Thu, Feb 19, 2026
[Archive]
Volume 22, Issue 3 (March 2024)
IJRM 2024, 22(3): 219-228 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khorsandi L, Heidari-Moghadam A, Younesi E, Khodayar M, Asadi-Fard Y. Naringenin ameliorates cytotoxic effects of bisphenol A on mouse Sertoli cells by suppressing oxidative stress and modulating mitophagy: An experimental study. IJRM 2024; 22 (3) :219-228
URL: http://ijrm.ir/article-1-3273-en.html
URL: http://ijrm.ir/article-1-3273-en.html
Layasadat Khorsandi1 

 , Abbas Heidari-Moghadam2
, Abbas Heidari-Moghadam2 

 , Elham Younesi3
, Elham Younesi3 

 , Mohammad-Javad Khodayar4
, Mohammad-Javad Khodayar4 

 , Yousef Asadi-Fard *5
, Yousef Asadi-Fard *5 




 , Abbas Heidari-Moghadam2
, Abbas Heidari-Moghadam2 

 , Elham Younesi3
, Elham Younesi3 

 , Mohammad-Javad Khodayar4
, Mohammad-Javad Khodayar4 

 , Yousef Asadi-Fard *5
, Yousef Asadi-Fard *5 


1- Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Department of Anatomical Sciences, School of Medicine, Dezful University of Medical Sciences, Dezful, Iran.
3- Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
4- Toxicology Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
5- Department of Anatomy, School of Medicine, Arak University of Medical Sciences, Arak, Iran. ,usef.fard@yahoo.com
2- Department of Anatomical Sciences, School of Medicine, Dezful University of Medical Sciences, Dezful, Iran.
3- Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
4- Toxicology Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
5- Department of Anatomy, School of Medicine, Arak University of Medical Sciences, Arak, Iran. ,
Keywords: Mitophagy, Naringenin, Sertoli cells, Bisphenol A, Reactive oxygen species, Pink1, Parkin.
Full-Text [PDF 2045 kb]
(884 Downloads)
| Abstract (HTML) (1122 Views)
1. Introduction
Bisphenol A (BPA), an endocrine and estrogenic disrupting agent, is widely used as polycarbonate plastics and epoxy resins for producing food containers, water bottles, medical devices, and other objects that must be made of flexible and lasting materials. It can leak into food and drinks from plastic containers and accumulate in humans' bodies (1). BPA exposure at environmentally relevant concentrations can cause reproductive disorders (2).
BPA involves multiple mechanisms, including interference with mitochondrial functions (3). The toxicity of BPA relates to the excessive production of reactive oxygen species (ROS) that overwhelm the intracellular antioxidants (4). Mitochondria is the primary source of ROS, and under physiological conditions, its production and clearance are regulated by antioxidant and oxidation systems (5). BPA can induce mitochondrial dysfunction by lowering mitochondrial membrane potential (MMP) (6).
Autophagic removal of dysfunctional mitochondria (mitophagy) is activated in response to various toxic agents (7). The PTEN-induced putative kinase 1 (Pink1)/Parkin signaling pathway is the primary mechanism of mitophagy. Pink1 and Parkin play an essential role in the final checkpoint of mitophagy (8). Sertoli cells are targeted for various chemicals, and their damage indicates testicular toxicity (9). Several investigations have evidenced that BPA affects Sertoli cell functions (10). In an experimental study, BPA time- and dose-dependency reduced Sertoli cell viability (11). The study further revealed that Sertoli cells treated with BPA in vitro at a concentration of 200 μM induced morphological distortions such as collapsing of the cytoskeleton, chromatin impairment, and DNA damage in the cells.
BPA induces Sertoli cell death in rodents by causing mitochondrial dysfunction and excessive ROS generation (4). Feng et al. showed that BPA induces cell cycle arrest and apoptosis by preventing ROS levels in the porcine Sertoli cells (12). It is, therefore, necessary to find preventive and therapeutic agents to address BPA-induced reproductive toxicity.
Natural antioxidants potentially inhibit the adverse impacts of BPA on the reproductive system (13). Naringenin (NG) is a member of bioflavonoids derived from citrus species (14).
NG has several biological and pharmacological properties, such as anti-cancer, lipid reduction, superoxide elimination, anti-atherosclerosis, and antioxidants (15-19). It has been evidenced that NG displays antioxidant effects both in vivo and in vitro (19). The beneficial impacts of NG on the reproductive system have been reported in previous studies (20, 21).
In a previous study, NG could ameliorate lead acetate-induced reproductive toxicity and increase germ cell survival (22).
Due to the widespread use of BPA and its negative effects on the human reproductive system, it seems necessary to find a compound that ameliorates its negative effects. Based on the beneficial impacts of the NG on the male reproductive system, the present work has examined the NG's impacts on BPA-induced toxicity, mitophagy, and oxidative stress in TM4 cells (a mouse Sertoli cell line).
2. Materials and Methods
2.1. Experimental design
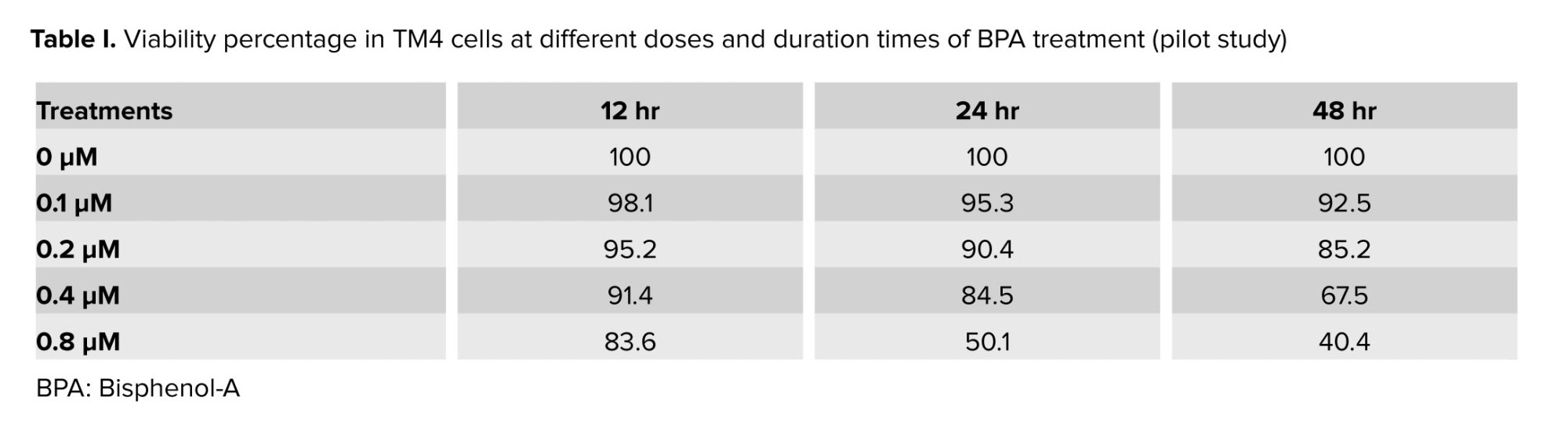
In this experimental study, the TM4 cells were purchased from the Iranian National Center for Genetic and Biological Resources. This study was performed in 2023 at the Cellular and Molecular Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The cells were cultured at 37°C in 5% CO2 in completed Dulbecco's Modified Eagle's Medium (DMEM, Merck, Germany) media. BPA and NG were dissolved in dimethyl sulfoxide (Sigma-Aldrich Chemie, Steinheim, Germany) and diluted in DMEM. The cells were treated with different concentrations of NG (Sigma-Aldrich, USA) and BPA (Sigma-Aldrich, USA). To explore the effect of NG on BPA-induced damage of TM4 cells, the cells were harvested in a medium containing various concentrations and times as a pilot study (Table I). The sublethal dose of BPA in TM4 cells was determined to be 0.8 μM. The protective dose of the NG was also identified based on a pilot study. The TM4 cells were divided into 4 groups:
2.2. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay
The cells were cultured in a 96-well plate (104 cells/well) for 24 hr. The media was discarded, and NG (25, 10, 20, 50, and 100 μg/ml) or BPA (0.8 μM/ml) for 24, 48, and 72 hr was added to indicate the concentration and duration efficacy. The cells were treated with an MTT (Sigma-Aldrich, USA) solution (0.5 mg/ml) for 3 hr. The supernatants were discarded, 100 μl dimethyl sulfoxide was added, and their absorbance was read at 570 nm.
2.3. Measurement of intracellular ROS, malondialdehyde (MDA), and antioxidant levels
ROS-producing was assessed by the florescent probe 2-7-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, USA). After treatment, the medium was removed, and the ROS indicator DCFH-DA (10 μg/ml) in media was added and incubated for 20 min (37oC). After washing the cells with PBS, the ROS levels were detected with a spectrofluorometer (LS50B, USA; Em: 570 nm; Ex: 490 nm). After homogenization, the TM4 cells were lysed, and their proteins were determined using a BCA protein assay kit (Sigma-Aldrich, USA). The MDA, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH) levels were measured based on the kit's directions (ZellBio, Germany).
2.4. Mitochondria isolation
The cells were re-suspended in an isolation buffer (250 mM sucrose, 20 mM HEPES-KOH, 10 mM KCl, 1.5 mM MgCl2, one mM EDTA, 1 mM EGTA, fat-free BSA 0.1%). The cells were homogenized and centrifuged (4°C) at 750 g for 10 min. The supernatants were again centrifuged (12,000 g) for 30 min. The pellets were then re-suspended in an isolation buffer.
2.5. MMP evaluation
Fluorescent cationic dye Rhodamine 123 (Beyotime, China) was used for the MMP measurement. The TM4 cells, after treatment, washed with PBS twice and exposed to Rhodamine 123 solution (2.5 μg/ml) for 25 min at 37oC. A spectrophotometer (LS50B, USA; emission: 535 nm; excitation: 490 nm) was used to determine fluorescence.
2.6. Real-time polymerase chain reaction
The RNeasy kit (Takara, Japan) was used to extract the RNAs from the TM4 cells (1 × 107 cells), and cDNA was made by a cDNA synthesis kit (Takara, Japan). The cDNA was amplified in PCR reaction buffer (25 μL), including SYBR green and primers. A 45 cycle was used for PCR amplification: 95°C: 10 sec; 95°C: 15 sec; 55-57°C: 20 sec; 60°C: 20 sec. Normalization of the relative gene expression was done using the housekeeping GAPDH gene. Data were analyzed by the 2-ΔΔCT method. The following primers were used in this study: GAGACGATACCGACAAACAC (forward Pink1), GGCATTTCCTCCAAGACTAAC (reverse Pink1); TGCTCGTCAACCTCTG TTC (forward Parkin), TCACTTTCTCCTTCCCATCC (reverse Parkin).
2.7. Measurement of Pink1 and Parkin protein levels
Pink1 and Parkin proteins were measured using ELISA kits (Biospes, China). Briefly, Pink1 and Parkin proteins of the mitochondrial fractions or cell lysates were bound to the primary antibodies and detected by a Horseradish peroxidase-conjugated secondary antibody. Quantification was done by recording the optical density at 450 nm. Pink1 sensitivity was more than 0.07 ng/ml in a range of 0.156-10 ng/ml, and Parkin sensitivity was 0.78 pg/ml in a range of 3.12-200 ng/ml.
1. Introduction
Bisphenol A (BPA), an endocrine and estrogenic disrupting agent, is widely used as polycarbonate plastics and epoxy resins for producing food containers, water bottles, medical devices, and other objects that must be made of flexible and lasting materials. It can leak into food and drinks from plastic containers and accumulate in humans' bodies (1). BPA exposure at environmentally relevant concentrations can cause reproductive disorders (2).
BPA involves multiple mechanisms, including interference with mitochondrial functions (3). The toxicity of BPA relates to the excessive production of reactive oxygen species (ROS) that overwhelm the intracellular antioxidants (4). Mitochondria is the primary source of ROS, and under physiological conditions, its production and clearance are regulated by antioxidant and oxidation systems (5). BPA can induce mitochondrial dysfunction by lowering mitochondrial membrane potential (MMP) (6).
Autophagic removal of dysfunctional mitochondria (mitophagy) is activated in response to various toxic agents (7). The PTEN-induced putative kinase 1 (Pink1)/Parkin signaling pathway is the primary mechanism of mitophagy. Pink1 and Parkin play an essential role in the final checkpoint of mitophagy (8). Sertoli cells are targeted for various chemicals, and their damage indicates testicular toxicity (9). Several investigations have evidenced that BPA affects Sertoli cell functions (10). In an experimental study, BPA time- and dose-dependency reduced Sertoli cell viability (11). The study further revealed that Sertoli cells treated with BPA in vitro at a concentration of 200 μM induced morphological distortions such as collapsing of the cytoskeleton, chromatin impairment, and DNA damage in the cells.
BPA induces Sertoli cell death in rodents by causing mitochondrial dysfunction and excessive ROS generation (4). Feng et al. showed that BPA induces cell cycle arrest and apoptosis by preventing ROS levels in the porcine Sertoli cells (12). It is, therefore, necessary to find preventive and therapeutic agents to address BPA-induced reproductive toxicity.
Natural antioxidants potentially inhibit the adverse impacts of BPA on the reproductive system (13). Naringenin (NG) is a member of bioflavonoids derived from citrus species (14).
NG has several biological and pharmacological properties, such as anti-cancer, lipid reduction, superoxide elimination, anti-atherosclerosis, and antioxidants (15-19). It has been evidenced that NG displays antioxidant effects both in vivo and in vitro (19). The beneficial impacts of NG on the reproductive system have been reported in previous studies (20, 21).
In a previous study, NG could ameliorate lead acetate-induced reproductive toxicity and increase germ cell survival (22).
Due to the widespread use of BPA and its negative effects on the human reproductive system, it seems necessary to find a compound that ameliorates its negative effects. Based on the beneficial impacts of the NG on the male reproductive system, the present work has examined the NG's impacts on BPA-induced toxicity, mitophagy, and oxidative stress in TM4 cells (a mouse Sertoli cell line).
2. Materials and Methods
2.1. Experimental design
In this experimental study, the TM4 cells were purchased from the Iranian National Center for Genetic and Biological Resources. This study was performed in 2023 at the Cellular and Molecular Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The cells were cultured at 37°C in 5% CO2 in completed Dulbecco's Modified Eagle's Medium (DMEM, Merck, Germany) media. BPA and NG were dissolved in dimethyl sulfoxide (Sigma-Aldrich Chemie, Steinheim, Germany) and diluted in DMEM. The cells were treated with different concentrations of NG (Sigma-Aldrich, USA) and BPA (Sigma-Aldrich, USA). To explore the effect of NG on BPA-induced damage of TM4 cells, the cells were harvested in a medium containing various concentrations and times as a pilot study (Table I). The sublethal dose of BPA in TM4 cells was determined to be 0.8 μM. The protective dose of the NG was also identified based on a pilot study. The TM4 cells were divided into 4 groups:
2.2. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay
The cells were cultured in a 96-well plate (104 cells/well) for 24 hr. The media was discarded, and NG (25, 10, 20, 50, and 100 μg/ml) or BPA (0.8 μM/ml) for 24, 48, and 72 hr was added to indicate the concentration and duration efficacy. The cells were treated with an MTT (Sigma-Aldrich, USA) solution (0.5 mg/ml) for 3 hr. The supernatants were discarded, 100 μl dimethyl sulfoxide was added, and their absorbance was read at 570 nm.
2.3. Measurement of intracellular ROS, malondialdehyde (MDA), and antioxidant levels
ROS-producing was assessed by the florescent probe 2-7-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, USA). After treatment, the medium was removed, and the ROS indicator DCFH-DA (10 μg/ml) in media was added and incubated for 20 min (37oC). After washing the cells with PBS, the ROS levels were detected with a spectrofluorometer (LS50B, USA; Em: 570 nm; Ex: 490 nm). After homogenization, the TM4 cells were lysed, and their proteins were determined using a BCA protein assay kit (Sigma-Aldrich, USA). The MDA, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH) levels were measured based on the kit's directions (ZellBio, Germany).
2.4. Mitochondria isolation
The cells were re-suspended in an isolation buffer (250 mM sucrose, 20 mM HEPES-KOH, 10 mM KCl, 1.5 mM MgCl2, one mM EDTA, 1 mM EGTA, fat-free BSA 0.1%). The cells were homogenized and centrifuged (4°C) at 750 g for 10 min. The supernatants were again centrifuged (12,000 g) for 30 min. The pellets were then re-suspended in an isolation buffer.
2.5. MMP evaluation
Fluorescent cationic dye Rhodamine 123 (Beyotime, China) was used for the MMP measurement. The TM4 cells, after treatment, washed with PBS twice and exposed to Rhodamine 123 solution (2.5 μg/ml) for 25 min at 37oC. A spectrophotometer (LS50B, USA; emission: 535 nm; excitation: 490 nm) was used to determine fluorescence.
2.6. Real-time polymerase chain reaction
The RNeasy kit (Takara, Japan) was used to extract the RNAs from the TM4 cells (1 × 107 cells), and cDNA was made by a cDNA synthesis kit (Takara, Japan). The cDNA was amplified in PCR reaction buffer (25 μL), including SYBR green and primers. A 45 cycle was used for PCR amplification: 95°C: 10 sec; 95°C: 15 sec; 55-57°C: 20 sec; 60°C: 20 sec. Normalization of the relative gene expression was done using the housekeeping GAPDH gene. Data were analyzed by the 2-ΔΔCT method. The following primers were used in this study: GAGACGATACCGACAAACAC (forward Pink1), GGCATTTCCTCCAAGACTAAC (reverse Pink1); TGCTCGTCAACCTCTG TTC (forward Parkin), TCACTTTCTCCTTCCCATCC (reverse Parkin).
2.7. Measurement of Pink1 and Parkin protein levels
Pink1 and Parkin proteins were measured using ELISA kits (Biospes, China). Briefly, Pink1 and Parkin proteins of the mitochondrial fractions or cell lysates were bound to the primary antibodies and detected by a Horseradish peroxidase-conjugated secondary antibody. Quantification was done by recording the optical density at 450 nm. Pink1 sensitivity was more than 0.07 ng/ml in a range of 0.156-10 ng/ml, and Parkin sensitivity was 0.78 pg/ml in a range of 3.12-200 ng/ml.
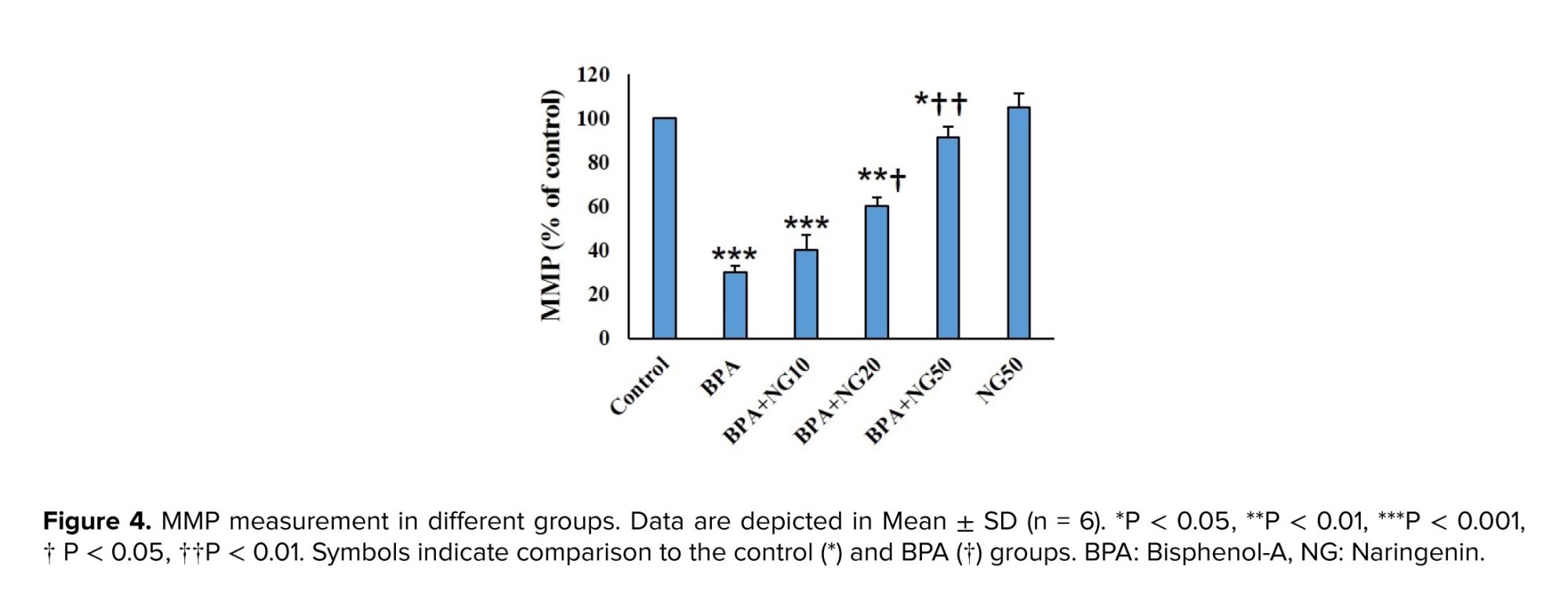
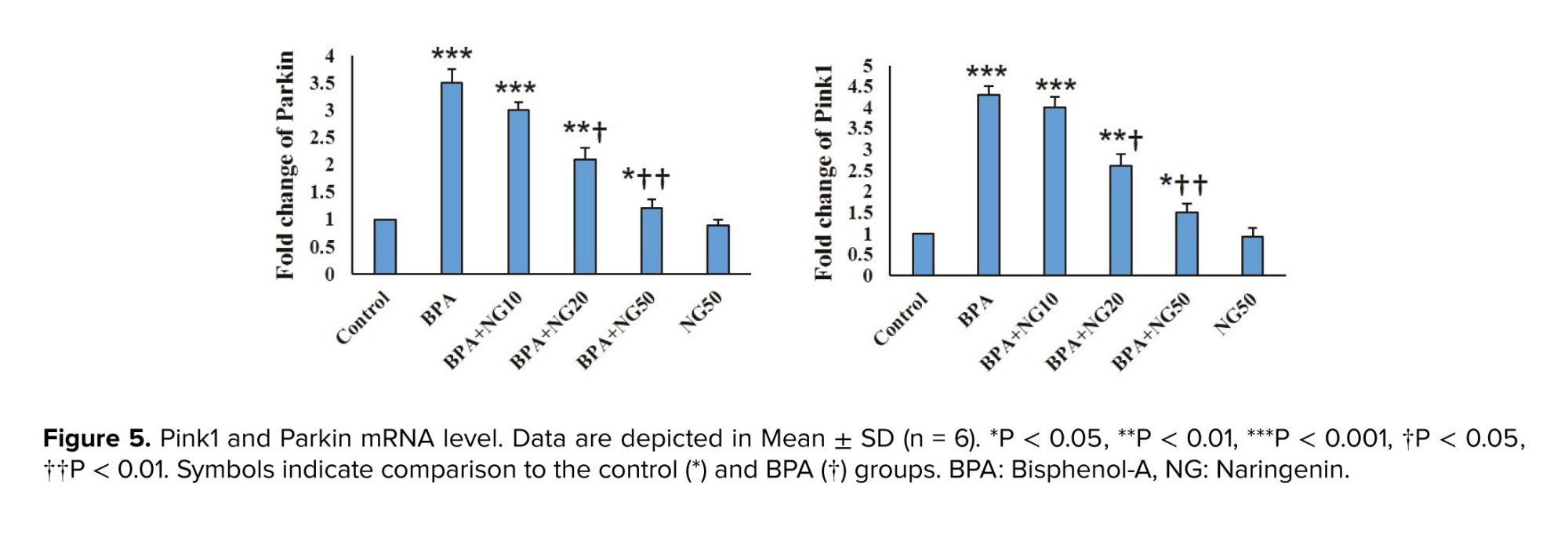
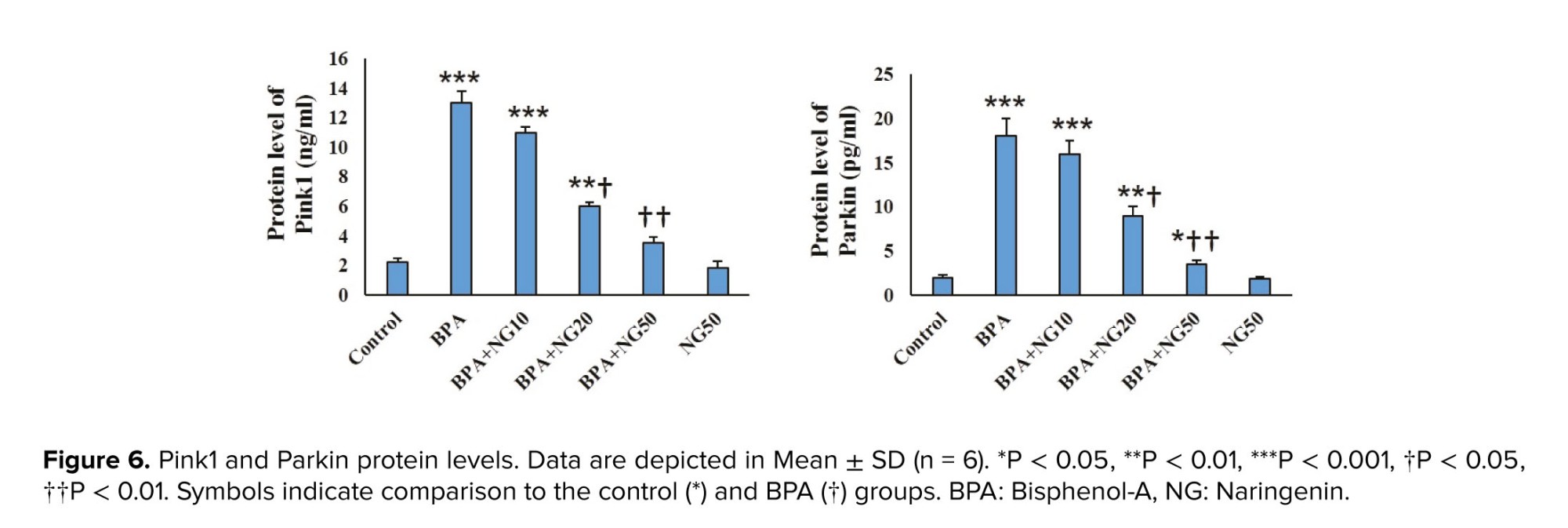
4. Discussion
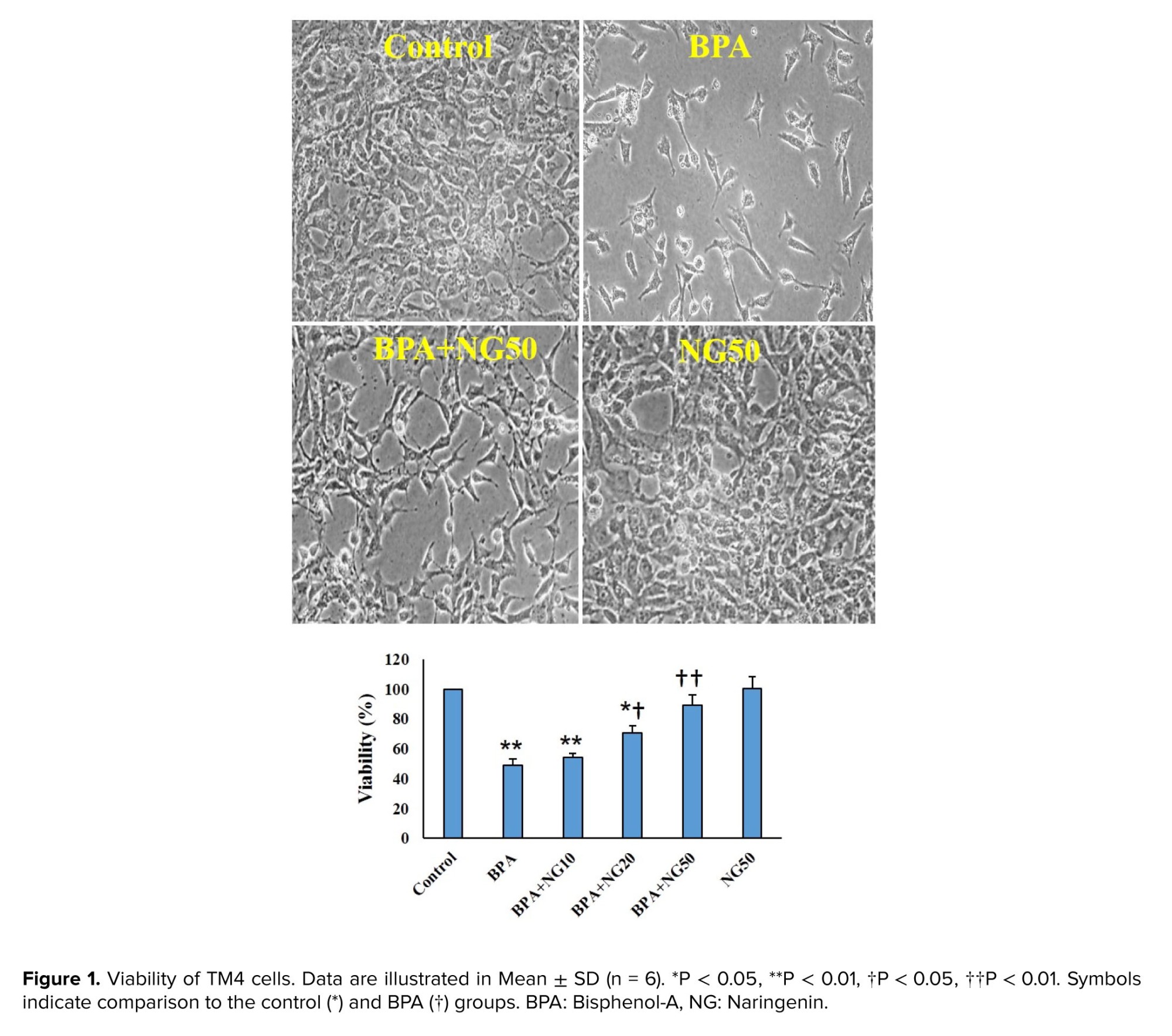
In the present study, we show the protective potential of NG against BPA-induced TM4 cell toxicity. BPA induced cytotoxicity by decreasing cell viability, enhancing ROS production, stimulating mitophagy, and disrupting mitochondria in the Sertoli cells. The reduced viability demonstrated the toxic effects of BPA on the TM4 Sertoli cells. In line with our results, a previous study showed that BPA reduced the survival rate of the TM4 cells (23).
NG dose-dependently reversed the viability of the BPA-exposed cells. In parallel to our findings, have reported the protective impacts of NG against permethrin-induced testicular toxicity in rats (24). Have demonstrated that NG improves some reproductive parameters, apoptosis, and oxidative stress in acrylamide-induced testis toxicity of rats (25).
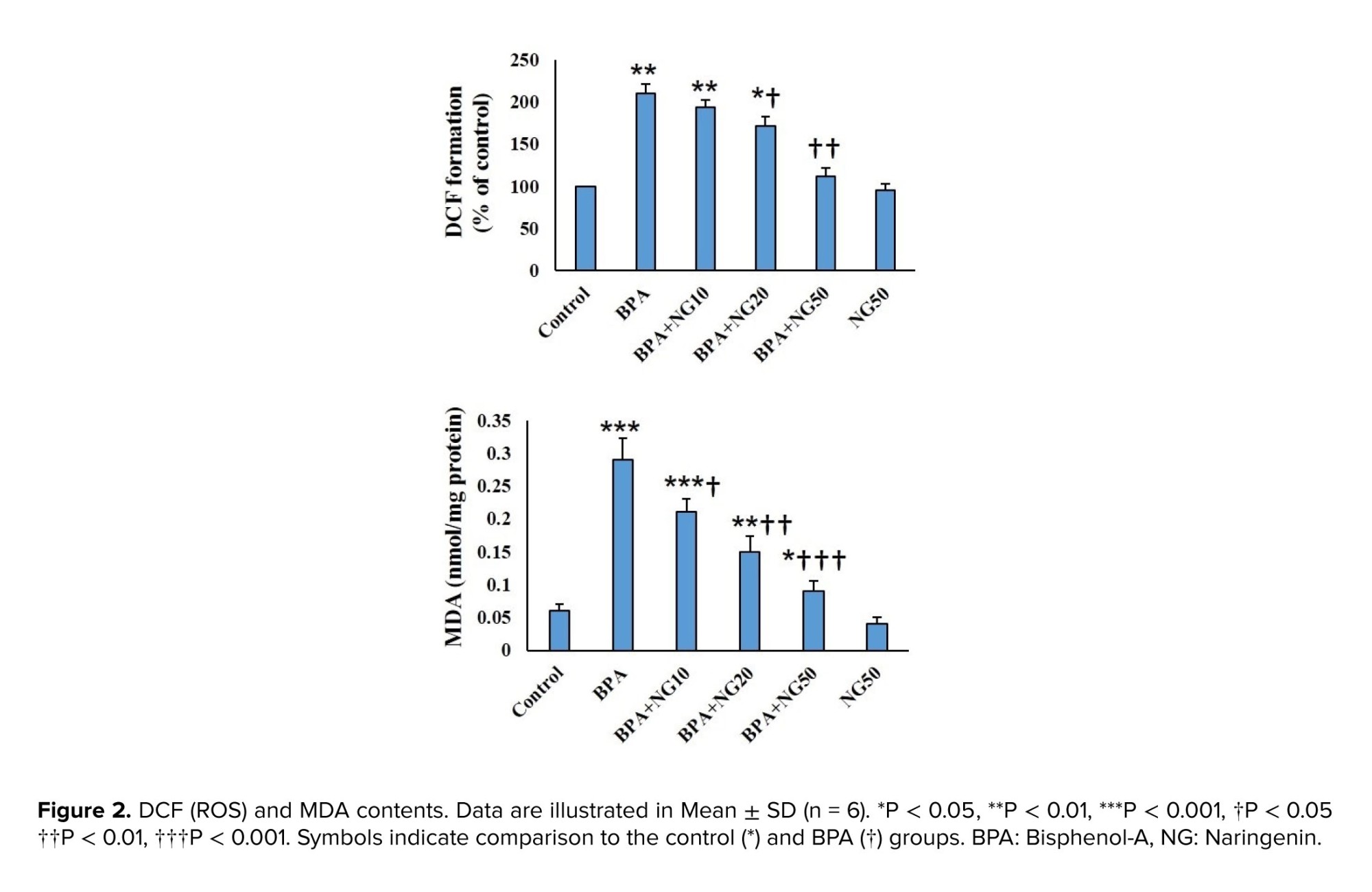
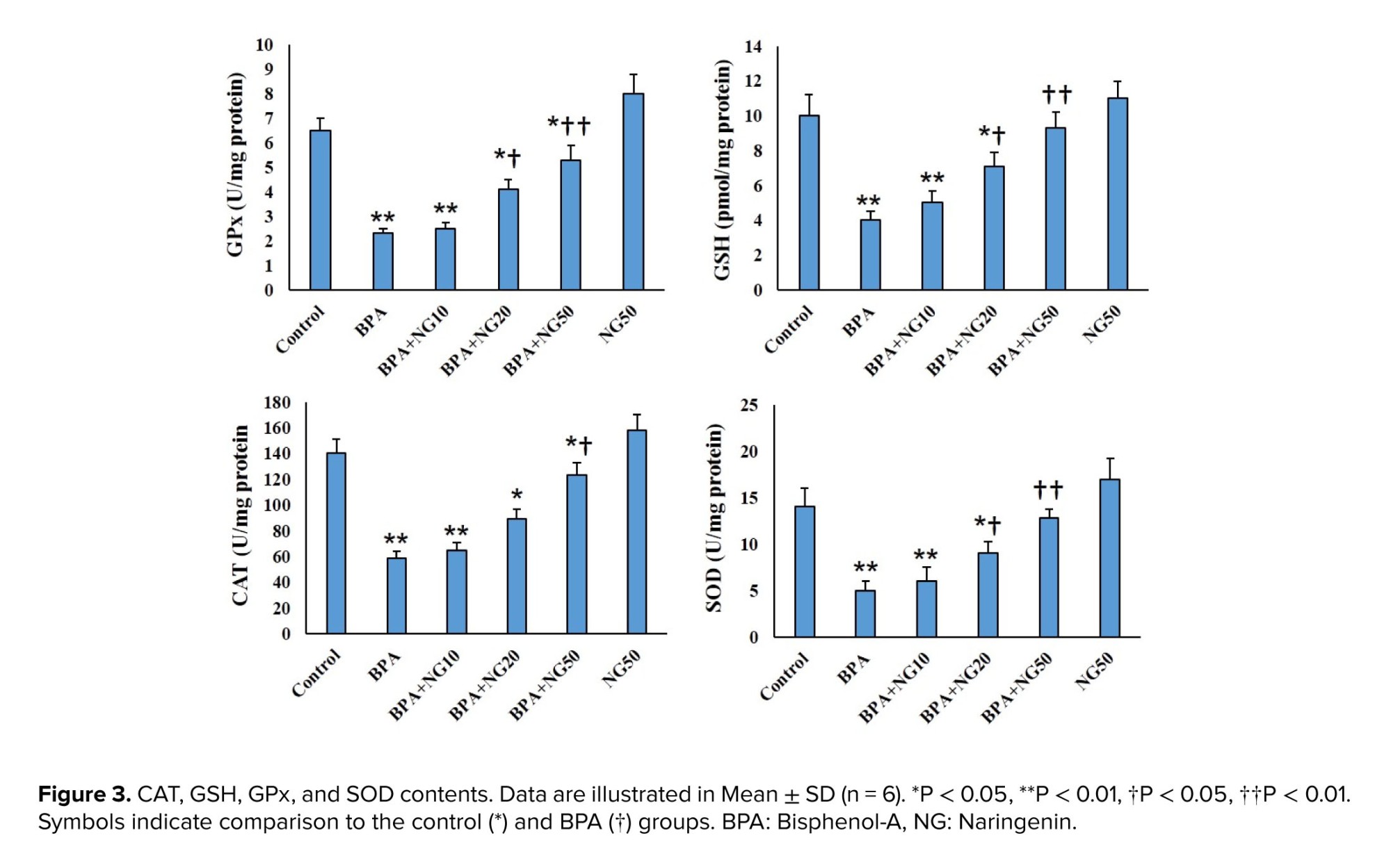
The present study showed that BPA increases oxidative stress in TM4 cells, manifested by rising MDA and ROS levels and decreasing antioxidant GSH, GPx, SOD, and CAT levels. The present observation agrees with previous findings showing an increase in MDA level and a decrease in the antioxidant activity in BPA-induced TM4 cell toxicity (26).
NG ameliorated BPA toxicity by reducing the elevated MDA and ROS levels and increased GSH, GPx, SOD, and CAT levels. Previous studies indicated that NG has potent antioxidant activity (13, 18, 21). For example, Mehranfard et al. mentioned that NG has a protective effect on gentamycin/antibiotic-induced testicular oxidative stress (21). Accordingly, NG may have a protective effect on BPA-induced toxicity by modulating the antioxidant system. In this study, BPA significantly diminished the MMP of the TM4 cells, and NG could concentration-dependently reverse the MMP level. BPA resulted in a loss of MMP in goat Sertoli cells (23). BPA causes hepatotoxicity by inducing mitochondrial disruption in rats (3).
Dysfunctional mitochondria are cleared using the mitophagy process (27). Hence, we explored the impact of BPA on the Parkin and Pink1 levels, which are involved in mitochondrial quality control (28). BPA enhanced the protein level of Pink1 and Parkin in mitochondrial fractions. Anand et al. evidenced that BPA stimulates mitophagy by the accumulation of Pink1 and translocation of Parkin to damaged mitochondria of primary rat hepatocytes (29). The reduction of MMP was associated with BPA-induced cell death (30). Overexpression of Parkin induces mitochondria removal by mitophagy when MMP is depleted (8). The enhanced expression of Pink1 and Parkin proteins indicates that reducing Sertoli cell viability by BPA is due to the mitophagy stimulation.
The mitophagy process closely relates to cell death (31, 32). The abnormal excessive mitophagy eliminates mitochondria, and cells lose their essential functions and undergo death (33, 34). In high-toxicity conditions, mitochondria cannot be repaired by mitophagy (35). Overexpression of Pink1 activates mitophagy and inhibits apoptosis (36).
In a previous study, Pink1/Parkin-mediated mitophagy could inhibit apoptosis in osteoblasts (37). Wei et al. showed that naringin (another bioactive polyphenol in citrus fruits) decreased the inflammatory response, oxidative stress, and apoptosis via regulating endoplasmic reticulum stress and mitophagy in a mouse model of pulmonary fibrosis (38). Feng et al. showed that naringin reduced cerebral ischemia-reperfusion damage in rats through mitophagy activation. In their study, naringin could suppress apoptosis and inhibit the translocation of Parkin to the mitochondria in the ischemia-reperfused rat brains (12). Conversely, excessive mitophagy induces apoptosis in cancerous cells, in particular (39). Chen et al. showed that ketoconazole encourages mitophagy to induce apoptosis in hepatocellular carcinoma. In another study, pardaxin induced excessive mitophagy and apoptosis in human ovarian cancer cells (40).
Our results showed that NG concentration-dependently reduced Pink1 and Parkin expression. Therefore, NG can reduce mitochondria dysfunction and mitophagy induced by BPA.
5. Conclusion
This study demonstrated that exposure to BPA raised Pink1 and Parkin levels in TM4 cells, which increased mitochondrial dysfunction and mitophagy. This study has also revealed that NG attenuates the cytotoxic impacts of BPA on the TM4 Sertoli cells. NG effectively increased the activity of antioxidant enzymes and the production of ROS in cells treated with BPA. NG inhibited mitophagy by reducing the expression of Pink1 and Parkin while simultaneously enhancing MMP in the exposed cells to BPA.
Data availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author contributions
YAF and LKh devised the project, the main conceptual ideas, and proof outline. EY and AHM monitored, evaluated, and analyzed the results of the study. Further, YAF and MJKh wrote the manuscript with support from LKh. All authors discussed the results and approved the final manuscript and took responsibility for the integrity of the data.
Acknowledgments
This work has received a grant from the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant Number: CMRC-0207). We thank our colleagues from the Cellular and Molecular Research Center.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (172 Views)
1. Introduction
Bisphenol A (BPA), an endocrine and estrogenic disrupting agent, is widely used as polycarbonate plastics and epoxy resins for producing food containers, water bottles, medical devices, and other objects that must be made of flexible and lasting materials. It can leak into food and drinks from plastic containers and accumulate in humans' bodies (1). BPA exposure at environmentally relevant concentrations can cause reproductive disorders (2).
BPA involves multiple mechanisms, including interference with mitochondrial functions (3). The toxicity of BPA relates to the excessive production of reactive oxygen species (ROS) that overwhelm the intracellular antioxidants (4). Mitochondria is the primary source of ROS, and under physiological conditions, its production and clearance are regulated by antioxidant and oxidation systems (5). BPA can induce mitochondrial dysfunction by lowering mitochondrial membrane potential (MMP) (6).
Autophagic removal of dysfunctional mitochondria (mitophagy) is activated in response to various toxic agents (7). The PTEN-induced putative kinase 1 (Pink1)/Parkin signaling pathway is the primary mechanism of mitophagy. Pink1 and Parkin play an essential role in the final checkpoint of mitophagy (8). Sertoli cells are targeted for various chemicals, and their damage indicates testicular toxicity (9). Several investigations have evidenced that BPA affects Sertoli cell functions (10). In an experimental study, BPA time- and dose-dependency reduced Sertoli cell viability (11). The study further revealed that Sertoli cells treated with BPA in vitro at a concentration of 200 μM induced morphological distortions such as collapsing of the cytoskeleton, chromatin impairment, and DNA damage in the cells.
BPA induces Sertoli cell death in rodents by causing mitochondrial dysfunction and excessive ROS generation (4). Feng et al. showed that BPA induces cell cycle arrest and apoptosis by preventing ROS levels in the porcine Sertoli cells (12). It is, therefore, necessary to find preventive and therapeutic agents to address BPA-induced reproductive toxicity.
Natural antioxidants potentially inhibit the adverse impacts of BPA on the reproductive system (13). Naringenin (NG) is a member of bioflavonoids derived from citrus species (14).
NG has several biological and pharmacological properties, such as anti-cancer, lipid reduction, superoxide elimination, anti-atherosclerosis, and antioxidants (15-19). It has been evidenced that NG displays antioxidant effects both in vivo and in vitro (19). The beneficial impacts of NG on the reproductive system have been reported in previous studies (20, 21).
In a previous study, NG could ameliorate lead acetate-induced reproductive toxicity and increase germ cell survival (22).
Due to the widespread use of BPA and its negative effects on the human reproductive system, it seems necessary to find a compound that ameliorates its negative effects. Based on the beneficial impacts of the NG on the male reproductive system, the present work has examined the NG's impacts on BPA-induced toxicity, mitophagy, and oxidative stress in TM4 cells (a mouse Sertoli cell line).
2. Materials and Methods
2.1. Experimental design
In this experimental study, the TM4 cells were purchased from the Iranian National Center for Genetic and Biological Resources. This study was performed in 2023 at the Cellular and Molecular Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The cells were cultured at 37°C in 5% CO2 in completed Dulbecco's Modified Eagle's Medium (DMEM, Merck, Germany) media. BPA and NG were dissolved in dimethyl sulfoxide (Sigma-Aldrich Chemie, Steinheim, Germany) and diluted in DMEM. The cells were treated with different concentrations of NG (Sigma-Aldrich, USA) and BPA (Sigma-Aldrich, USA). To explore the effect of NG on BPA-induced damage of TM4 cells, the cells were harvested in a medium containing various concentrations and times as a pilot study (Table I). The sublethal dose of BPA in TM4 cells was determined to be 0.8 μM. The protective dose of the NG was also identified based on a pilot study. The TM4 cells were divided into 4 groups:
- Control: received only culture medium for 24 hr.
- NG: received culture medium and 50 μg/ml NG for 24 hr.
- BPA: received culture medium and 0.8 μM/ml BPA for 24 hr.
- NG + BPA groups: received culture medium supplemented with BPA and NG at 10, 20, and 50 μg/ml concentrations for 24 hr.
2.2. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay
The cells were cultured in a 96-well plate (104 cells/well) for 24 hr. The media was discarded, and NG (25, 10, 20, 50, and 100 μg/ml) or BPA (0.8 μM/ml) for 24, 48, and 72 hr was added to indicate the concentration and duration efficacy. The cells were treated with an MTT (Sigma-Aldrich, USA) solution (0.5 mg/ml) for 3 hr. The supernatants were discarded, 100 μl dimethyl sulfoxide was added, and their absorbance was read at 570 nm.
2.3. Measurement of intracellular ROS, malondialdehyde (MDA), and antioxidant levels
ROS-producing was assessed by the florescent probe 2-7-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, USA). After treatment, the medium was removed, and the ROS indicator DCFH-DA (10 μg/ml) in media was added and incubated for 20 min (37oC). After washing the cells with PBS, the ROS levels were detected with a spectrofluorometer (LS50B, USA; Em: 570 nm; Ex: 490 nm). After homogenization, the TM4 cells were lysed, and their proteins were determined using a BCA protein assay kit (Sigma-Aldrich, USA). The MDA, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH) levels were measured based on the kit's directions (ZellBio, Germany).
2.4. Mitochondria isolation
The cells were re-suspended in an isolation buffer (250 mM sucrose, 20 mM HEPES-KOH, 10 mM KCl, 1.5 mM MgCl2, one mM EDTA, 1 mM EGTA, fat-free BSA 0.1%). The cells were homogenized and centrifuged (4°C) at 750 g for 10 min. The supernatants were again centrifuged (12,000 g) for 30 min. The pellets were then re-suspended in an isolation buffer.
2.5. MMP evaluation
Fluorescent cationic dye Rhodamine 123 (Beyotime, China) was used for the MMP measurement. The TM4 cells, after treatment, washed with PBS twice and exposed to Rhodamine 123 solution (2.5 μg/ml) for 25 min at 37oC. A spectrophotometer (LS50B, USA; emission: 535 nm; excitation: 490 nm) was used to determine fluorescence.
2.6. Real-time polymerase chain reaction
The RNeasy kit (Takara, Japan) was used to extract the RNAs from the TM4 cells (1 × 107 cells), and cDNA was made by a cDNA synthesis kit (Takara, Japan). The cDNA was amplified in PCR reaction buffer (25 μL), including SYBR green and primers. A 45 cycle was used for PCR amplification: 95°C: 10 sec; 95°C: 15 sec; 55-57°C: 20 sec; 60°C: 20 sec. Normalization of the relative gene expression was done using the housekeeping GAPDH gene. Data were analyzed by the 2-ΔΔCT method. The following primers were used in this study: GAGACGATACCGACAAACAC (forward Pink1), GGCATTTCCTCCAAGACTAAC (reverse Pink1); TGCTCGTCAACCTCTG TTC (forward Parkin), TCACTTTCTCCTTCCCATCC (reverse Parkin).
2.7. Measurement of Pink1 and Parkin protein levels
Pink1 and Parkin proteins were measured using ELISA kits (Biospes, China). Briefly, Pink1 and Parkin proteins of the mitochondrial fractions or cell lysates were bound to the primary antibodies and detected by a Horseradish peroxidase-conjugated secondary antibody. Quantification was done by recording the optical density at 450 nm. Pink1 sensitivity was more than 0.07 ng/ml in a range of 0.156-10 ng/ml, and Parkin sensitivity was 0.78 pg/ml in a range of 3.12-200 ng/ml.

1. Introduction
Bisphenol A (BPA), an endocrine and estrogenic disrupting agent, is widely used as polycarbonate plastics and epoxy resins for producing food containers, water bottles, medical devices, and other objects that must be made of flexible and lasting materials. It can leak into food and drinks from plastic containers and accumulate in humans' bodies (1). BPA exposure at environmentally relevant concentrations can cause reproductive disorders (2).
BPA involves multiple mechanisms, including interference with mitochondrial functions (3). The toxicity of BPA relates to the excessive production of reactive oxygen species (ROS) that overwhelm the intracellular antioxidants (4). Mitochondria is the primary source of ROS, and under physiological conditions, its production and clearance are regulated by antioxidant and oxidation systems (5). BPA can induce mitochondrial dysfunction by lowering mitochondrial membrane potential (MMP) (6).
Autophagic removal of dysfunctional mitochondria (mitophagy) is activated in response to various toxic agents (7). The PTEN-induced putative kinase 1 (Pink1)/Parkin signaling pathway is the primary mechanism of mitophagy. Pink1 and Parkin play an essential role in the final checkpoint of mitophagy (8). Sertoli cells are targeted for various chemicals, and their damage indicates testicular toxicity (9). Several investigations have evidenced that BPA affects Sertoli cell functions (10). In an experimental study, BPA time- and dose-dependency reduced Sertoli cell viability (11). The study further revealed that Sertoli cells treated with BPA in vitro at a concentration of 200 μM induced morphological distortions such as collapsing of the cytoskeleton, chromatin impairment, and DNA damage in the cells.
BPA induces Sertoli cell death in rodents by causing mitochondrial dysfunction and excessive ROS generation (4). Feng et al. showed that BPA induces cell cycle arrest and apoptosis by preventing ROS levels in the porcine Sertoli cells (12). It is, therefore, necessary to find preventive and therapeutic agents to address BPA-induced reproductive toxicity.
Natural antioxidants potentially inhibit the adverse impacts of BPA on the reproductive system (13). Naringenin (NG) is a member of bioflavonoids derived from citrus species (14).
NG has several biological and pharmacological properties, such as anti-cancer, lipid reduction, superoxide elimination, anti-atherosclerosis, and antioxidants (15-19). It has been evidenced that NG displays antioxidant effects both in vivo and in vitro (19). The beneficial impacts of NG on the reproductive system have been reported in previous studies (20, 21).
In a previous study, NG could ameliorate lead acetate-induced reproductive toxicity and increase germ cell survival (22).
Due to the widespread use of BPA and its negative effects on the human reproductive system, it seems necessary to find a compound that ameliorates its negative effects. Based on the beneficial impacts of the NG on the male reproductive system, the present work has examined the NG's impacts on BPA-induced toxicity, mitophagy, and oxidative stress in TM4 cells (a mouse Sertoli cell line).
2. Materials and Methods
2.1. Experimental design
In this experimental study, the TM4 cells were purchased from the Iranian National Center for Genetic and Biological Resources. This study was performed in 2023 at the Cellular and Molecular Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The cells were cultured at 37°C in 5% CO2 in completed Dulbecco's Modified Eagle's Medium (DMEM, Merck, Germany) media. BPA and NG were dissolved in dimethyl sulfoxide (Sigma-Aldrich Chemie, Steinheim, Germany) and diluted in DMEM. The cells were treated with different concentrations of NG (Sigma-Aldrich, USA) and BPA (Sigma-Aldrich, USA). To explore the effect of NG on BPA-induced damage of TM4 cells, the cells were harvested in a medium containing various concentrations and times as a pilot study (Table I). The sublethal dose of BPA in TM4 cells was determined to be 0.8 μM. The protective dose of the NG was also identified based on a pilot study. The TM4 cells were divided into 4 groups:
- Control: received only culture medium for 24 hr.
- NG: received culture medium and 50 μg/ml NG for 24 hr.
- BPA: received culture medium and 0.8 μM/ml BPA for 24 hr.
- NG + BPA groups: received culture medium supplemented with BPA and NG at 10, 20, and 50 μg/ml concentrations for 24 hr.
2.2. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay
The cells were cultured in a 96-well plate (104 cells/well) for 24 hr. The media was discarded, and NG (25, 10, 20, 50, and 100 μg/ml) or BPA (0.8 μM/ml) for 24, 48, and 72 hr was added to indicate the concentration and duration efficacy. The cells were treated with an MTT (Sigma-Aldrich, USA) solution (0.5 mg/ml) for 3 hr. The supernatants were discarded, 100 μl dimethyl sulfoxide was added, and their absorbance was read at 570 nm.
2.3. Measurement of intracellular ROS, malondialdehyde (MDA), and antioxidant levels
ROS-producing was assessed by the florescent probe 2-7-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, USA). After treatment, the medium was removed, and the ROS indicator DCFH-DA (10 μg/ml) in media was added and incubated for 20 min (37oC). After washing the cells with PBS, the ROS levels were detected with a spectrofluorometer (LS50B, USA; Em: 570 nm; Ex: 490 nm). After homogenization, the TM4 cells were lysed, and their proteins were determined using a BCA protein assay kit (Sigma-Aldrich, USA). The MDA, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH) levels were measured based on the kit's directions (ZellBio, Germany).
2.4. Mitochondria isolation
The cells were re-suspended in an isolation buffer (250 mM sucrose, 20 mM HEPES-KOH, 10 mM KCl, 1.5 mM MgCl2, one mM EDTA, 1 mM EGTA, fat-free BSA 0.1%). The cells were homogenized and centrifuged (4°C) at 750 g for 10 min. The supernatants were again centrifuged (12,000 g) for 30 min. The pellets were then re-suspended in an isolation buffer.
2.5. MMP evaluation
Fluorescent cationic dye Rhodamine 123 (Beyotime, China) was used for the MMP measurement. The TM4 cells, after treatment, washed with PBS twice and exposed to Rhodamine 123 solution (2.5 μg/ml) for 25 min at 37oC. A spectrophotometer (LS50B, USA; emission: 535 nm; excitation: 490 nm) was used to determine fluorescence.
2.6. Real-time polymerase chain reaction
The RNeasy kit (Takara, Japan) was used to extract the RNAs from the TM4 cells (1 × 107 cells), and cDNA was made by a cDNA synthesis kit (Takara, Japan). The cDNA was amplified in PCR reaction buffer (25 μL), including SYBR green and primers. A 45 cycle was used for PCR amplification: 95°C: 10 sec; 95°C: 15 sec; 55-57°C: 20 sec; 60°C: 20 sec. Normalization of the relative gene expression was done using the housekeeping GAPDH gene. Data were analyzed by the 2-ΔΔCT method. The following primers were used in this study: GAGACGATACCGACAAACAC (forward Pink1), GGCATTTCCTCCAAGACTAAC (reverse Pink1); TGCTCGTCAACCTCTG TTC (forward Parkin), TCACTTTCTCCTTCCCATCC (reverse Parkin).
2.7. Measurement of Pink1 and Parkin protein levels
Pink1 and Parkin proteins were measured using ELISA kits (Biospes, China). Briefly, Pink1 and Parkin proteins of the mitochondrial fractions or cell lysates were bound to the primary antibodies and detected by a Horseradish peroxidase-conjugated secondary antibody. Quantification was done by recording the optical density at 450 nm. Pink1 sensitivity was more than 0.07 ng/ml in a range of 0.156-10 ng/ml, and Parkin sensitivity was 0.78 pg/ml in a range of 3.12-200 ng/ml.






4. Discussion
In the present study, we show the protective potential of NG against BPA-induced TM4 cell toxicity. BPA induced cytotoxicity by decreasing cell viability, enhancing ROS production, stimulating mitophagy, and disrupting mitochondria in the Sertoli cells. The reduced viability demonstrated the toxic effects of BPA on the TM4 Sertoli cells. In line with our results, a previous study showed that BPA reduced the survival rate of the TM4 cells (23).
NG dose-dependently reversed the viability of the BPA-exposed cells. In parallel to our findings, have reported the protective impacts of NG against permethrin-induced testicular toxicity in rats (24). Have demonstrated that NG improves some reproductive parameters, apoptosis, and oxidative stress in acrylamide-induced testis toxicity of rats (25).
The present study showed that BPA increases oxidative stress in TM4 cells, manifested by rising MDA and ROS levels and decreasing antioxidant GSH, GPx, SOD, and CAT levels. The present observation agrees with previous findings showing an increase in MDA level and a decrease in the antioxidant activity in BPA-induced TM4 cell toxicity (26).
NG ameliorated BPA toxicity by reducing the elevated MDA and ROS levels and increased GSH, GPx, SOD, and CAT levels. Previous studies indicated that NG has potent antioxidant activity (13, 18, 21). For example, Mehranfard et al. mentioned that NG has a protective effect on gentamycin/antibiotic-induced testicular oxidative stress (21). Accordingly, NG may have a protective effect on BPA-induced toxicity by modulating the antioxidant system. In this study, BPA significantly diminished the MMP of the TM4 cells, and NG could concentration-dependently reverse the MMP level. BPA resulted in a loss of MMP in goat Sertoli cells (23). BPA causes hepatotoxicity by inducing mitochondrial disruption in rats (3).
Dysfunctional mitochondria are cleared using the mitophagy process (27). Hence, we explored the impact of BPA on the Parkin and Pink1 levels, which are involved in mitochondrial quality control (28). BPA enhanced the protein level of Pink1 and Parkin in mitochondrial fractions. Anand et al. evidenced that BPA stimulates mitophagy by the accumulation of Pink1 and translocation of Parkin to damaged mitochondria of primary rat hepatocytes (29). The reduction of MMP was associated with BPA-induced cell death (30). Overexpression of Parkin induces mitochondria removal by mitophagy when MMP is depleted (8). The enhanced expression of Pink1 and Parkin proteins indicates that reducing Sertoli cell viability by BPA is due to the mitophagy stimulation.
The mitophagy process closely relates to cell death (31, 32). The abnormal excessive mitophagy eliminates mitochondria, and cells lose their essential functions and undergo death (33, 34). In high-toxicity conditions, mitochondria cannot be repaired by mitophagy (35). Overexpression of Pink1 activates mitophagy and inhibits apoptosis (36).
In a previous study, Pink1/Parkin-mediated mitophagy could inhibit apoptosis in osteoblasts (37). Wei et al. showed that naringin (another bioactive polyphenol in citrus fruits) decreased the inflammatory response, oxidative stress, and apoptosis via regulating endoplasmic reticulum stress and mitophagy in a mouse model of pulmonary fibrosis (38). Feng et al. showed that naringin reduced cerebral ischemia-reperfusion damage in rats through mitophagy activation. In their study, naringin could suppress apoptosis and inhibit the translocation of Parkin to the mitochondria in the ischemia-reperfused rat brains (12). Conversely, excessive mitophagy induces apoptosis in cancerous cells, in particular (39). Chen et al. showed that ketoconazole encourages mitophagy to induce apoptosis in hepatocellular carcinoma. In another study, pardaxin induced excessive mitophagy and apoptosis in human ovarian cancer cells (40).
Our results showed that NG concentration-dependently reduced Pink1 and Parkin expression. Therefore, NG can reduce mitochondria dysfunction and mitophagy induced by BPA.
5. Conclusion
This study demonstrated that exposure to BPA raised Pink1 and Parkin levels in TM4 cells, which increased mitochondrial dysfunction and mitophagy. This study has also revealed that NG attenuates the cytotoxic impacts of BPA on the TM4 Sertoli cells. NG effectively increased the activity of antioxidant enzymes and the production of ROS in cells treated with BPA. NG inhibited mitophagy by reducing the expression of Pink1 and Parkin while simultaneously enhancing MMP in the exposed cells to BPA.
Data availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author contributions
YAF and LKh devised the project, the main conceptual ideas, and proof outline. EY and AHM monitored, evaluated, and analyzed the results of the study. Further, YAF and MJKh wrote the manuscript with support from LKh. All authors discussed the results and approved the final manuscript and took responsibility for the integrity of the data.
Acknowledgments
This work has received a grant from the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant Number: CMRC-0207). We thank our colleagues from the Cellular and Molecular Research Center.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
References
1. Manzoor MF, Tariq T, Fatima B, Sahar A, Tariq F, Munir S, et al. An insight into bisphenol A, food exposure and its adverse effects on health: A review. Front Nutr 2022; 9: 1047827. [DOI:10.3389/fnut.2022.1047827] [PMID] [PMCID]
2. Asadi-Fard Y, Zohour Soleimani M, Khodayar MJ, Khorsandi L, Shirani M, Samimi A. Morin improves bisphenol-A-induced toxicity in the rat testicular mitochondria and sperms. JBRA Assist Reprod 2023; 27: 174-179. [DOI:10.5935/1518-0557.20220010] [PMID] [PMCID]
3. Khan S, Beigh S, Chaudhari BP, Sharma S, Aliul Hasan Abdi S, Ahmad S, et al. Mitochondrial dysfunction induced by bisphenol A is a factor of its hepatotoxicity in rats. Environ Toxicol 2016; 31: 1922-1934. [DOI:10.1002/tox.22193] [PMID]
4. Wang Ch, Qi S, Liu Ch, Yang A, Fu W, Quan C, et al. Mitochondrial dysfunction and Ca2+ overload in injured Sertoli cells exposed to bisphenol A. Environ Toxicol 2017; 32: 823-831. [DOI:10.1002/tox.22282] [PMID]
5. Tirichen H, Yaigoub H, Xu W, Wu C, Li R, Li Y. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front Physiol 2021; 12: 627837. [DOI:10.3389/fphys.2021.627837] [PMID] [PMCID]
6. Kaur S, Saluja M, Bansal MP. Bisphenol A induced oxidative stress and apoptosis in mice testes: Modulation by selenium. Andrologia 2018; 50: e12834. [DOI:10.1111/and.12834] [PMID]
7. Lemasters JJ. Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (type 3). Redox Biol 2014; 2: 749-754. [DOI:10.1016/j.redox.2014.06.004] [PMID] [PMCID]
8. Koyano F, Yamano K, Kosako H, Tanaka K, Matsuda N. Parkin recruitment to impaired mitochondria for nonselective ubiquitylation is facilitated by MITOL. J Biol Chem 2019; 294: 10300-10314. [DOI:10.1074/jbc.RA118.006302] [PMID] [PMCID]
9. Choi M-S, Park H-J, Oh J-H, Lee E-H, Park S-M, Yoon S. Nonylphenol-induced apoptotic cell death in mouse TM4 Sertoli cells via the generation of reactive oxygen species and activation of the ERK signaling pathway. J Appl Toxicol 2014; 34: 628-636. [DOI:10.1002/jat.2886] [PMID]
10. Gao Y, Mruk DD, Cheng CY. Sertoli cells are the target of environmental toxicants in the testis- a mechanistic and therapeutic insight. Expert Opin Ther Targets 2015; 19: 1073-1090. [DOI:10.1517/14728222.2015.1039513] [PMID] [PMCID]
11. Qi S, Fu W, Wang C, Liu C, Quan C, Kourouma A, et al. BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod Toxicol 2014; 50: 108-116. [DOI:10.1016/j.reprotox.2014.10.013] [PMID]
12. Feng Y, Wu J, Lei R, Zhang Y, Qiao M, Zhou J, et al. N-acetyl-L-cysteine ameliorates BPAF-induced porcine Sertoli cell apoptosis and cell cycle arrest via inhibiting the ROS level. Toxics 2023; 11: 923-937. [DOI:10.3390/toxics11110923] [PMID] [PMCID]
13. Amjad S, Rahman MS, Pang MG. Role of antioxidants in alleviating bisphenol A toxicity. Biomolecules 2020; 10: 1105-1131. [DOI:10.3390/biom10081105] [PMID] [PMCID]
14. Han X, Parker TL. Antiinflammatory activity of cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytother Res 2017; 31: 1034-1038. [DOI:10.1002/ptr.5822] [PMID] [PMCID]
15. Qiu M, Wei W, Zhang J, Wang H, Bai Y, Guo DA. A scientometric study to a critical review on promising anticancer and neuroprotective compounds: Citrus flavonoids. Antioxidants (Basel) 2023; 12: 669-692. [DOI:10.3390/antiox12030669] [PMID] [PMCID]
16. Pengnet S, Prommaouan S, Sumarithum P, Malakul W. Naringin reverses high-cholesterol diet-induced vascular dysfunction and oxidative stress in rats via regulating LOX-1 and NADPH oxidase subunit expression. Biomed Res Int 2019; 2019: 3708497. [DOI:10.1155/2019/3708497] [PMID] [PMCID]
17. Li F, Zhan Z, Qian J, Cao C, Yao W, Wang N. Naringin attenuates rat myocardial ischemia/reperfusion injury via PI3K/Akt pathway-mediated inhibition of apoptosis, oxidative stress and autophagy. Exp Ther Med 2021; 22: 811-819. [DOI:10.3892/etm.2021.10243] [PMID] [PMCID]
18. Hsueh TP, Sheen JM, Pang JH, Bi KW, Huang CC, Wu HT, et al. The anti-atherosclerotic effect of naringin is associated with reduced expressions of cell adhesion molecules and chemokines through NF-κB pathway. Molecules 2016; 21: 195-210. [DOI:10.3390/molecules21020195] [PMID] [PMCID]
19. Mohamed EE, Ahmed OM, Abdel-Moneim A, Zoheir KMA, Elesawy BH, Al Askary A, et al. Protective effects of naringin-dextrin nanoformula against chemically induced hepatocellular carcinoma in Wistar rats: Roles of oxidative stress, inflammation, cell apoptosis, and proliferation. Pharmaceuticals (Basel) 2022; 15: 1558-1582. [DOI:10.3390/ph15121558] [PMID] [PMCID]
20. Alboghobeish S, Mahdavinia M, Zeidooni L, Samimi A, Oroojan AA, Alizadeh S, et al. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran J Basic Med Sci 2019; 22: 315-325.
21. Mehranfard N, Ghasemi M, Rajabian A, Ansari L. Protective potential of naringenin and its nanoformulations in redox mechanisms of injury and disease. Heliyon 2023; 9: e22820. [DOI:10.1016/j.heliyon.2023.e22820] [PMID] [PMCID]
22. Elhemiely AA, Yahia R, Gad AM. Naringenin alleviate reproductive toxicity evoked by lead acetate via attenuation of sperm profile and biochemical alterations in male Wistar rat: Involvement of TGFβ/AKT/mTOR pathway. J Biochem Mol Toxicol 2023; 37: e23335. [DOI:10.1002/jbt.23335] [PMID]
23. Zhang Y, Han L, Yang H, Pang J, Li P, Zhang G, et al. Bisphenol A affects cell viability involved in autophagy and apoptosis in goat testis sertoli cell. Environ Toxicol Pharmacol 2017; 55: 137-147. [DOI:10.1016/j.etap.2017.07.014] [PMID]
24. Mostafa H-S, Abd El-Baset SA, Kattaia AA, Zidan RA, Al Sadek MM. Efficacy of naringenin against permethrin-induced testicular toxicity in rats. Int J Exp Pathol 2016; 97: 37-49. [DOI:10.1111/iep.12168] [PMID] [PMCID]
25. Sengul E, Gelen V, Yildirim S, Cinar İ, Aksu EH. Effects of naringin on oxidative stress, inflammation, some reproductive parameters, and apoptosis in acrylamide-induced testis toxicity in rat. Environ Toxicol 2023; 38: 798-808. [DOI:10.1002/tox.23728] [PMID]
26. Gassman NR. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ Mol Mutagen 2017; 58: 60-71. [DOI:10.1002/em.22072] [PMID] [PMCID]
27. Matic I, Strobbe D, Frison M, Campanella M. Controlled and impaired mitochondrial quality in neurons: Molecular physiology and prospective pharmacology. Pharmacol Res 2015; 99: 410-424. [DOI:10.1016/j.phrs.2015.03.021] [PMID]
28. Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 2013; 20: 31-42. [DOI:10.1038/cdd.2012.81] [PMID] [PMCID]
29. Anand SK, Sharma A, Singh N, Kakkar P. Activation of autophagic flux via LKB1/AMPK/mTOR axis against xenoestrogen Bisphenol-A exposure in primary rat hepatocytes. Food Chem Toxicol 2020; 141: 111314-111329. [DOI:10.1016/j.fct.2020.111314] [PMID]
30. Morshed AKMH, Paul S, Hossain A, Basak T, Hossain MS, Hasan MM, et al. Baicalein as promising anticancer agent: A comprehensive analysis on molecular mechanisms and therapeutic perspectives. Cancers (Basel) 2023; 15: 2128-2156. [DOI:10.3390/cancers15072128] [PMID] [PMCID]
31. Li S, Zhang J, Liu C, Wang Q, Yan J, Hui L, et al. The role of mitophagy in regulating cell death. Oxid Med Cell Longev 2021; 2021: 6617256. [DOI:10.1155/2021/6617256] [PMID] [PMCID]
32. Decker ST, Matias AA, Bannon ST, Madden JP, Alexandrou-Majaj N, Layec G. Effects of cigarette smoke on in situ mitochondrial substrate oxidation of slow- and fast-twitch skeletal muscles. Life Sci 2023; 315: 121376. [DOI:10.1016/j.lfs.2023.121376] [PMID]
33. Song Y, Ding W, Xiao Y, Lu KJ. The progress of mitophagy and related pathogenic mechanisms of the neurodegenerative diseases and tumor. Neurosci J 2015; 2015: 543758. [DOI:10.1155/2015/543758] [PMID] [PMCID]
34. Sun T, Ding W, Xu T, Ao X, Yu T, Li M, et al. Parkin regulates programmed necrosis and myocardial ischemia/reperfusion INGury by targeting cyclophilin-D. Antioxid Redox Signal 2019; 31: 1177-1193. [DOI:10.1089/ars.2019.7734] [PMID]
35. Paech F, Mingard C, Grünig D, Abegg VF, Bouitbir J, Krähenbühl S. Mechanisms of mitochondrial toxicity of the kinase inhibitors ponatinib, regorafenib and sorafenib in human hepatic HepG2 cells. Toxicology 2018; 395: 34-44. [DOI:10.1016/j.tox.2018.01.005] [PMID]
36. Wang M, Luan S, Fan X, Wang J, Huang J, Gao X, et al. The emerging multifaceted role of PINK1 in cancer biology. Cancer Sci 2022; 113: 4037-4047. [DOI:10.1111/cas.15568] [PMID] [PMCID]
37. Li W, Jiang WS, Su YR, Tu KW, Zou L, Liao CR, et al. PINK1/Parkin-mediated mitophagy inhibits osteoblast apoptosis induced by advanced oxidation protein products. Cell Death Dis 2023; 14: 88-100. [DOI:10.1038/s41419-023-05595-5] [PMID] [PMCID]
38. Wei Y, Sun L, Liu C, Li L. Naringin regulates endoplasmic reticulum stress and mitophagy through the ATF3/PINK1 signaling axis to alleviate pulmonary fibrosis. Naunyn Schmiedebergs Arch Pharmacol 2023; 396: 1155-1169. [DOI:10.1007/s00210-023-02390-z] [PMID]
39. Praharaj PP, Naik PP, Panigrahi DP, Bhol CS, Mahapatra KK, Patra S, et al. Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: Its implication in cancer therapeutics. Cell Mol Life Sci 2019; 76: 1641-1652. [DOI:10.1007/s00018-018-2990-x] [PMID] [PMCID]
40. Chen Y, Chen HN, Wang K, Zhang L, Huang Z, Liu J, et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol 2019; 70: 66-77. [DOI:10.1016/j.jhep.2018.09.022] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




