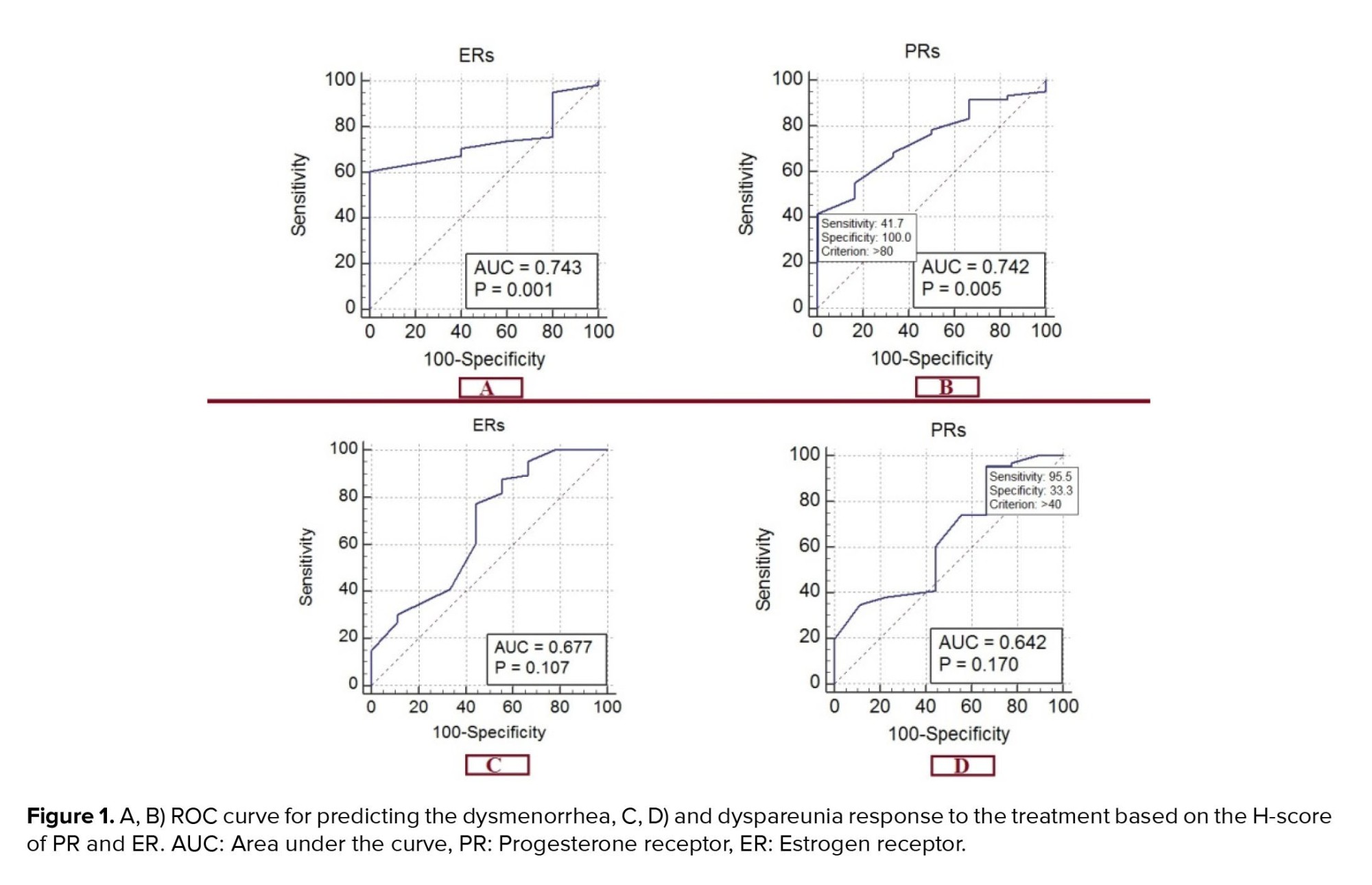
As shown in figure 1, the ROC curve for predicting the dysmenorrhea response was based on the H-score, which shows an area below the curve of 0.677 for ER (95% CI: 0.559-0.780). This predicts a good response of dysmenorrhea to treatment with a sensitivity of 77.27% and a specificity of 55.56% in the presence of 60% ER in the tissue sample (p = 0.1065). For PR, the area below the curve was 0.642 (95% CI: 0.523-0.750), predicting the response to the treatment of dysmenorrhea with a sensitivity of 95.45% and a specificity of 33.33% in the presence of 40% PR in the tissue sample (p = 0.1699).
The ROC curve for predicting the dyspareunia response is based on the H-score for the ER, which shows an area below the curve of 0.743 (95% CI: 0.620-0.842). This predicts the response of dyspareunia to treatment with a sensitivity of 60.66% and a specificity of 100% in the presence of 70% ER in the tissue sample (p = 0.001). The area under the curve is 0.742 (95% CI: 0.619-0.842) for PR. This predicts the response of dyspareunia to the treatment with a sensitivity of 41.67% and a specificity of 100% in the presence of 80% of PR in the tissue sample (p = 0.005).
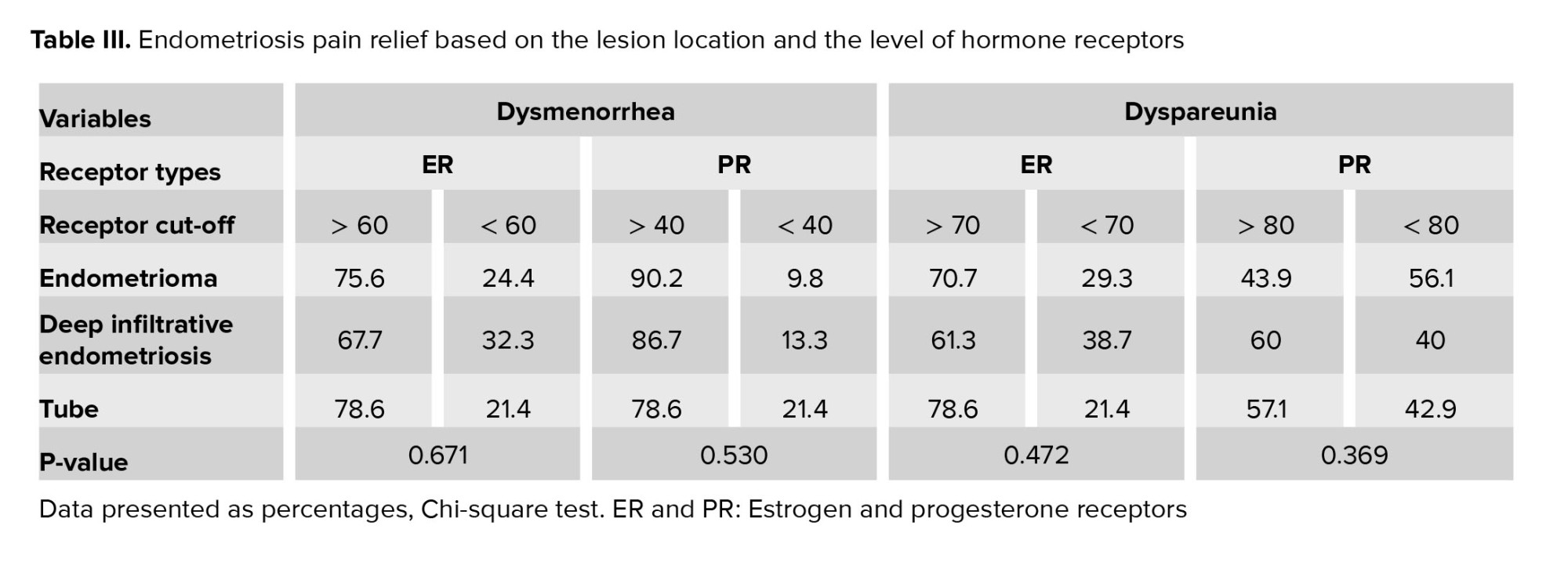
The rates of improvement for dysmenorrhea and dyspareunia are summarized in table III based on the cutoff presented in ROC curve for 3 types of endometriosis lesions.
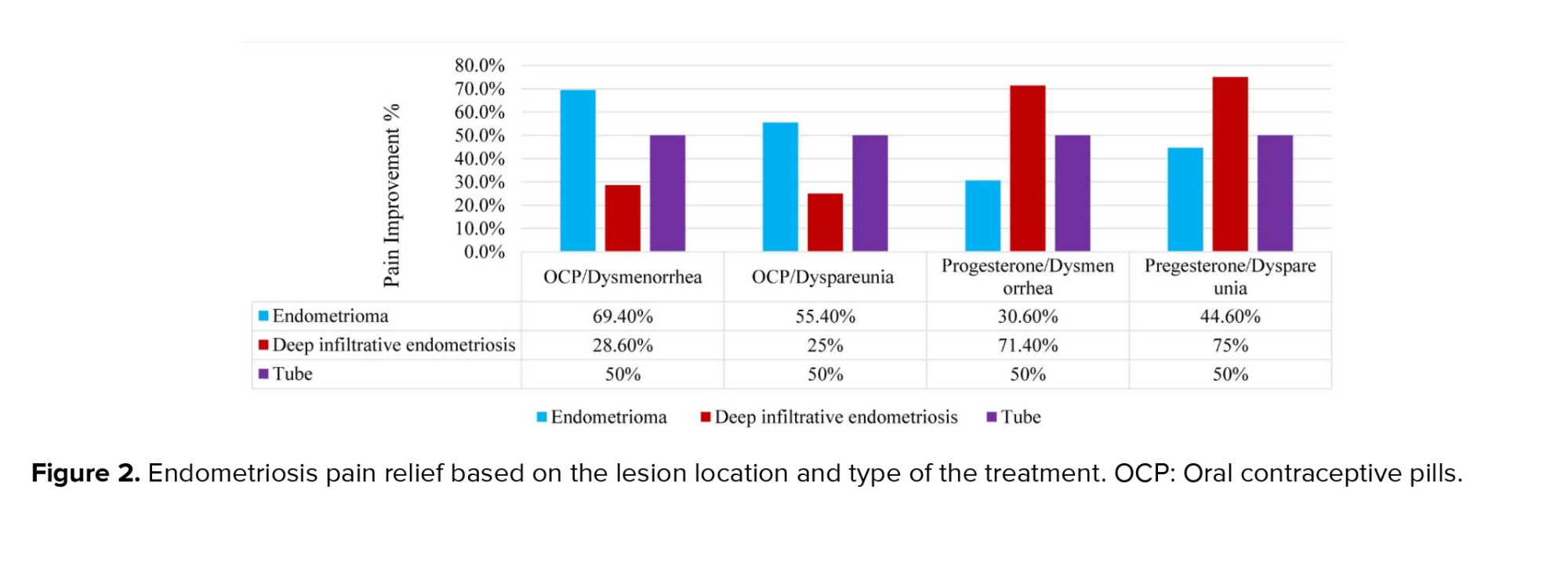
Based on figure 2, while the type of treatment for tubal lesions does not show a difference in pain reduction, OCP treatment appears to yield a better pain response in endometrioma lesions, and progesterone treatment demonstrates a better pain response in DIE lesions, although neither of these differences are statistically significant (p > 0.05).
4. Discussion
This study showed that the levels of progesterone and ERs in endometriotic lesions could strongly predict the response of these patients to drug therapy (including OCP and progesterone). Our results revealed that the response to treatment of dysmenorrhea and dyspareunia is directly related to the increase in H-score. Based on previous studies in this field and those on breast lesions, we decided to set a threshold for the number of estrogen and PR in endometriotic lesions (17-24).
Therefore, we set a cut-off point to predict the response to the treatment of these lesions with the following goals: to improve the quality of life in these women and reduce the financial burden of this disease on society given the long-term need for treatment and follow-up. Accordingly, for predicting the appropriate response of dyspareunia to the treatment based on the ROC curve (p < 0.05), we set a 70% threshold for tissue estrogen and an 80% threshold for PRs, along with a 60% threshold for tissue estrogen and a 40% for PR (p > 0.05) to predict the appropriate response of dyspareunia and dysmenorrhea to the treatment.
Based on our study results, in the case of endometrioma (OMA), DIE, and tubal lesions pain symptoms responded better to the treatment once the estrogen and PR levels were higher than the cut-off point. Comparing the pain symptoms improvement and the role of determining tissue hormone receptors, it should be said that in most OMA lesions, tissue ERs were determined more than the cut-off point. While in the DIE lesions, tissue PR were determined more than the cut-off point, which justifies the response to the treatment of lesions to OCP or progesterone treatment; however, this difference was not statistically significant.
A few studies have investigated the status of PR and ER and its role in predicting the treatment response in patients with endometriosis (21, 23-28). Similarly, Flores et al., 2018 examined the status of PR and its role in predicting the response of endometriotic lesions to progesterone treatment. In their study, H-score was used to determine the qualitative status of PR. H-score was higher in responsive patients, and the treatment response status was strongly associated with PR. They concluded that depending on the number of receptors in endometriosis, different hormone-based therapies could be pursued after surgery (15).
In 2017, Hou et al., examined the role of predictive biomarkers in the accurate treatment of endometriosis. They investigated the effect of bazedoxifene and medroxyprogesterone acetate on the expression of PR, ER, and aromatase enzyme (CYP19A1) genes in the cell culture media obtained from the patient biopsy with endometriosis. They concluded that the degree of PR expression might predict progesterone resistance as well as response to treatment of endometriotic lesions (29).
It is yet to be determined, whether the heterogeneity observed in the expression of hormone receptors in different types of tissues can explain the difference in patients' response to hormone therapy (30). Based on table III, concerning OMA, DIE, and tubal lesions dysmenorrhea, and especially dyspareunia, responded better to the treatment if estrogen and PR levels were higher than the cut-off point. Our obtained data also confirmed previous findings that showed variable levels of PR in various endometriotic lesions. Thus, it is best to treat all endometriotic lesions based on the expression of their hormone receptors (31).
In line with the present study, Colón-Caraballo et al., 2019 conducted their research aiming at expressing the concentrations of steroid receptor hormones in different types of endometriotic lesions and also eutopic endometrium. This comparison was performed between the endometriosis and normal women (control group) using the tissue microarray method. Their results indicated that ovarian lesions had the lowest expression of ER1 (alpha estrogen) and PGR (progesterone) and the highest ER2 (beta estrogen) expression, while the highest expression of all 3 receptors belonged to the fallopian tube lesions. The highest ER2: ER1 ratio was observed in ovarian and endometrial secretory lesions (32). Other studies in this area also show overexpression of ER2/ERS1 in endometriosis tissues, which leads to increased proliferation in lesions and also induces progesterone resistance (13, 14, 33, 34).
This shows a clear association between ER and PR expression, and its secretion cycle with the development and progression of endometriosis. Increased expression of B ER leads to more local production of estrogen as well as suppression of PR in endometriosis tissue. In addition, a lack of PR in these tissues was observed, which leads to progesterone resistance, commonly reported as estrogen-dependent and progesterone-resistant in the endometriosis tissue (32). Thus, the IHC expression characteristics of nuclear isoforms of ER and PR in target tissue sampled during surgery can predict the response to commonly prescribed drugs.
Given the fact that the overexpression of ER2/ER1 in hormone-dependent malignancies, such as breast and prostate cancer, and also endometrial cancers, are associated with a high-grade tumoral process and predicts clinical outcomes, including the overall survival rate of patients, we can use the results of this study for the same purposes in patients with endometriosis (13, 35-37). In our study, the improvement of dysmenorrhea and dyspareunia in tubular lesions did not depend on the type of treatment, but in the case of the OMA, dysmenorrhea responded better to the treatment with OCP. Additionally, in the case of DIE lesions, the dysmenorrhea and dyspareunia responded better to the treatment with progesterone compared with those in OCP. Hence, we found a difference in PR levels between the patients and even in an individual, based on the type of endometriotic lesions which can predict the treatment response in endometriotic people and help to choose a better treatment for each individual. It may also prevent the recurrence of the disease following surgery.
The significant improvement in dyspareunia compared to dysmenorrhea in our study confirmed the higher PR levels of pelvic endometriotic lesions compared with ovarian endometrioma lesions (13).
According to our findings, a gynecologist can opt for the right hormonal treatment (such as OCP and progesterone) based on the specific pattern of IHC staining obtained from the patients surgical specimens, resulting in improved quality of life and effective pain reduction. Therefore, disease recurrence in the reproductive age may be prevented by prescribing an appropriate treatment. The limitation of our study is the lack of estrogen subtype determination, since through comparing the difference in ER2/1 ratio in endometrioma, other DIE lesions, and the short length and follow-up time for evaluation of the recurrence rate based on the type of treatment and concentration of hormone receptor in these lesions.
5. Conclusion
Prescribing the correct hormone therapy based on a specific IHC staining pattern can enhance the quality of life for postoperative endometriosis patients and decrease the likelihood of pain recurrence. Therefore, gynecologists can recommend appropriate hormonal treatments (like OCP and progesterone) by analyzing the specific pattern of IHC staining from patients' surgical samples, resulting in improved quality of life and effective pain relief postsurgery.
Data availability
Study data can be shared electronically through the corresponding author's email if necessary.
Author contributions
Tahereh Poordast: Conception and design of study, Saeed Alborzi: Conception and design of study, Navid Omidfar: Conception, design of study, and patient recruitment, Kefayat Chamanara: Patient recruitment, and manuscript preparation, Mansoureh Shokripour: Conception and design of study, and patient recruitment. Ziba Kiani: Patient recruitment & data collection. Elham Askary: Conception, design of study, final approach, data interpretation, and manuscript preparation. Alimohammad Keshtvarz Hesam Abadi: Data analysis, interpretation and final approach.
Acknowledgments
The authors would like to thank all the staff members of the surgical and laboratory units of Shahid Faghihi and Hazrate Zeinab hospitals, Shiraz, Iran for their expert assistance in data collection. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We did not use of artificial intelligence for producing this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Endometriosis is a complex disease commonly seen in about 10% of women of reproductive age with presence of endometrial glands and stroma outside the uterine cavity (1). Symptoms like dysmenorrhea, dyschezia, dyspareunia, as well as infertility are associated with endometriosis, all of which can affect a person's quality of life significantly (2, 3).
The objective of endometriosis treatment is to reduce inflammation, disease activity, and alleviate the pain. For this purpose, several treatments have been suggested, including hormonal and nonhormonal therapies, (such as gonadotropin-releasing hormone, oral contraceptive pill [OCP], progestin, and nonsteroidal anti-inflammatory drugs) (4). Several hypotheses justify the onset and recurrence of endometriotic lesions; however, estrogen dependence and progesterone resistance have been shown to play a role in the recurrence of endometriotic lesions after surgery (5). In the endometrial implants, estradiol typically stimulates cell proliferation and progesterone stimulates cell differentiation. The function of ovarian hormones is mediated by estrogen (α and β) or (1 and 2) and progesterone receptors (PRs) (A and B). Previous studies have shown that the overexpression of hormone receptors, such as high estrogen receptor (ER)2/ER1 ratio and reduced PR expression play a role in endometriotic lesions. This receptor-mediated signal disorder affects cellular behavior and causes different responses to hormonal therapies (6-8).
One of the most common hormonal treatments for endometriosis is the use of progesterone or the compounds containing it. Progesterone (preg-4-ene-3, 20-dione) is a natural cholesterol catabolite of cyclopentanephydrofentanthrene (cyclopentanoperhydrophenanthrene) that is naturally produced in the corpus luteum (7). Progesterone functions by regulating endometrial decidualization and inhibiting estrogen-derived endometrial proliferation (7, 8). However, in some patients it has been observed that despite the similarity of serum progesterone levels in healthy and endometriotic women, endometriotic lesions do not respond adequately to progesterone (9). In endometriotic lesions, PR expression seems to undergo some changes (10-13).
On the other hand, available sources strongly support the benefits associated with the long term use of hormone therapy after surgery to prevent the recurrence of endometriosis and related symptoms, particularly dysmenorrhea. The lack of improvement in some patients may suggest an inappropriate response to the routine hormonal therapies in these cases. This is, especially true for those who relapse despite receiving hormone therapy after surgery (7, 10-15).
In addition, the production of estradiol (intracrine and paracrine) in endometriotic lesions increases the concentration of steroid hormones and enhances the estrogenic effect. Endometriotic lesions exert a lower level of estradiol inactivation compared to eutopic endometrium, which may further enhance the local effects. A few studies have investigated the role of PR in patients with endometriosis and its effect on treatment failure and disease recurrence (7, 13, 15-17). These studies have mainly focused on dysmenorrhea and endometrioma recurrence rather than dyspareunia and deep infiltrating endometriosis (DIE) lesions (16).
Therefore, the present research aimed to measure the levels of PR and ER in endometriotic lesions and determine a cut-off point for selecting the appropriate treatment based on the hormone receptors of these lesions to help improve the quality of life in patients with endometriosis.
The current article titled "Evaluation of progesterone and estrogen receptor (PR & ER) levels and their role in medical treatment of endometriosis patients" was published on Research Square's preprint site in October 2021 with DOI: 10.21203/rs.3.rs991753/v1.
2. Materials and Methods
In this cross-sectional study, from March 2017-2019, 1500 endometriotic women were referred to Shahid Faghihi and Hazrate Zeinab hospitals, Shiraz, Iran for endometriosis surgery based on the inclusion and exclusion criteria. By reviewing the medical records of patients and testing their archive samples and phone interview if needed, only 86 people were ultimately included in the study.
All women were divided into 2 groups: responsiveness to medical treatment and surgery, and unresponsiveness to surgery and medical treatment. Nonresponse criteria consisted of individuals whose endometriosis pain did not show improvement before the surgery, or experienced pain recurrence with a visual analogue scale (VAS) score > 5 during the 6- and 12-month follow-up visits. They showed no signs of recurrence in the subsequent ultrasound. All individuals were examined and analyzed separately regarding 2 common symptoms of endometriosis, that is, dysmenorrhea and dyspareunia. The relationship between the response to pain of each group was investigated and reported based on the amount of tissue hormone receptors as a result.
The inclusion criteria were: Women with a definitive diagnosis of endometriosis based on a pathology report, complete demographic, and follow-up information retrieved over a phone call (every 6 months, for at least 1 yr after the surgery), those whose VAS score in all the pain symptoms of endometriosis (dysmenorrhea or dyspareunia) were moderate to severe before the surgery (18), those with data about pain response to progesterone-based therapies after surgical treatment, unwillingness to conceive until at least 2 yr after the surgery, a tendency to continue the treatment, despite knowing that they will not have a menstruation period during this time, and women with a regular follow-up after the surgery, and no contraindication for hormonal treatment.
The exclusion criteria were: Incomplete records and follow-up, women with gastrointestinal or urinary tract diseases, or pelvic inflammatory disease, were on hormonal or infertility drugs up to 6 months before the surgery, had previous endometriosis surgery, follicle-stimulating hormone > 10 before the operation, age > 45 at the time of operation, subjects who for some reason stopped taking medications following the operation or used them irregularly, the existence of concomitant malignancy, and unavailability of pathology slides.
2.1. Sample size
Out of 1500 women who underwent surgery, 96 of them met the inclusion criteria, whose medical records were analyzed to assess their response to medical treatment. 100% of them complained of dysmenorrhea and 80% complained of simultaneous preoperative dyspareunia. Meanwhile, in only 86 individuals, the tissue samples were sufficient for pathological examination.
2.2. Data collection
Data were extracted from participants’ medical records. Demographic information, such as age, body mass index, pain symptoms, and the stage of endometriosis disease according to the American Society for Reproductive Medicine classification, the affected area, the type of hormone therapies, treatment duration, and response to the treatment according to VAS were recorded in a checklist.
After all, adhesions were lysed and excised by sharp dissection to fully mobilize the ovaries and ovarian cystectomy; all the areas of superficial active endometriosis involving the other ovary or the pelvic peritoneum were fulgurated. Deep infiltrative endometriotic lesions located in the uterosacral, retrocervical, and rectovaginal area, Douglas pouch, rectum, and bladder were separated and resected from the surrounding normal tissue; while preserving important structures such as the ureter, uterine vessels, and pelvic nerves.
The participants were aware of the 2 treatment methods before their operation and were informed that none had been proven to be superior yet. All the participants were either at stage 3 or 4 of the disease according to the American Society for Reproductive Medicine classification. Medical treatment was initiated on the day of discharge based on the patient’s preference, which included 30 mg daily medroxyprogesterone (Medrofem tablet 5 mg, Iran Hormone Co., Iran) or contraceptive pills with 0.03 mg ethinyl estradiol and 15 ug desogestrel (Desoceptive tablet, Iran Hormone Co., Iran). Medication was administered on a seasonal basis. The women were screened for the recurrence of the disease every 6 months (by ultrasound imaging). During the follow-up period, pain symptoms were checked and recorded at each visit. All the patients were followed-up for at least 12 month after the surgery (8-25 month). Failure in response to medical treatment was defined as new onset or persistence of endometriosis-related pain symptoms in the absence of recurrence of disease in 6 and 12 month of follow-up visits as a VAS score > 5. No recurrence was reported during the follow-up period.
After selecting the subjects based on the inclusion and exclusion criteria and extracting the required data from the patients' records, patients' surgical pathological slides were re-examined and stained for PR and ER for immunohistochemistry.
Primarily, the samples in paraffin were cut down to a size of 5 µm and placed on a slide (19, 20). The slides were deparaffinized and hydrated by a series of washes with xylene and ethanol. After rinsing in distilled water for 5 min, the slides were immersed in 0.01 M sodium citrate buffer for 15 min and then cooled down for 45 min.
The slides were then rinsed in phosphate-buffered saline (PBS) 1%, phosphate-buffered saline tween 20 (PBST) for 5 min (Thermo Scientific Pierce 20X PBS tween-20 was a space-saving stock solution ideal for preparing PBS-tween [PBS-T] wash buffers for the enzyme-linked immunosorbent assay, Western, and other immunoassays as well as a blocking buffer for plate-based assays) and cut with a hydrophobic pen. Endogenous peroxidase was quenched for 5 min with 3% hydrogen peroxide and then rinsed with PBST for 5 min. Nonspecific binding with 5% natural goat serum in PBST was blocked for 1 hr at room temperature.
The primarily used antibodies (PR H-190) (sc_7208; 1: 800) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The slides were incubated with the initial antibody at 4°C overnight. Normal goat IgG (Biotechnology Santa Cruz, CA) was used as a negative control.
Natural endometrium on day 14 was also considered a positive control. Goat-antirabbit biotinylated-secondary antibody was utilized for PR (Vector Laboratories, Burlingame, CA) for 1 hr at room temperature. The slides were washed in 1% PBS and incubated at ABC Elite (Vector Laboratories) for 30 min at room temperature, then washed again with 1% PBS, and incubated with diaminobenzidine (Vector Laboratories) for 41 sec.
They were subsequently exposed to hematoxylin as a counterstain for 30 sec, and finally, rinsed with ethanol and xylene for 5 min and washed and mounted with permount (Thermo Fisher Scientific, Waltham, MA) (7, 21, 22). For each slide, the histopathologic score (H-score) for immunohistochemical staining was determined based on the receptor staining percentage. Each slide was scored separately by 2 pathologists unaware of the patients, and the H-scores were average. The H-score was calculated using the modified version, expressed as negative (score 0), weakly positive (score 1), positive (score 2), and strongly positive (score 3) (8, 22, 23).
2.3. Ethical considerations
The Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran approved this research (Code: IR.SUMS.REC.1398.1395). All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the participants for using their medical information on the first visit after surgery. This was done so that their information files and histological samples archived in the pathology department at the first visit in both clinics could be used.
2.4. Statistical analysis
The collected data were entered in Statistical Package for the Social Sciences, version 20.0, Statistical Package for the Social Sciences (SPSS) Inc., Chicago, Illinois, USA. For the final analysis, the participants were divided into 2 groups based on their response to the treatment, the data of the 2 groups were then compared. Qualitative data were compared using the Chi-square test and if necessary, Fisher’s exact test, the quantitative data were compared using the t test. P < 0.05 were considered statistically significant.
3. Results
In this study, 96 women met the inclusion criteria, whose medical records were analyzed to assess their response to medical treatment. 100% of them complained of dysmenorrhea and 80% complained of simultaneous preoperative dyspareunia. Meanwhile, in only 86 individuals, the tissue samples were sufficient for pathological examination.
Demographic characteristics based on the improvement of dysmenorrhea or dyspareunia were the same for the responsive and nonresponsive groups (Table I). The women’s mean age and mean body mass index were 34.71 ± 6.01 and 24.04 ± 4.16, respectively. Based on receiver operating characteristic (ROC) curve analysis with 2 threshold strategies, all individuals were categorized into 3 groups based on estrogen and PR density: low, moderate, and high receptor density as shown in the table II.
According to the data analysis, an H-score ≤ 5 was selected owing to high sensitivity (100%), and an H-score ≥ 80 for the PR and ≥ 70 for the ER were selected because of high specificity (100%).
Response to treatment of dysmenorrhea and dyspareunia (VAS < 3) was directly related to the increase of H-score, which is shown in table II. By elevating the PR and estrogen H-score from medium to high, the treatment response rises. While this enhanced response to treatment with increased tissue receptor was statistically significant solely in the high ER group (p < 0.05).
Due to a small sample size in the group with a low H-score, the results obtained from this group could not be considered valid.
As shown in figure 1, the ROC curve for predicting the dysmenorrhea response was based on the H-score, which shows an area below the curve of 0.677 for ER (95% CI: 0.559-0.780). This predicts a good response of dysmenorrhea to treatment with a sensitivity of 77.27% and a specificity of 55.56% in the presence of 60% ER in the tissue sample (p = 0.1065). For PR, the area below the curve was 0.642 (95% CI: 0.523-0.750), predicting the response to the treatment of dysmenorrhea with a sensitivity of 95.45% and a specificity of 33.33% in the presence of 40% PR in the tissue sample (p = 0.1699).
The ROC curve for predicting the dyspareunia response is based on the H-score for the ER, which shows an area below the curve of 0.743 (95% CI: 0.620-0.842). This predicts the response of dyspareunia to treatment with a sensitivity of 60.66% and a specificity of 100% in the presence of 70% ER in the tissue sample (p = 0.001). The area under the curve is 0.742 (95% CI: 0.619-0.842) for PR. This predicts the response of dyspareunia to the treatment with a sensitivity of 41.67% and a specificity of 100% in the presence of 80% of PR in the tissue sample (p = 0.005).
The rates of improvement for dysmenorrhea and dyspareunia are summarized in table III based on the cutoff presented in ROC curve for 3 types of endometriosis lesions.
Based on figure 2, while the type of treatment for tubal lesions does not show a difference in pain reduction, OCP treatment appears to yield a better pain response in endometrioma lesions, and progesterone treatment demonstrates a better pain response in DIE lesions, although neither of these differences are statistically significant (p > 0.05).





4. Discussion
This study showed that the levels of progesterone and ERs in endometriotic lesions could strongly predict the response of these patients to drug therapy (including OCP and progesterone). Our results revealed that the response to treatment of dysmenorrhea and dyspareunia is directly related to the increase in H-score. Based on previous studies in this field and those on breast lesions, we decided to set a threshold for the number of estrogen and PR in endometriotic lesions (17-24).
Therefore, we set a cut-off point to predict the response to the treatment of these lesions with the following goals: to improve the quality of life in these women and reduce the financial burden of this disease on society given the long-term need for treatment and follow-up. Accordingly, for predicting the appropriate response of dyspareunia to the treatment based on the ROC curve (p < 0.05), we set a 70% threshold for tissue estrogen and an 80% threshold for PRs, along with a 60% threshold for tissue estrogen and a 40% for PR (p > 0.05) to predict the appropriate response of dyspareunia and dysmenorrhea to the treatment.
Based on our study results, in the case of endometrioma (OMA), DIE, and tubal lesions pain symptoms responded better to the treatment once the estrogen and PR levels were higher than the cut-off point. Comparing the pain symptoms improvement and the role of determining tissue hormone receptors, it should be said that in most OMA lesions, tissue ERs were determined more than the cut-off point. While in the DIE lesions, tissue PR were determined more than the cut-off point, which justifies the response to the treatment of lesions to OCP or progesterone treatment; however, this difference was not statistically significant.
A few studies have investigated the status of PR and ER and its role in predicting the treatment response in patients with endometriosis (21, 23-28). Similarly, Flores et al., 2018 examined the status of PR and its role in predicting the response of endometriotic lesions to progesterone treatment. In their study, H-score was used to determine the qualitative status of PR. H-score was higher in responsive patients, and the treatment response status was strongly associated with PR. They concluded that depending on the number of receptors in endometriosis, different hormone-based therapies could be pursued after surgery (15).
In 2017, Hou et al., examined the role of predictive biomarkers in the accurate treatment of endometriosis. They investigated the effect of bazedoxifene and medroxyprogesterone acetate on the expression of PR, ER, and aromatase enzyme (CYP19A1) genes in the cell culture media obtained from the patient biopsy with endometriosis. They concluded that the degree of PR expression might predict progesterone resistance as well as response to treatment of endometriotic lesions (29).
It is yet to be determined, whether the heterogeneity observed in the expression of hormone receptors in different types of tissues can explain the difference in patients' response to hormone therapy (30). Based on table III, concerning OMA, DIE, and tubal lesions dysmenorrhea, and especially dyspareunia, responded better to the treatment if estrogen and PR levels were higher than the cut-off point. Our obtained data also confirmed previous findings that showed variable levels of PR in various endometriotic lesions. Thus, it is best to treat all endometriotic lesions based on the expression of their hormone receptors (31).
In line with the present study, Colón-Caraballo et al., 2019 conducted their research aiming at expressing the concentrations of steroid receptor hormones in different types of endometriotic lesions and also eutopic endometrium. This comparison was performed between the endometriosis and normal women (control group) using the tissue microarray method. Their results indicated that ovarian lesions had the lowest expression of ER1 (alpha estrogen) and PGR (progesterone) and the highest ER2 (beta estrogen) expression, while the highest expression of all 3 receptors belonged to the fallopian tube lesions. The highest ER2: ER1 ratio was observed in ovarian and endometrial secretory lesions (32). Other studies in this area also show overexpression of ER2/ERS1 in endometriosis tissues, which leads to increased proliferation in lesions and also induces progesterone resistance (13, 14, 33, 34).
This shows a clear association between ER and PR expression, and its secretion cycle with the development and progression of endometriosis. Increased expression of B ER leads to more local production of estrogen as well as suppression of PR in endometriosis tissue. In addition, a lack of PR in these tissues was observed, which leads to progesterone resistance, commonly reported as estrogen-dependent and progesterone-resistant in the endometriosis tissue (32). Thus, the IHC expression characteristics of nuclear isoforms of ER and PR in target tissue sampled during surgery can predict the response to commonly prescribed drugs.
Given the fact that the overexpression of ER2/ER1 in hormone-dependent malignancies, such as breast and prostate cancer, and also endometrial cancers, are associated with a high-grade tumoral process and predicts clinical outcomes, including the overall survival rate of patients, we can use the results of this study for the same purposes in patients with endometriosis (13, 35-37). In our study, the improvement of dysmenorrhea and dyspareunia in tubular lesions did not depend on the type of treatment, but in the case of the OMA, dysmenorrhea responded better to the treatment with OCP. Additionally, in the case of DIE lesions, the dysmenorrhea and dyspareunia responded better to the treatment with progesterone compared with those in OCP. Hence, we found a difference in PR levels between the patients and even in an individual, based on the type of endometriotic lesions which can predict the treatment response in endometriotic people and help to choose a better treatment for each individual. It may also prevent the recurrence of the disease following surgery.
The significant improvement in dyspareunia compared to dysmenorrhea in our study confirmed the higher PR levels of pelvic endometriotic lesions compared with ovarian endometrioma lesions (13).
According to our findings, a gynecologist can opt for the right hormonal treatment (such as OCP and progesterone) based on the specific pattern of IHC staining obtained from the patients surgical specimens, resulting in improved quality of life and effective pain reduction. Therefore, disease recurrence in the reproductive age may be prevented by prescribing an appropriate treatment. The limitation of our study is the lack of estrogen subtype determination, since through comparing the difference in ER2/1 ratio in endometrioma, other DIE lesions, and the short length and follow-up time for evaluation of the recurrence rate based on the type of treatment and concentration of hormone receptor in these lesions.
5. Conclusion
Prescribing the correct hormone therapy based on a specific IHC staining pattern can enhance the quality of life for postoperative endometriosis patients and decrease the likelihood of pain recurrence. Therefore, gynecologists can recommend appropriate hormonal treatments (like OCP and progesterone) by analyzing the specific pattern of IHC staining from patients' surgical samples, resulting in improved quality of life and effective pain relief postsurgery.
Data availability
Study data can be shared electronically through the corresponding author's email if necessary.
Author contributions
Tahereh Poordast: Conception and design of study, Saeed Alborzi: Conception and design of study, Navid Omidfar: Conception, design of study, and patient recruitment, Kefayat Chamanara: Patient recruitment, and manuscript preparation, Mansoureh Shokripour: Conception and design of study, and patient recruitment. Ziba Kiani: Patient recruitment & data collection. Elham Askary: Conception, design of study, final approach, data interpretation, and manuscript preparation. Alimohammad Keshtvarz Hesam Abadi: Data analysis, interpretation and final approach.
Acknowledgments
The authors would like to thank all the staff members of the surgical and laboratory units of Shahid Faghihi and Hazrate Zeinab hospitals, Shiraz, Iran for their expert assistance in data collection. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We did not use of artificial intelligence for producing this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Surgery
References
1. Olive DL, Schwartz LB. Endometriosis. N Engl J Med 1993; 328: 1759-1769. [DOI:10.1056/NEJM199306173282407] [PMID]
2. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012; 98: 511-519. [DOI:10.1016/j.fertnstert.2012.06.029] [PMID] [PMCID]
3. Rossini R, Lisi G, Pesci A, Ceccaroni M, Zamboni G, Gentile I, et al. Depth of intestinal wall infiltration and clinical presentation of deep infiltrating endometriosis: Evaluation of 553 consecutive cases. J Laparoendosc Adv Surg Tech A 2018; 28: 152-156. [DOI:10.1089/lap.2017.0440] [PMID]
4. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: Endometriosis. Hum Reprod Open 2022; 2022: hoac009.
5. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: Where are we and where are we going? Reproduction 2016; 152: R63-R78. [DOI:10.1530/REP-16-0052] [PMID] [PMCID]
6. Bergqvist A, Fernö M. Estrogen and progesterone receptors in endometriotic tissue and endometrium: Comparison according to localization and recurrence. Fertil Steril 1993; 60: 63-68. [DOI:10.1016/S0015-0282(16)56037-3] [PMID]
7. García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbón M. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol 2020; 10: 935. [DOI:10.3389/fendo.2019.00935] [PMID] [PMCID]
8. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96: 623-632. [DOI:10.1111/aogs.13156] [PMID]
9. Ng S-W, Norwitz GA, Pavlicev M, Tilburgs T, Simón C, Norwitz ER. Endometrial decidualization: The primary driver of pregnancy health. Int J Mol Sci 2020; 21: 4092. [DOI:10.3390/ijms21114092] [PMID] [PMCID]
10. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 2000; 85: 2897-2902.
https://doi.org/10.1210/jcem.85.8.6739 [DOI:10.1210/jc.85.8.2897] [PMID]
11. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol Cell Endocrinol 2006; 248: 94-103. [DOI:10.1016/j.mce.2005.11.041] [PMID]
12. Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: The role of progesterone-hox gene interactions. Semin Reprod Med 2010; 28: 69-74. [DOI:10.1055/s-0029-1242996] [PMID] [PMCID]
13. Reis FM, Coutinho LM, Vannuccini S, Batteux F, Chapron C, Petraglia F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum Reprod Update 2020; 26: 565-585. [DOI:10.1093/humupd/dmaa009] [PMID] [PMCID]
14. Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med 2010; 28: 36-43. [DOI:10.1055/s-0029-1242991] [PMID] [PMCID]
15. Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone receptor status predicts response to progestin therapy in endometriosis. J Clin Endocrinol Metab 2018; 103: 4561-4568. [DOI:10.1210/jc.2018-01227] [PMID] [PMCID]
16. Somigliana E, Vercellini P, Vigano P, Benaglia L, Busnelli A, Fedele L. Postoperative medical therapy after surgical treatment of endometriosis: From adjuvant therapy to tertiary prevention. J Minim Invasive Gynecol 2014; 21: 328-334. [DOI:10.1016/j.jmig.2013.10.007] [PMID]
17. van Diest PJ, van Dam P, Henzen-Logmans SC, Berns E, van der Burg ME, Green J, et al. A scoring system for immunohistochemical staining: Consensus report of the task force for basic research of the EORTC-GCCG. J Clin Pathol 1997; 50: 801-804. [DOI:10.1136/jcp.50.10.801] [PMID] [PMCID]
18. Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, et al. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine 2018; 97: e9536. [DOI:10.1097/MD.0000000000009536] [PMID] [PMCID]
19. Nagini S. Breast cancer: Current molecular therapeutic targets and new players. Anticancer Agents Med Chem 2017; 17: 152-163. [DOI:10.2174/1871520616666160502122724] [PMID]
20. Seracchioli R, Mabrouk M, Frascà C, Manuzzi L, Savelli L, Venturoli S. Long-term oral contraceptive pills and postoperative pain management after laparoscopic excision of ovarian endometrioma: A randomized controlled trial. Fertil Steril 2010; 94: 464-471. [DOI:10.1016/j.fertnstert.2009.03.083] [PMID]
21. Miró AM, Sastre-Serra J, Pons DG, Valle A, Roca P, Oliver J. 17β-Estradiol regulates oxidative stress in prostate cancer cell lines according to ERalpha/ERbeta ratio. J Steroid Biochem Mol Biol 2011; 123: 133-139. [DOI:10.1016/j.jsbmb.2010.12.004] [PMID]
22. Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril 2019; 111: 629-640. [DOI:10.1016/j.fertnstert.2019.02.008] [PMID]
23. Torrisi R, Marrazzo E, Agostinetto E, De Sanctis R, Losurdo A, Masci G, et al. Neoadjuvant chemotherapy in hormone receptor-positive/HER2-negative early breast cancer: When, why and what? Crit Rev Oncol Hematol 2021; 160: 103280. [DOI:10.1016/j.critrevonc.2021.103280] [PMID]
24. Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol 2016; 2: 1477-1486. [DOI:10.1001/jamaoncol.2016.1897] [PMID] [PMCID]
25. Bruner-Tran KL, Herington JL, Duleba AJ, Taylor HS, Osteen KG. Medical management of endometriosis: Emerging evidence linking inflammation to disease pathophysiology. Minerva Ginecol 2013; 65: 199-213.
26. Cohen DA, Dabbs DJ, Cooper KL, Amin M, Jones TE, Jones MW, et al. Interobserver agreement among pathologists for semiquantitative hormone receptor scoring in breast carcinoma. Am J Clin Pathol 2012; 138: 796-802. [DOI:10.1309/AJCP6DKRND5CKVDD] [PMID]
27. Garcia-Alonso L, Handfield L-F, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet 2021; 53: 1698-1711. [DOI:10.1038/s41588-021-00972-2] [PMID] [PMCID]
28. Vassilopoulou L, Matalliotakis M, Zervou MI, Matalliotaki C, Spandidos DA, Matalliotakis I, et al. Endometriosis and in vitro fertilisation. Exp Ther Med 2018; 16: 1043-1051. [DOI:10.3892/etm.2018.6307] [PMID] [PMCID]
29. Hou Zh, Mamillapalli R, Taylor HS. Predictive biomarkers may allow precision therapy of endometriosis. J Endometr Pelvic Pain Disord 2017; 9: 279-285. [DOI:10.5301/jeppd.5000311] [PMID] [PMCID]
30. Nothnick WB. MicroRNAs and progesterone receptor signaling in endometriosis pathophysiology. Cells 2022; 11: 1096. [DOI:10.3390/cells11071096] [PMID] [PMCID]
31. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148: 3814-3826. [DOI:10.1210/en.2006-1692] [PMID]
32. Colón-Caraballo M, García M, Mendoza A, Flores I. Human endometriosis tissue microarray reveals site-specific expression of estrogen receptors, progesterone receptor, and Ki67. Appl Immunohistochem Mol Morphol 2019; 27: 491-500. [DOI:10.1097/PAI.0000000000000663] [PMID] [PMCID]
33. Tang Z-R, Zhang R, Lian Z-X, Deng S-L, Yu K. Estrogen-receptor expression and function in female reproductive disease. Cells 2019; 8: 1123. [DOI:10.3390/cells8101123] [PMID] [PMCID]
34. Zannoni GF, Monterossi G, De Stefano I, Gargini A, Salerno MG, Farulla I, et al. The expression ratios of estrogen receptor α (ERα) to estrogen receptor β1 (ERβ1) and ERα to ERβ2 identify poor clinical outcome in endometrioid endometrial cancer. Hum Pathol 2013; 44: 1047-1054. [DOI:10.1016/j.humpath.2012.09.007] [PMID]
35. Chantalat E, Valera MC, Vaysse C, Noirrit E, Rusidze M, Weyl A, et al. Estrogen receptors and endometriosis. Int J Mol Sci 2020; 21: 2815. [DOI:10.3390/ijms21082815] [PMID] [PMCID]
36. Peng H, Weng L, Lei S, Hou S, Yang S, Li M, et al. Hypoxia-hindered methylation of PTGIS in endometrial stromal cells accelerates endometriosis progression by inducing CD16- NK-cell differentiation. Exp Mol Med 2022; 54: 890-905. [DOI:10.1038/s12276-022-00793-1] [PMID] [PMCID]
37. Shao R, Cao S, Wang X, Feng Y, Billig H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res 2014; 6: 104-113.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




