Wed, Feb 4, 2026
[Archive]
Volume 22, Issue 7 (July 2024)
IJRM 2024, 22(7): 553-566 |
Back to browse issues page
Ethics code: IR.ARAKU.REC.1400.003
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shariatzadeh S M A, Salmani F, Moghanlo H, Mahmoodi M. Stereological and biochemical effects of thymoquinone on ovarian tissue toxicity induced by silver nanoparticles in NMRI mice: An experimental study. IJRM 2024; 22 (7) :553-566
URL: http://ijrm.ir/article-1-3301-en.html
URL: http://ijrm.ir/article-1-3301-en.html
1- Department of Biology, Faculty of Sciences, Arak University, Arak, Iran. , s-shariatzadeh@araku.ac.ir
2- Department of Biology, Faculty of Sciences, Arak University, Arak, Iran.
2- Department of Biology, Faculty of Sciences, Arak University, Arak, Iran.
Full-Text [PDF 3407 kb]
(1938 Downloads)
| Abstract (HTML) (2541 Views)
Full-Text: (145 Views)
- Introduction
Nowadays, several factors, such as changing lifestyles, exposure to environmental toxins, underlying medical conditions, and genetic predispositions, have impacted couples' fertility (1). The specific biological properties of silver nanoparticles (AgNPs) have led to their widespread use in various industries, such as medicine, plastics, textiles, water refineries, air filters, and agriculture (2). In medical science, AgNPs are used for wound dressing, skin ointments, disinfectants, drug delivery systems, and dentistry due to their antiviral, antibacterial, and antifungal properties (3). Despite these benefits, many studies show that long-term exposure to AgNPs induces cellular and tissue toxicity in various tissues and reproductive organs (4, 5). AgNPs enter cells by crossing blood barriers and lead to cytotoxicity due to damage to cell organelles, especially the mitochondrial electron transport chain (6). The reproductive organs are vulnerable to AgNPs in a dose- and time-dependent manner (7).
Regarding the female reproductive system, AgNPs in different sizes, depending on the dose, cause severe damage to ovarian tissue and follicular cells (8). Currently, laboratory studies have demonstrated that AgNPs can cause developmental toxicities in the fetus (9). Exposure to AgNPs by disrupting the process of aromatization and steroidogenesis causes a decrease in estrogen, degeneration of follicles, and induction of apoptosis in ovarian cells (10). AgNPs have toxic effects on the development of mouse embryos; histopathological studies have shown that the toxic dose of AgNPs passes through the placenta, causing a decrease in cell viability and damaging the follicular cells in the ovarian tissue (11). Through the induction of lipid peroxidation and reactive oxygen species, AgNPs decreased Cyp19a1 enzyme (cytochrome P450, family 19, subfamily a, polypeptide 1) activity, estrogen levels, and destruction of preantral and antral follicles (12).
Herbal medicines are of particular importance as alternative medicines. They have reduced the concern caused by the side effects of synthetic drugs. One of the effective compounds is thymoquinone (TQ). TQ (2-isopropyl-5-methylbenzo-1,4-quinone), as a natural antioxidant, is a bioactive ingredient of Nigella sativa (13). Molecular signaling pathways show that TQ protects cells against oxidative stress by regulating the activity of antioxidant enzymes. They can also play an essential role in cell survival or apoptosis by regulating inflammatory cytokines and factors involved in cell cycle regulation (14). The pharmacological effects of TQ, including anti-inflammatory, modulation of the immune system, and anticancer effects, have been proven in previous studies. In addition, TQ improves cardiac, neurological (neurological inflammation, Alzheimer's and Parkinson's diseases), respiratory, digestive, liver, kidney, and other disorders. Besides, it is very effective in antiobesity and antidiabetes (15). Previous studies have shown the therapeutic effect of TQ on the male and female reproductive system (16). Recent findings have shown that TQ has a protective impact on polycystic ovary syndrome (17).
Based on recent searches, there was no stereological analyses of the effects of TQ on the toxic effects of AgNPs on mouse ovarian structure. The present study evaluated the effectiveness of TQ against the toxicity induced by AgNPs on ovarian tissue in mice. The stereological analysis of ovarian tissue, the follicular count and their volume at different developmental stages, the measurement of oxidative stress indices, and the level of sex hormones evaluated.
2. Materials and Methods
2.1. Preparation of chemicals
The commercial AgNPs (US Research Nanomaterials, Inc., USA) were purchased from Pishgaman Nanomaterials Co., Iran. The authenticity of the quality of AgNPs (spherical morphology, 99.99% purity, average diameter 20 nm, metal base) was evaluated and confirmed through X-ray diffraction and transmission electron microscopy by Pishgaman Nanomaterials Co., Iran. Also, TQ with characteristics (2-Isopropyl-5-methyl-1, 4-benzoquinone, ≥ 98.0%, molecular formula: C10H12O2, molecular weight: 164.20 g.mol-1) (Sigma-Aldrich, USA) was purchased.
2.2. Sample size
According to previous studies, alpha error was considered 5% (4, 15). Subsequently, the sample size was determined using the N = DF/K + 1 formula, where N, DF, and K denote the number of mice per group, degrees of freedom, and the number of groups, respectively. Therefore, 24 mice (n = 6/each) were utilized for the experiment.
2.3. Experimental design and mice
In this experiment, 24 healthy 5-6 wk-old NMRI female mice with an average weight of 33 ± 1.5 gr were used. Mice were purchased from the Pasteur Institute (Tehran, Iran). 1 wk before the beginning of the experiment, the animals were kept in the animal house of Arak University under standard conditions for 12 hr light/dark cycle at a temperature of 21 ± 2°C with free access to a standard diet and water. We closely monitored the mice’s nutritional behavior, body weight, behavior, and physical appearance changes during the treatment period. Therefore, we committed to halting the treatment immediately if we noticed any signs of suffering.
Mice were randomly divided into 4 groups (n = 6/each): control group, AgNPs group (500 mg/kg) (4, 7), TQ group (2.5 mg/kg) (15), and co-administration of AgNPs + TQ group (2.5 mg/kg TQ + 500 mg/kg AgNPs). According to the average weight of female mice (33 gr), solutions of 500 mg/kg bwt AgNPs in distilled water and 2.5 mg/kg bwt TQ in dimethyl sulfoxide were prepared daily. Thus, mice were treated with AgNPs orally (via gavage tube) and TQ via intraperitoneal injection for 35 consecutive days (12). At the end of the treatment period, the percentage of changes in the body weight of mice was calculated using the formula (W2-W1)
2.4. Tissue processing and stereological analysis
Tissue processing was performed via an automatic processor (Histokinette, Leica, Germany). In brief, spherical and cylindrical tissue blocks were obtained from the paraffin dispenser (DS-4L, Didsabz, Iran). Sections were prepared using the isotropic uniform random method and a Leitz 1512 rotary microtome (Leitz, USA). For stereological studies, 10-12 sections per ovary are required (18). Approximately 120 sections (i.e., 60 sections of 5-micron and 60 sections of 20-micron) alternately were obtained, per ovary. Subsequently, using the systematic sampling and K = N/n formula, 20 sections were selected for each ovary (i.e., 10 sections of 20-micron and 10 sections of 5-micron per ovary) to the hematoxylin and eosin staining. K, N, and n denote the uniform distance between 2 samples, the total number of samples, and the number of samples, respectively.
2.4.1. Estimation of volume (ovary, medulla, cortex, and corpus luteum)
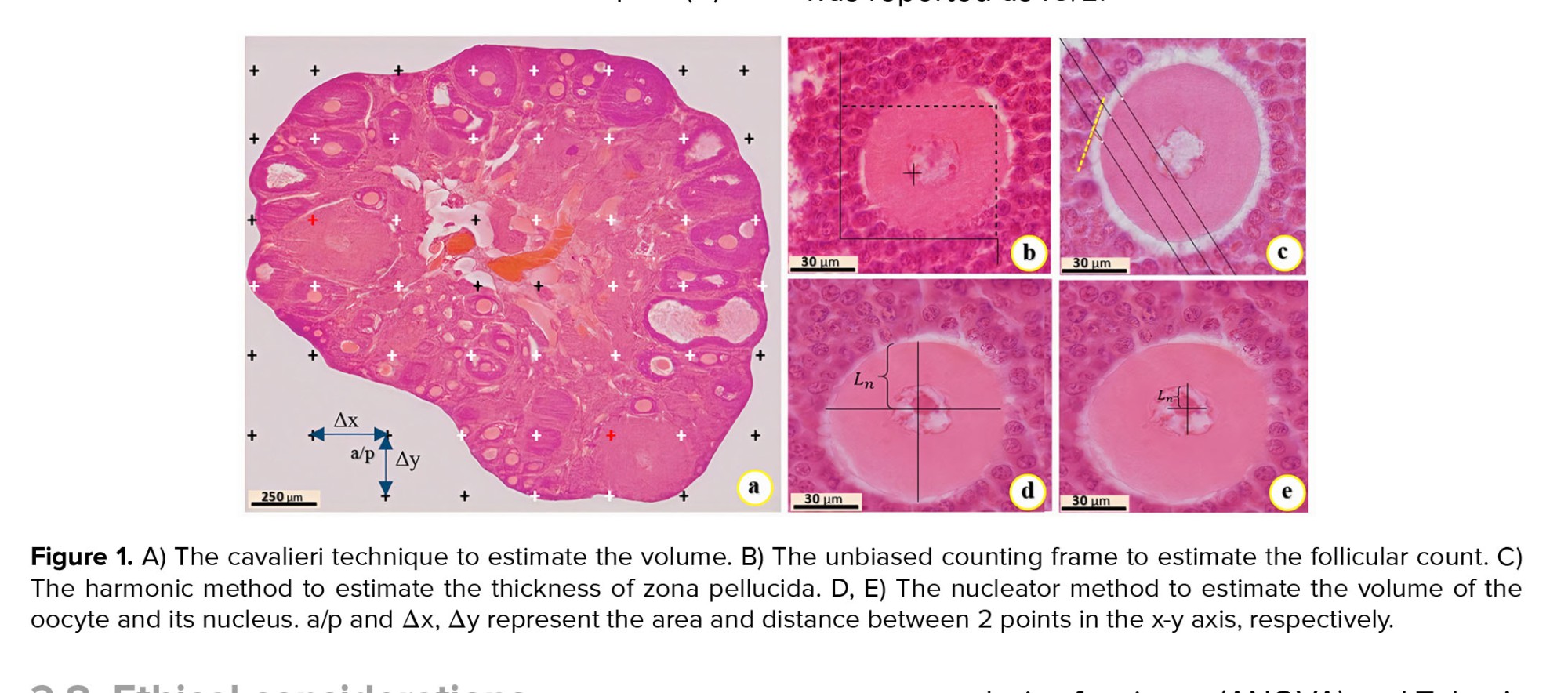
The Cavalieri technique and 10 sections of 5-micron per ovary were employed to estimate the volume of ovarian structure. Briefly, an optical microscope (BX41, Olympus, Japan) equipped with a camera (DP12, Olympus, Japan) and the images of ovarian tissue were reflected on the monitor with ×40 magnification. The point probe was randomly dropped on the images. In each ovary, the total number of collision points with all images of the sections and 200 collision points for each section of the cortex, medulla, and corpus luteum were counted. Based on the following formulas, ‘∑p’ is the total collision points between the probe and images, ‘a(p)’ is the area of each point on the probe, ‘t’ is the thickness of the sections, ‘Δx’ and ‘Δy’ are the distance between 2 points (Figure 1a), ‘M’ denotes the microscope magnification, ‘∑p medulla’ is probe collisions with the medulla, ‘∑p cortex’ is probe collisions with the cortex, ‘∑p corpus luteum’ denotes the probe collisions with the corpus luteum (18).

2.4.2. Estimation of the number of follicles
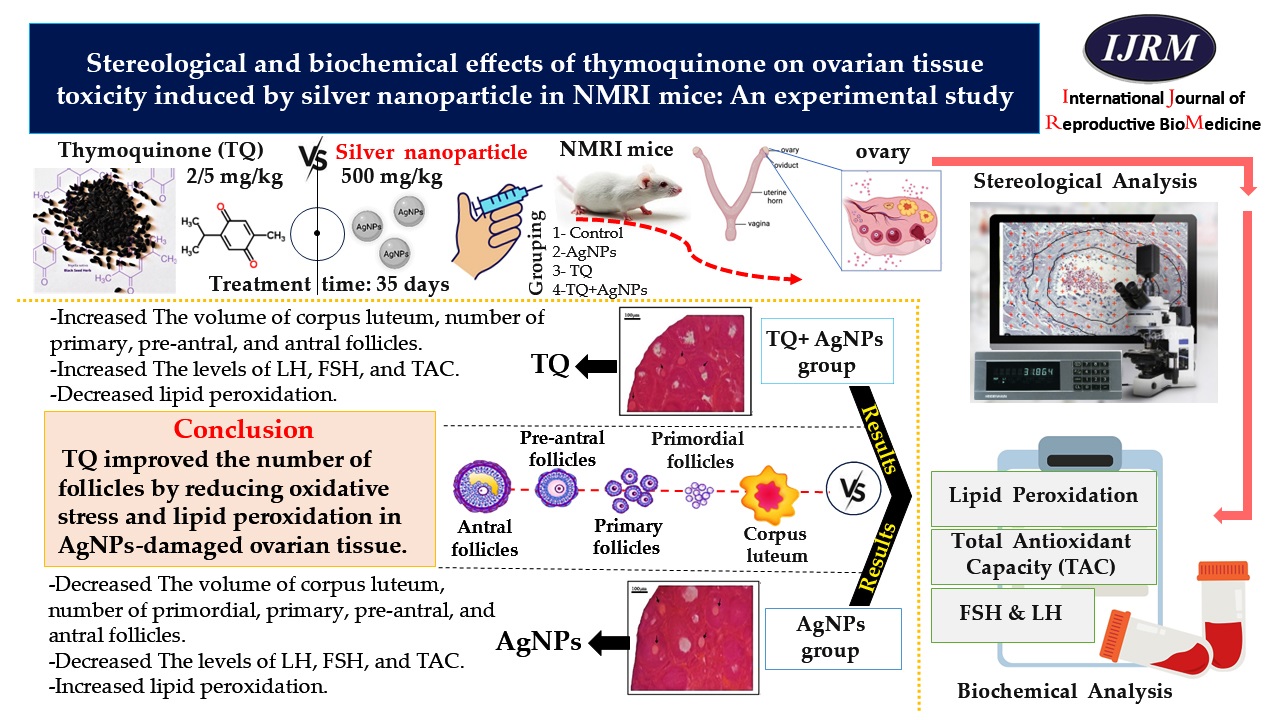
The optical disector method and optical microscope (BX41, Olympus, Japan) equipped with a microcator device (ND221B, Heidenhain, Germany) were used to estimate the follicular count at different developmental stages. The images of 20-micron sections were reflected on the monitor with ×1000 magnification. The number of cells at a depth of 10 µm was estimated using a microcator and an unbiased counting frame (Figure 1b). 5 µm from the top and bottom of the 20 µm sections as guard zones were omitted. 100-150 cells were counted for each ovary. According to the following formula, the number of follicles, including primordial, primary, preantral, and antral were estimated in ovarian tissue. Where ‘∑Qi’, ‘∑p’, and ‘a/f’ denote the number of cells counted, the total number of counting frames that hit the fields of view, and the area of the unbiased counting frame, respectively. Also, ‘h’ is the height of the disector which was 10 μm, and ‘Vtotal ovary’ represents the total volume of the ovary calculated by the Cavalieri method (18).
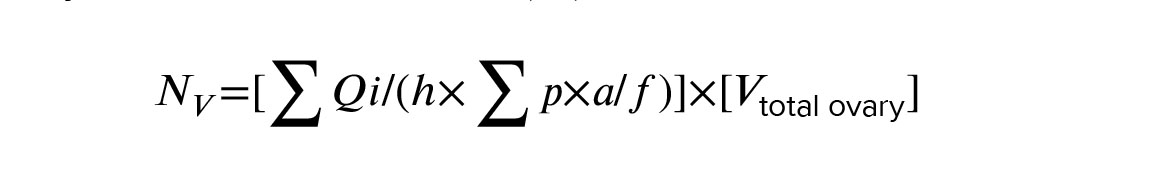
2.4.3. Estimation of zona pellucida thickness in the preantral and antral follicles
At first, microscopic images from 5-micron sections were randomly selected, and the harmonic method was used to measure the thickness of the zona pellucida. Then, a special probe with 3 parallel and equal lines was randomly dropped on the microscopic images with ×1000 magnification (Figure 1c). In this method, from the intersection of probe lines with the inner surface of the zona pellucida, a vertical line was drawn to the tangent line of the outer surface of the zona pellucida. The length of the vertical line (orthogonal intercept) was measured by Motic Images software. An average of 120 orthogonal intercepts (oi1, oi2, oi3, …., oi119, oi120) were measured for each ovary. Finally, the mean thickness of the zona pellucida (ZP) was estimated using the following formula: Where ‘N’ represents the total number of measurements, and ‘oi’ shows the length of each vertical line from the inner surface to the outer surface of the zona pellucida (18) (π = 3.14).
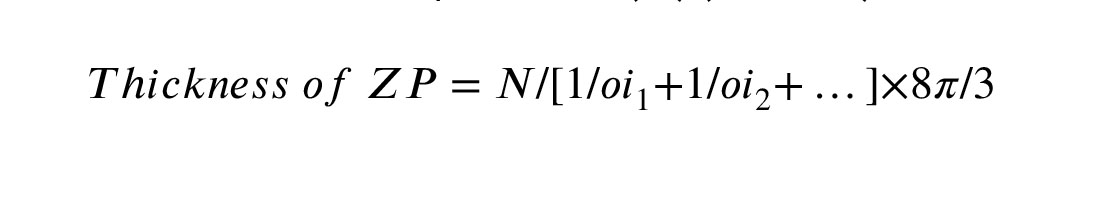
2.4.4. Estimation of the volume of the oocyte and its nucleus
According to the nucleator method, images of 20-micron sections were randomly selected using an optical microscope at ×1000 magnifications (BX41, Olympus, Japan), equipped with a camera (DP12, Olympus, Japan) and an unbiased counting frame. In brief, probing in the depth of sections, the oocyte's nucleus in maximum focus was selected (if the nucleus is not apparent, the center of the nucleus was considered as the virtual center of the nucleus) (18). 2 straight lines were drawn in different directions from the nucleus's center to the nuclear membrane (to estimate the volume of the nucleus) and to the oocyte membrane (to estimate the volume of the oocyte) (Figure 1d, e). Finally, the following formula was used, where ‘Vn’ represents the volume of the oocyte or nucleus and ‘Ln’ displays the distance from the nucleus's center to the nucleus membrane or from the nucleus's center to the oocyte membrane. The results were reported as µm3 (π = 3.14).
2.5. Lipid peroxidation assay
Lipid peroxidation in the damaged cell membrane increases the production of malondialdehyde (MDA). Therefore, MDA was measured as an index of lipid peroxidation. MDA assay was performed via the Aust and Buege method. Briefly, a reagent solution, including 0.375% thiobarbituric acid (Merck, Germany), 15% trichloroacetic acid (Merck, Germany), and 0.25 N hydrochloric acid. 200 μl of reagent and 100 μl of serum were mixed and placed in a bain-marie at 95°C for 15 min, then cooled and centrifuged (10 min, 1000 rpm). Lastly, the supernatant solution obtained was measured at 532 nm by spectrophotometer (PG Instruments Ltd, UK). Finally, the MDA level was calculated using the extinction coefficient of 1.56 × 105 M-1 cm-1 (5).
2.6. Ferric reducing antioxidant power (FRAP) assay
The FRAP method was applied to measure the total antioxidant capacity (TAC) (FRAP method presented by Benzie and Strain). Briefly, the FRAP reagent was prepared in a 1:1:10 ratio of 20 mM ferric chloride, 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ; Sigma-Aldrich, USA), and 300 mM sodium acetate buffer (pH 3.6). Also, different concentrations of FeSO4.7H2O were prepared. 1.5 ml of FRAP reagent was added to 50 μl of serum sample and FeSO4.7H2O. It was then incubated for 4 min at 37°C bain-marie, and absorbance at a wavelength of 593 nm by a spectrophotometer (PG Instruments Ltd, UK) was measured. The standard curve of different concentrations of FeSO4.7H2O was drawn using GraphPad Prism software. Finally, TAC levels were calculated in serum samples (5).
2.7. Hormonal assay
The levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the experimental groups were measured by the enzyme-linked immunosorbent assay (ELISA, Pishtazteb, Iran). Based on the kit protocol, hormonal levels were measured by sandwich ELISA method and using the monoclonal antibodies. Finally, the concentration of hormones was reported as IU/L.
2.8. Ethical considerations
According to the principles approved by the Research Ethics Committee of Arak University, Arak, Iran (Code: IR.ARAKU.REC.1400.003), laboratory mice were employed in this study. All procedures were carried out following the ethical and ARRIVE guidelines.
2.9. Statistical analysis
Data were analyzed using the SPSS statistics software (Version 26, IBM Inc., USA). Shapiro-Wilk and Kolmogorov-Smirnov statistical tests were used to evaluate the normality of the data. Finally, considering the significance level p < 0.05 and 95% confidence interval, data were analyzed using one-way analysis of variance (ANOVA) and Tukey's post hoc test.
3. Results
In this experimental study, all 24 healthy 5-6 wk-old NMRI female mice (Pasteur Institute, Tehran, Iran) were treated in 4 groups until the end of the treatment period for 35 days. Hence, all participants data were reported.
3.1. Body and right ovary weight
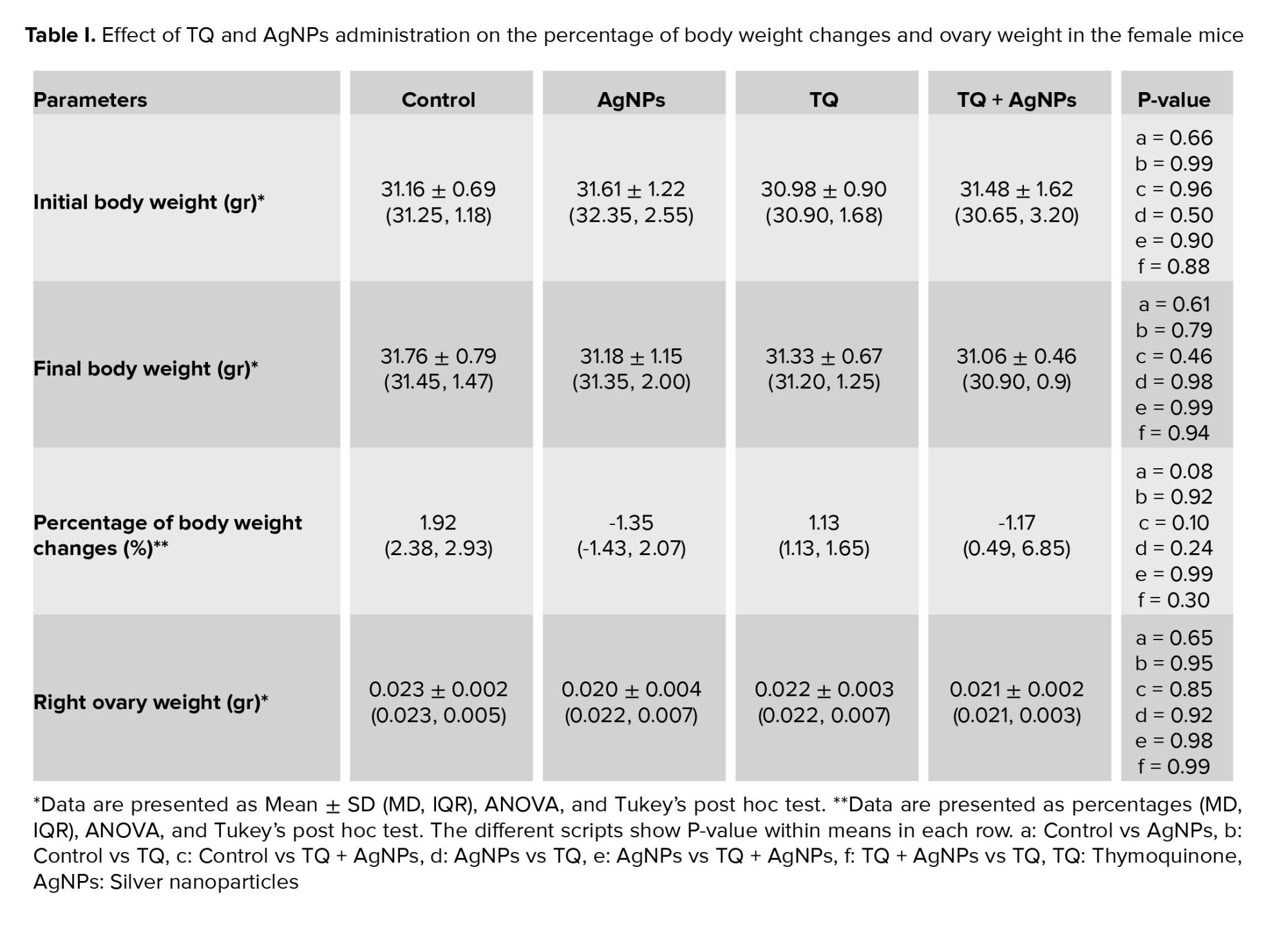
No significant changes were observed in the body and ovary weight in the AgNPs group compared to the control group. Also, no difference was observed between the experimental groups. The detailed data are shown in table I.
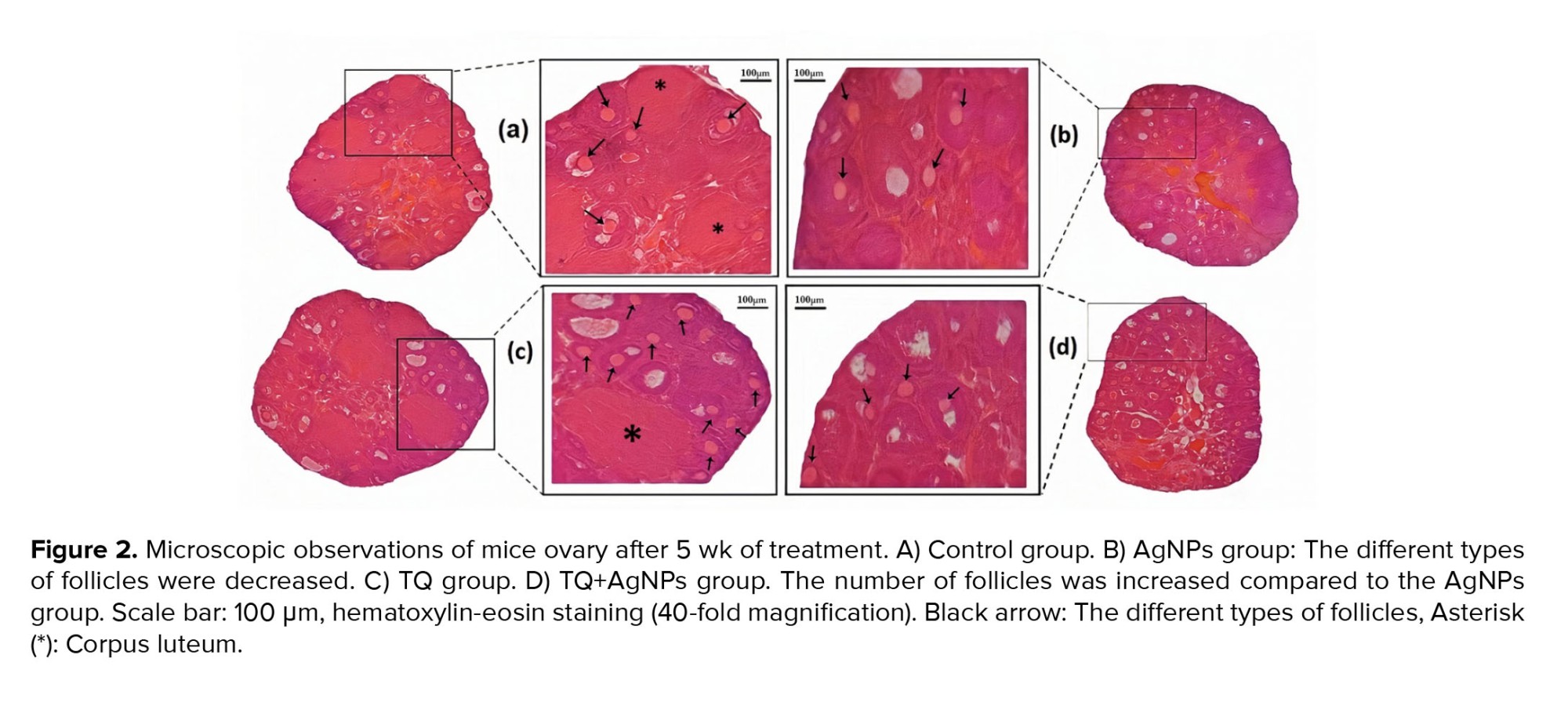
3.2. Histological findings
Based on microscopic studies (Figure 2), folliculogenesis, the number of follicles, and corpus luteum in control and TQ groups were normal. In contrast, fewer follicles were observed in the AgNPs group. Meanwhile, the different types of follicles were observed in the co-administration group (TQ+AgNPs), similar to the control group.
3.3. Volume of the ovary, medulla, cortex, and corpus luteum
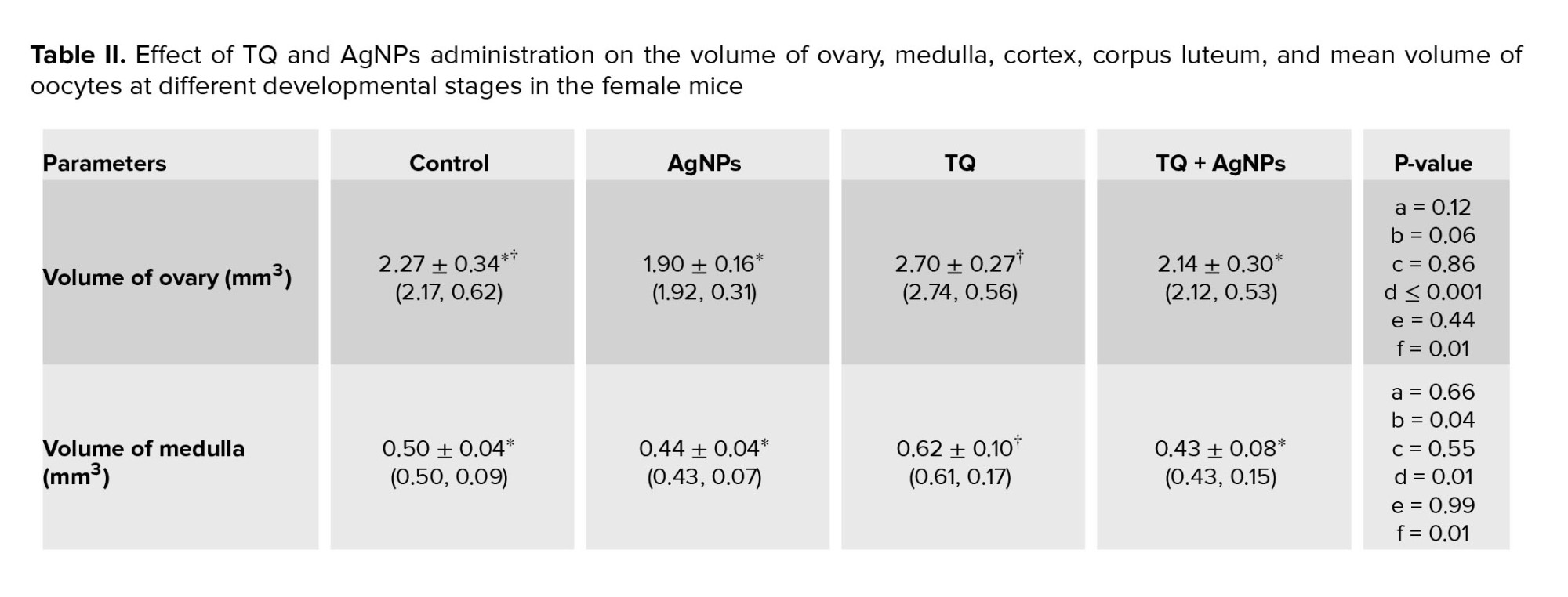
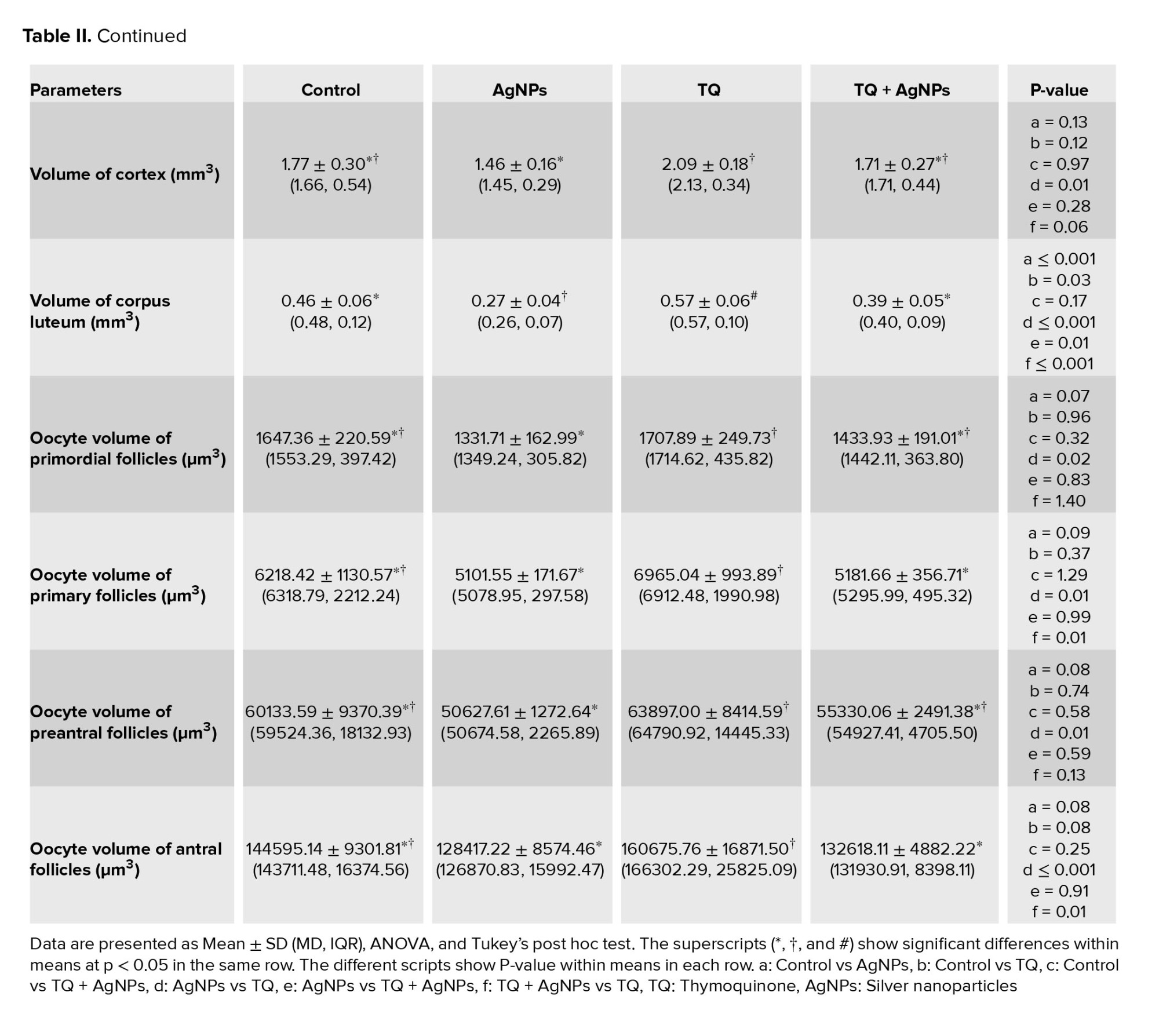
No differences were observed in the mean total volume of the ovary, medulla, and cortex in the AgNPs group compared to the control group. In contrast, an increase in the mean total volume of the ovary, medulla, and cortex in the TQ group compared to the AgNPs group, were observed (Table II). However, AgNPs caused a decrease in the volume of the corpus luteum in the AgNPs group compared to the control group. On the other hand, the volume of corpus luteum in the co-administration group (TQ+AgNPs) was improved compared to the AgNPs group, so it was observed at the level of the control group. Also, an increase in corpus luteum volume was observed in the TQ group compared to the control group (Table II).
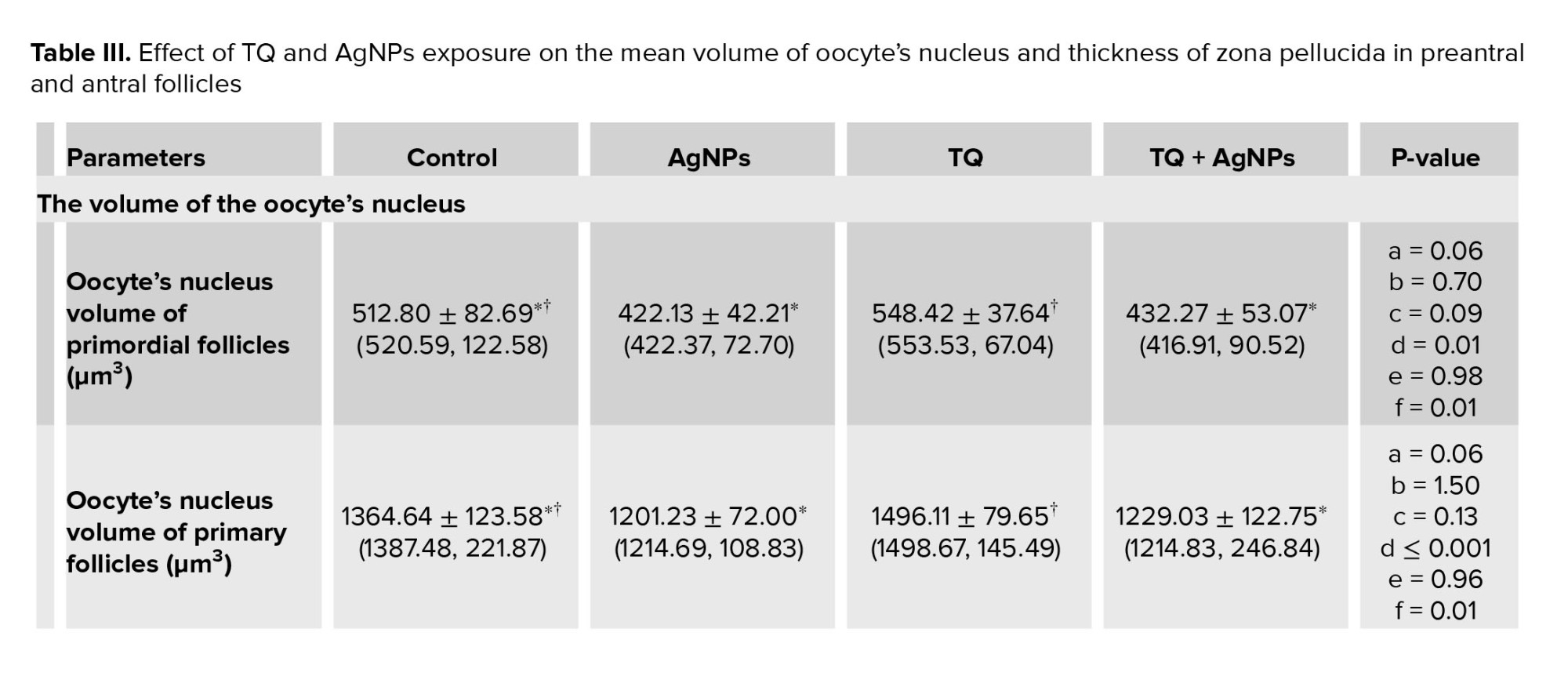
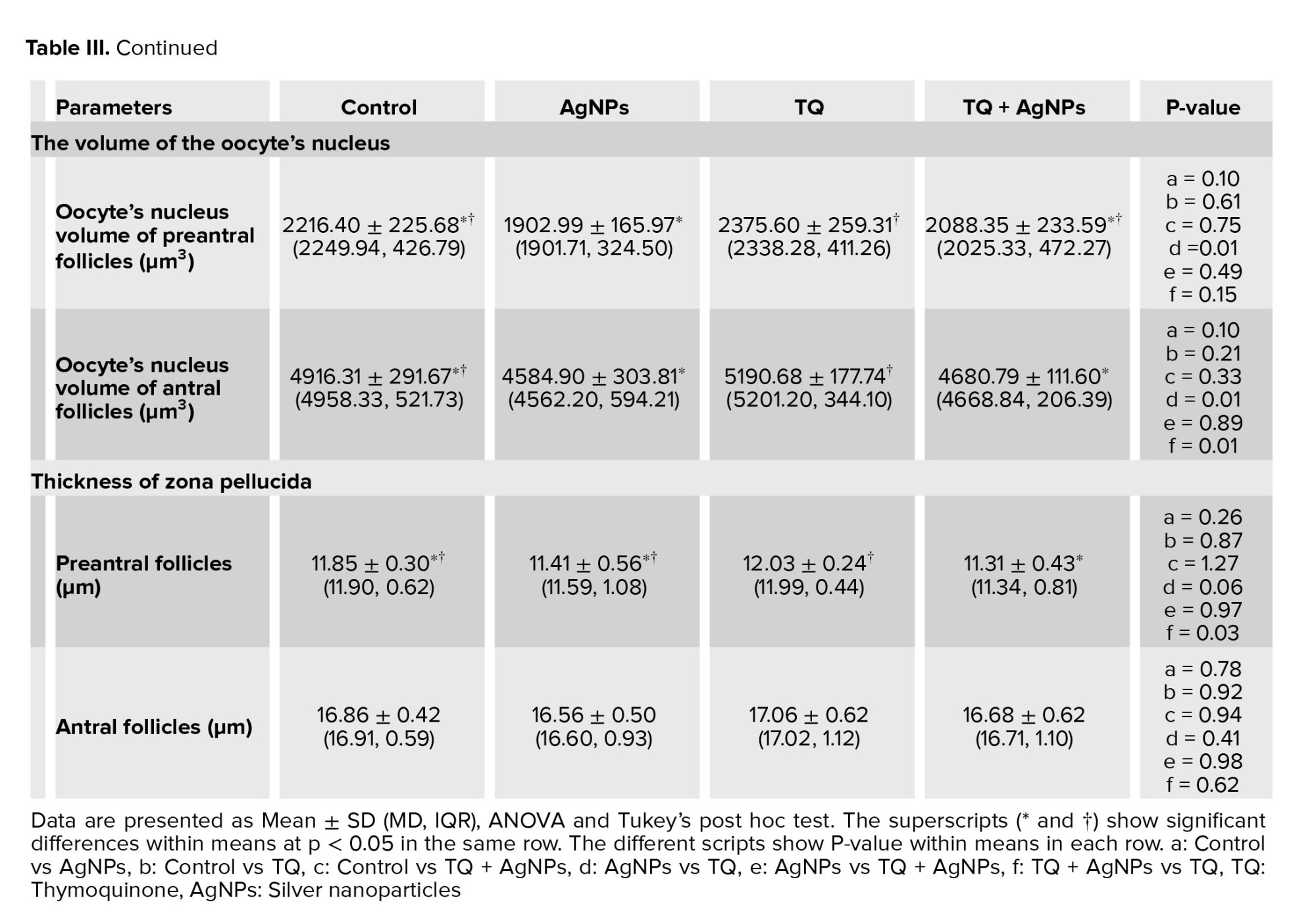
3.4. Volume of the oocyte and its nucleus, and thickness of zona pellucida
Compared to the control group, no differences were observed in the mean volume of oocytes in primary, primordial, preantral, and antral follicles in the AgNPs group (Table II). Compared to the control group, no differences were observed in the mean volume of the oocyte nucleus in primary, primordial, preantral, and antral follicles in the AgNPs group (Table III). Also, no difference was observed in the mean thickness of the zona pellucida in preantral and antral follicles in the AgNPs group when compared with the control group (Table III).
3.5. Follicular count
A decrease in the number of primordial, primary, preantral, and antral follicles was seen in the AgNPs group compared to the control group. In the TQ+AgNPs group, an increase in the number of primary, preantral, and antral follicles was observed compared to the AgNPs group. Although the number of primordial follicles in the TQ+AgNPs group improved compared to the AgNPs group, this increase was insignificant. In addition, an increase in the number of primary, preantral, and antral follicles was observed in the TQ group compared to the control group (Table IV).
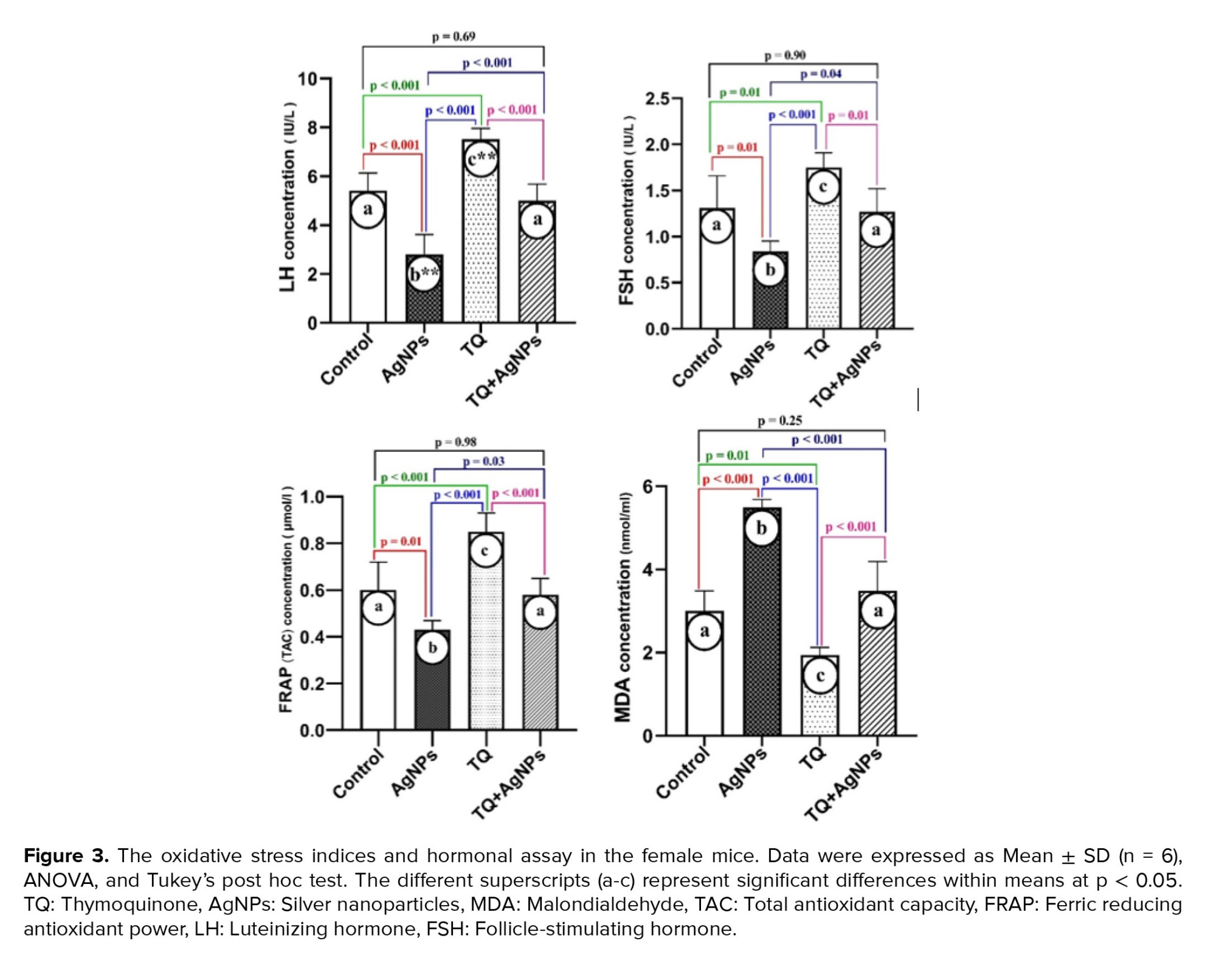
3.6. FRAP and lipid peroxidation
As shown in figure 3, the results of oxidative stress indices showed that the level of MDA increased in the AgNPs group. However, it was decreased in the co-administration group (TQ + AgNPs) compared to the AgNPs group. In addition, a decrease in MDA was observed in the TQ group compared to the control group. Also, the results of FRAP showed a decrease in TAC in the AgNPs group compared to the control group. In contrast, the level of TAC in the TQ + AgNPs group was increased compared to the AgNPs group.
3.7. The sexual hormones levels: LH and FSH
As shown in figure 3, compared to the control group, the levels of LH and FSH hormones were decreased in the AgNPs group. In parallel, in the TQ + AgNPs group, improvement of LH and FSH were observed compared to the AgNPs group. Also, TQ increased the concentration of LH and FSH compared to the control group.

2.4.2. Estimation of the number of follicles
The optical disector method and optical microscope (BX41, Olympus, Japan) equipped with a microcator device (ND221B, Heidenhain, Germany) were used to estimate the follicular count at different developmental stages. The images of 20-micron sections were reflected on the monitor with ×1000 magnification. The number of cells at a depth of 10 µm was estimated using a microcator and an unbiased counting frame (Figure 1b). 5 µm from the top and bottom of the 20 µm sections as guard zones were omitted. 100-150 cells were counted for each ovary. According to the following formula, the number of follicles, including primordial, primary, preantral, and antral were estimated in ovarian tissue. Where ‘∑Qi’, ‘∑p’, and ‘a/f’ denote the number of cells counted, the total number of counting frames that hit the fields of view, and the area of the unbiased counting frame, respectively. Also, ‘h’ is the height of the disector which was 10 μm, and ‘Vtotal ovary’ represents the total volume of the ovary calculated by the Cavalieri method (18).
N V [ ∑ /(h ∑ p a f ) V total ovary ]
2.4.3. Estimation of zona pellucida thickness in the preantral and antral follicles
At first, microscopic images from 5-micron sections were randomly selected, and the harmonic method was used to measure the thickness of the zona pellucida. Then, a special probe with 3 parallel and equal lines was randomly dropped on the microscopic images with ×1000 magnification (Figure 1c). In this method, from the intersection of probe lines with the inner surface of the zona pellucida, a vertical line was drawn to the tangent line of the outer surface of the zona pellucida. The length of the vertical line (orthogonal intercept) was measured by Motic Images software. An average of 120 orthogonal intercepts (oi1, oi2, oi3, …., oi119, oi120) were measured for each ovary. Finally, the mean thickness of the zona pellucida (ZP) was estimated using the following formula: Where ‘N’ represents the total number of measurements, and ‘oi’ shows the length of each vertical line from the inner surface to the outer surface of the zona pellucida (18) (π = 3.14).
T h ickness of ZP /[1/ oi 1 1/ oi 2 …
2.4.4. Estimation of the volume of the oocyte and its nucleus
According to the nucleator method, images of 20-micron sections were randomly selected using an optical microscope at ×1000 magnifications (BX41, Olympus, Japan), equipped with a camera (DP12, Olympus, Japan) and an unbiased counting frame. In brief, probing in the depth of sections, the oocyte's nucleus in maximum focus was selected (if the nucleus is not apparent, the center of the nucleus was considered as the virtual center of the nucleus) (18). 2 straight lines were drawn in different directions from the nucleus's center to the nuclear membrane (to estimate the volume of the nucleus) and to the oocyte membrane (to estimate the volume of the oocyte) (Figure 1d, e). Finally, the following formula was used, where ‘Vn’ represents the volume of the oocyte or nucleus and ‘Ln’ displays the distance from the nucleus's center to the nucleus membrane or from the nucleus's center to the oocyte membrane. The results were reported as µm3 (π = 3.14).
V n 4 /3 L n 3
2.5. Lipid peroxidation assay
Lipid peroxidation in the damaged cell membrane increases the production of malondialdehyde (MDA). Therefore, MDA was measured as an index of lipid peroxidation. MDA assay was performed via the Aust and Buege method. Briefly, a reagent solution, including 0.375% thiobarbituric acid (Merck, Germany), 15% trichloroacetic acid (Merck, Germany), and 0.25 N hydrochloric acid. 200 μl of reagent and 100 μl of serum were mixed and placed in a bain-marie at 95°C for 15 min, then cooled and centrifuged (10 min, 1000 rpm). Lastly, the supernatant solution obtained was measured at 532 nm by spectrophotometer (PG Instruments Ltd, UK). Finally, the MDA level was calculated using the extinction coefficient of 1.56 × 105 M-1 cm-1 (5).
2.6. Ferric reducing antioxidant power (FRAP) assay
The FRAP method was applied to measure the total antioxidant capacity (TAC) (FRAP method presented by Benzie and Strain). Briefly, the FRAP reagent was prepared in a 1:1:10 ratio of 20 mM ferric chloride, 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ; Sigma-Aldrich, USA), and 300 mM sodium acetate buffer (pH 3.6). Also, different concentrations of FeSO4.7H2O were prepared. 1.5 ml of FRAP reagent was added to 50 μl of serum sample and FeSO4.7H2O. It was then incubated for 4 min at 37°C bain-marie, and absorbance at a wavelength of 593 nm by a spectrophotometer (PG Instruments Ltd, UK) was measured. The standard curve of different concentrations of FeSO4.7H2O was drawn using GraphPad Prism software. Finally, TAC levels were calculated in serum samples (5).
2.7. Hormonal assay
The levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the experimental groups were measured by the enzyme-linked immunosorbent assay (ELISA, Pishtazteb, Iran). Based on the kit protocol, hormonal levels were measured by sandwich ELISA method and using the monoclonal antibodies. Finally, the concentration of hormones was reported as IU/L.
Figure 1. A) The cavalieri technique to estimate the volume. B) The unbiased counting frame to estimate the follicular count. C) The harmonic method to estimate the thickness of zona pellucida. D, E) The nucleator method to estimate the volume of the oocyte and its nucleus. a/p and Δx, Δy represent the area and distance between 2 points in the x-y axis, respectively.
2.8. Ethical considerations
According to the principles approved by the Research Ethics Committee of Arak University, Arak, Iran (Code: IR.ARAKU.REC.1400.003), laboratory mice were employed in this study. All procedures were carried out following the ethical and ARRIVE guidelines.
2.9. Statistical analysis
Data were analyzed using the SPSS statistics software (Version 26, IBM Inc., USA). Shapiro-Wilk and Kolmogorov-Smirnov statistical tests were used to evaluate the normality of the data. Finally, considering the significance level p < 0.05 and 95% confidence interval, data were analyzed using one-way analysis of variance (ANOVA) and Tukey's post hoc test.
3. Results
In this experimental study, all 24 healthy 5-6 wk-old NMRI female mice (Pasteur Institute, Tehran, Iran) were treated in 4 groups until the end of the treatment period for 35 days. Hence, all participants data were reported.
3.1. Body and right ovary weight
No significant changes were observed in the body and ovary weight in the AgNPs group compared to the control group. Also, no difference was observed between the experimental groups. The detailed data are shown in table I.
3.2. Histological findings
Based on microscopic studies (Figure 2), folliculogenesis, the number of follicles, and corpus luteum in control and TQ groups were normal. In contrast, fewer follicles were observed in the AgNPs group. Meanwhile, the different types of follicles were observed in the co-administration group (TQ+AgNPs), similar to the control group.
3.3. Volume of the ovary, medulla, cortex, and corpus luteum
No differences were observed in the mean total volume of the ovary, medulla, and cortex in the AgNPs group compared to the control group. In contrast, an increase in the mean total volume of the ovary, medulla, and cortex in the TQ group compared to the AgNPs group, were observed (Table II). However, AgNPs caused a decrease in the volume of the corpus luteum in the AgNPs group compared to the control group. On the other hand, the volume of corpus luteum in the co-administration group (TQ+AgNPs) was improved compared to the AgNPs group, so it was observed at the level of the control group. Also, an increase in corpus luteum volume was observed in the TQ group compared to the control group (Table II).
3.4. Volume of the oocyte and its nucleus, and thickness of zona pellucida
Compared to the control group, no differences were observed in the mean volume of oocytes in primary, primordial, preantral, and antral follicles in the AgNPs group (Table II). Compared to the control group, no differences were observed in the mean volume of the oocyte nucleus in primary, primordial, preantral, and antral follicles in the AgNPs group (Table III). Also, no difference was observed in the mean thickness of the zona pellucida in preantral and antral follicles in the AgNPs group when compared with the control group (Table III).
3.5. Follicular count
A decrease in the number of primordial, primary, preantral, and antral follicles was seen in the AgNPs group compared to the control group. In the TQ+AgNPs group, an increase in the number of primary, preantral, and antral follicles was observed compared to the AgNPs group. Although the number of primordial follicles in the TQ+AgNPs group improved compared to the AgNPs group, this increase was insignificant. In addition, an increase in the number of primary, preantral, and antral follicles was observed in the TQ group compared to the control group (Table IV).
3.6. FRAP and lipid peroxidation
As shown in figure 3, the results of oxidative stress indices showed that the level of MDA increased in the AgNPs group. However, it was decreased in the co-administration group (TQ + AgNPs) compared to the AgNPs group. In addition, a decrease in MDA was observed in the TQ group compared to the control group. Also, the results of FRAP showed a decrease in TAC in the AgNPs group compared to the control group. In contrast, the level of TAC in the TQ + AgNPs group was increased compared to the AgNPs group.
3.7. The sexual hormones levels: LH and FSH
As shown in figure 3, compared to the control group, the levels of LH and FSH hormones were decreased in the AgNPs group. In parallel, in the TQ + AgNPs group, improvement of LH and FSH were observed compared to the AgNPs group. Also, TQ increased the concentration of LH and FSH compared to the control group.
Regarding the female reproductive system, AgNPs in different sizes, depending on the dose, cause severe damage to ovarian tissue and follicular cells (8). Currently, laboratory studies have demonstrated that AgNPs can cause developmental toxicities in the fetus (9). Exposure to AgNPs by disrupting the process of aromatization and steroidogenesis causes a decrease in estrogen, degeneration of follicles, and induction of apoptosis in ovarian cells (10). AgNPs have toxic effects on the development of mouse embryos; histopathological studies have shown that the toxic dose of AgNPs passes through the placenta, causing a decrease in cell viability and damaging the follicular cells in the ovarian tissue (11). Through the induction of lipid peroxidation and reactive oxygen species, AgNPs decreased Cyp19a1 enzyme (cytochrome P450, family 19, subfamily a, polypeptide 1) activity, estrogen levels, and destruction of preantral and antral follicles (12).
Herbal medicines are of particular importance as alternative medicines. They have reduced the concern caused by the side effects of synthetic drugs. One of the effective compounds is thymoquinone (TQ). TQ (2-isopropyl-5-methylbenzo-1,4-quinone), as a natural antioxidant, is a bioactive ingredient of Nigella sativa (13). Molecular signaling pathways show that TQ protects cells against oxidative stress by regulating the activity of antioxidant enzymes. They can also play an essential role in cell survival or apoptosis by regulating inflammatory cytokines and factors involved in cell cycle regulation (14). The pharmacological effects of TQ, including anti-inflammatory, modulation of the immune system, and anticancer effects, have been proven in previous studies. In addition, TQ improves cardiac, neurological (neurological inflammation, Alzheimer's and Parkinson's diseases), respiratory, digestive, liver, kidney, and other disorders. Besides, it is very effective in antiobesity and antidiabetes (15). Previous studies have shown the therapeutic effect of TQ on the male and female reproductive system (16). Recent findings have shown that TQ has a protective impact on polycystic ovary syndrome (17).
Based on recent searches, there was no stereological analyses of the effects of TQ on the toxic effects of AgNPs on mouse ovarian structure. The present study evaluated the effectiveness of TQ against the toxicity induced by AgNPs on ovarian tissue in mice. The stereological analysis of ovarian tissue, the follicular count and their volume at different developmental stages, the measurement of oxidative stress indices, and the level of sex hormones evaluated.
2. Materials and Methods
2.1. Preparation of chemicals
The commercial AgNPs (US Research Nanomaterials, Inc., USA) were purchased from Pishgaman Nanomaterials Co., Iran. The authenticity of the quality of AgNPs (spherical morphology, 99.99% purity, average diameter 20 nm, metal base) was evaluated and confirmed through X-ray diffraction and transmission electron microscopy by Pishgaman Nanomaterials Co., Iran. Also, TQ with characteristics (2-Isopropyl-5-methyl-1, 4-benzoquinone, ≥ 98.0%, molecular formula: C10H12O2, molecular weight: 164.20 g.mol-1) (Sigma-Aldrich, USA) was purchased.
2.2. Sample size
According to previous studies, alpha error was considered 5% (4, 15). Subsequently, the sample size was determined using the N = DF/K + 1 formula, where N, DF, and K denote the number of mice per group, degrees of freedom, and the number of groups, respectively. Therefore, 24 mice (n = 6/each) were utilized for the experiment.
2.3. Experimental design and mice
In this experiment, 24 healthy 5-6 wk-old NMRI female mice with an average weight of 33 ± 1.5 gr were used. Mice were purchased from the Pasteur Institute (Tehran, Iran). 1 wk before the beginning of the experiment, the animals were kept in the animal house of Arak University under standard conditions for 12 hr light/dark cycle at a temperature of 21 ± 2°C with free access to a standard diet and water. We closely monitored the mice’s nutritional behavior, body weight, behavior, and physical appearance changes during the treatment period. Therefore, we committed to halting the treatment immediately if we noticed any signs of suffering.
Mice were randomly divided into 4 groups (n = 6/each): control group, AgNPs group (500 mg/kg) (4, 7), TQ group (2.5 mg/kg) (15), and co-administration of AgNPs + TQ group (2.5 mg/kg TQ + 500 mg/kg AgNPs). According to the average weight of female mice (33 gr), solutions of 500 mg/kg bwt AgNPs in distilled water and 2.5 mg/kg bwt TQ in dimethyl sulfoxide were prepared daily. Thus, mice were treated with AgNPs orally (via gavage tube) and TQ via intraperitoneal injection for 35 consecutive days (12). At the end of the treatment period, the percentage of changes in the body weight of mice was calculated using the formula (W2-W1)
2.4. Tissue processing and stereological analysis
Tissue processing was performed via an automatic processor (Histokinette, Leica, Germany). In brief, spherical and cylindrical tissue blocks were obtained from the paraffin dispenser (DS-4L, Didsabz, Iran). Sections were prepared using the isotropic uniform random method and a Leitz 1512 rotary microtome (Leitz, USA). For stereological studies, 10-12 sections per ovary are required (18). Approximately 120 sections (i.e., 60 sections of 5-micron and 60 sections of 20-micron) alternately were obtained, per ovary. Subsequently, using the systematic sampling and K = N/n formula, 20 sections were selected for each ovary (i.e., 10 sections of 20-micron and 10 sections of 5-micron per ovary) to the hematoxylin and eosin staining. K, N, and n denote the uniform distance between 2 samples, the total number of samples, and the number of samples, respectively.
2.4.1. Estimation of volume (ovary, medulla, cortex, and corpus luteum)
The Cavalieri technique and 10 sections of 5-micron per ovary were employed to estimate the volume of ovarian structure. Briefly, an optical microscope (BX41, Olympus, Japan) equipped with a camera (DP12, Olympus, Japan) and the images of ovarian tissue were reflected on the monitor with ×40 magnification. The point probe was randomly dropped on the images. In each ovary, the total number of collision points with all images of the sections and 200 collision points for each section of the cortex, medulla, and corpus luteum were counted. Based on the following formulas, ‘∑p’ is the total collision points between the probe and images, ‘a(p)’ is the area of each point on the probe, ‘t’ is the thickness of the sections, ‘Δx’ and ‘Δy’ are the distance between 2 points (Figure 1a), ‘M’ denotes the microscope magnification, ‘∑p medulla’ is probe collisions with the medulla, ‘∑p cortex’ is probe collisions with the cortex, ‘∑p corpus luteum’ denotes the probe collisions with the corpus luteum (18).

2.4.2. Estimation of the number of follicles
The optical disector method and optical microscope (BX41, Olympus, Japan) equipped with a microcator device (ND221B, Heidenhain, Germany) were used to estimate the follicular count at different developmental stages. The images of 20-micron sections were reflected on the monitor with ×1000 magnification. The number of cells at a depth of 10 µm was estimated using a microcator and an unbiased counting frame (Figure 1b). 5 µm from the top and bottom of the 20 µm sections as guard zones were omitted. 100-150 cells were counted for each ovary. According to the following formula, the number of follicles, including primordial, primary, preantral, and antral were estimated in ovarian tissue. Where ‘∑Qi’, ‘∑p’, and ‘a/f’ denote the number of cells counted, the total number of counting frames that hit the fields of view, and the area of the unbiased counting frame, respectively. Also, ‘h’ is the height of the disector which was 10 μm, and ‘Vtotal ovary’ represents the total volume of the ovary calculated by the Cavalieri method (18).
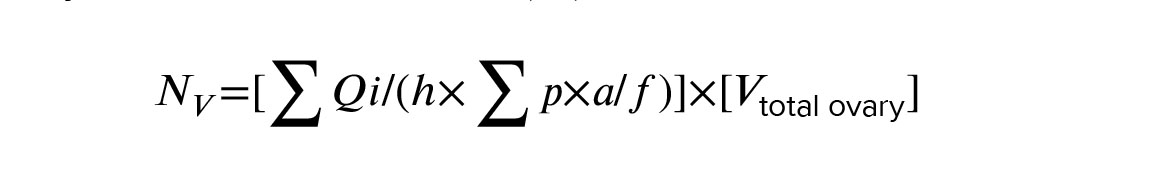
2.4.3. Estimation of zona pellucida thickness in the preantral and antral follicles
At first, microscopic images from 5-micron sections were randomly selected, and the harmonic method was used to measure the thickness of the zona pellucida. Then, a special probe with 3 parallel and equal lines was randomly dropped on the microscopic images with ×1000 magnification (Figure 1c). In this method, from the intersection of probe lines with the inner surface of the zona pellucida, a vertical line was drawn to the tangent line of the outer surface of the zona pellucida. The length of the vertical line (orthogonal intercept) was measured by Motic Images software. An average of 120 orthogonal intercepts (oi1, oi2, oi3, …., oi119, oi120) were measured for each ovary. Finally, the mean thickness of the zona pellucida (ZP) was estimated using the following formula: Where ‘N’ represents the total number of measurements, and ‘oi’ shows the length of each vertical line from the inner surface to the outer surface of the zona pellucida (18) (π = 3.14).
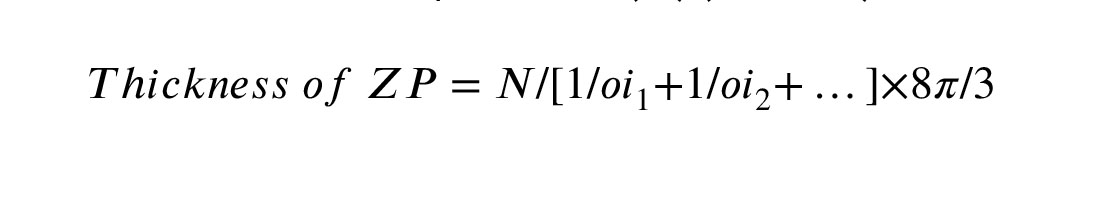
2.4.4. Estimation of the volume of the oocyte and its nucleus
According to the nucleator method, images of 20-micron sections were randomly selected using an optical microscope at ×1000 magnifications (BX41, Olympus, Japan), equipped with a camera (DP12, Olympus, Japan) and an unbiased counting frame. In brief, probing in the depth of sections, the oocyte's nucleus in maximum focus was selected (if the nucleus is not apparent, the center of the nucleus was considered as the virtual center of the nucleus) (18). 2 straight lines were drawn in different directions from the nucleus's center to the nuclear membrane (to estimate the volume of the nucleus) and to the oocyte membrane (to estimate the volume of the oocyte) (Figure 1d, e). Finally, the following formula was used, where ‘Vn’ represents the volume of the oocyte or nucleus and ‘Ln’ displays the distance from the nucleus's center to the nucleus membrane or from the nucleus's center to the oocyte membrane. The results were reported as µm3 (π = 3.14).
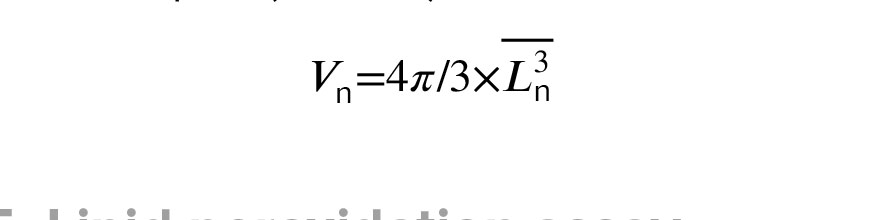
2.5. Lipid peroxidation assay
Lipid peroxidation in the damaged cell membrane increases the production of malondialdehyde (MDA). Therefore, MDA was measured as an index of lipid peroxidation. MDA assay was performed via the Aust and Buege method. Briefly, a reagent solution, including 0.375% thiobarbituric acid (Merck, Germany), 15% trichloroacetic acid (Merck, Germany), and 0.25 N hydrochloric acid. 200 μl of reagent and 100 μl of serum were mixed and placed in a bain-marie at 95°C for 15 min, then cooled and centrifuged (10 min, 1000 rpm). Lastly, the supernatant solution obtained was measured at 532 nm by spectrophotometer (PG Instruments Ltd, UK). Finally, the MDA level was calculated using the extinction coefficient of 1.56 × 105 M-1 cm-1 (5).
2.6. Ferric reducing antioxidant power (FRAP) assay
The FRAP method was applied to measure the total antioxidant capacity (TAC) (FRAP method presented by Benzie and Strain). Briefly, the FRAP reagent was prepared in a 1:1:10 ratio of 20 mM ferric chloride, 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ; Sigma-Aldrich, USA), and 300 mM sodium acetate buffer (pH 3.6). Also, different concentrations of FeSO4.7H2O were prepared. 1.5 ml of FRAP reagent was added to 50 μl of serum sample and FeSO4.7H2O. It was then incubated for 4 min at 37°C bain-marie, and absorbance at a wavelength of 593 nm by a spectrophotometer (PG Instruments Ltd, UK) was measured. The standard curve of different concentrations of FeSO4.7H2O was drawn using GraphPad Prism software. Finally, TAC levels were calculated in serum samples (5).
2.7. Hormonal assay
The levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the experimental groups were measured by the enzyme-linked immunosorbent assay (ELISA, Pishtazteb, Iran). Based on the kit protocol, hormonal levels were measured by sandwich ELISA method and using the monoclonal antibodies. Finally, the concentration of hormones was reported as IU/L.

2.8. Ethical considerations
According to the principles approved by the Research Ethics Committee of Arak University, Arak, Iran (Code: IR.ARAKU.REC.1400.003), laboratory mice were employed in this study. All procedures were carried out following the ethical and ARRIVE guidelines.
2.9. Statistical analysis
Data were analyzed using the SPSS statistics software (Version 26, IBM Inc., USA). Shapiro-Wilk and Kolmogorov-Smirnov statistical tests were used to evaluate the normality of the data. Finally, considering the significance level p < 0.05 and 95% confidence interval, data were analyzed using one-way analysis of variance (ANOVA) and Tukey's post hoc test.
3. Results
In this experimental study, all 24 healthy 5-6 wk-old NMRI female mice (Pasteur Institute, Tehran, Iran) were treated in 4 groups until the end of the treatment period for 35 days. Hence, all participants data were reported.
3.1. Body and right ovary weight
No significant changes were observed in the body and ovary weight in the AgNPs group compared to the control group. Also, no difference was observed between the experimental groups. The detailed data are shown in table I.
3.2. Histological findings
Based on microscopic studies (Figure 2), folliculogenesis, the number of follicles, and corpus luteum in control and TQ groups were normal. In contrast, fewer follicles were observed in the AgNPs group. Meanwhile, the different types of follicles were observed in the co-administration group (TQ+AgNPs), similar to the control group.
3.3. Volume of the ovary, medulla, cortex, and corpus luteum
No differences were observed in the mean total volume of the ovary, medulla, and cortex in the AgNPs group compared to the control group. In contrast, an increase in the mean total volume of the ovary, medulla, and cortex in the TQ group compared to the AgNPs group, were observed (Table II). However, AgNPs caused a decrease in the volume of the corpus luteum in the AgNPs group compared to the control group. On the other hand, the volume of corpus luteum in the co-administration group (TQ+AgNPs) was improved compared to the AgNPs group, so it was observed at the level of the control group. Also, an increase in corpus luteum volume was observed in the TQ group compared to the control group (Table II).
3.4. Volume of the oocyte and its nucleus, and thickness of zona pellucida
Compared to the control group, no differences were observed in the mean volume of oocytes in primary, primordial, preantral, and antral follicles in the AgNPs group (Table II). Compared to the control group, no differences were observed in the mean volume of the oocyte nucleus in primary, primordial, preantral, and antral follicles in the AgNPs group (Table III). Also, no difference was observed in the mean thickness of the zona pellucida in preantral and antral follicles in the AgNPs group when compared with the control group (Table III).
3.5. Follicular count
A decrease in the number of primordial, primary, preantral, and antral follicles was seen in the AgNPs group compared to the control group. In the TQ+AgNPs group, an increase in the number of primary, preantral, and antral follicles was observed compared to the AgNPs group. Although the number of primordial follicles in the TQ+AgNPs group improved compared to the AgNPs group, this increase was insignificant. In addition, an increase in the number of primary, preantral, and antral follicles was observed in the TQ group compared to the control group (Table IV).
3.6. FRAP and lipid peroxidation
As shown in figure 3, the results of oxidative stress indices showed that the level of MDA increased in the AgNPs group. However, it was decreased in the co-administration group (TQ + AgNPs) compared to the AgNPs group. In addition, a decrease in MDA was observed in the TQ group compared to the control group. Also, the results of FRAP showed a decrease in TAC in the AgNPs group compared to the control group. In contrast, the level of TAC in the TQ + AgNPs group was increased compared to the AgNPs group.
3.7. The sexual hormones levels: LH and FSH
As shown in figure 3, compared to the control group, the levels of LH and FSH hormones were decreased in the AgNPs group. In parallel, in the TQ + AgNPs group, improvement of LH and FSH were observed compared to the AgNPs group. Also, TQ increased the concentration of LH and FSH compared to the control group.








2.4.2. Estimation of the number of follicles
The optical disector method and optical microscope (BX41, Olympus, Japan) equipped with a microcator device (ND221B, Heidenhain, Germany) were used to estimate the follicular count at different developmental stages. The images of 20-micron sections were reflected on the monitor with ×1000 magnification. The number of cells at a depth of 10 µm was estimated using a microcator and an unbiased counting frame (Figure 1b). 5 µm from the top and bottom of the 20 µm sections as guard zones were omitted. 100-150 cells were counted for each ovary. According to the following formula, the number of follicles, including primordial, primary, preantral, and antral were estimated in ovarian tissue. Where ‘∑Qi’, ‘∑p’, and ‘a/f’ denote the number of cells counted, the total number of counting frames that hit the fields of view, and the area of the unbiased counting frame, respectively. Also, ‘h’ is the height of the disector which was 10 μm, and ‘Vtotal ovary’ represents the total volume of the ovary calculated by the Cavalieri method (18).
2.4.3. Estimation of zona pellucida thickness in the preantral and antral follicles
At first, microscopic images from 5-micron sections were randomly selected, and the harmonic method was used to measure the thickness of the zona pellucida. Then, a special probe with 3 parallel and equal lines was randomly dropped on the microscopic images with ×1000 magnification (Figure 1c). In this method, from the intersection of probe lines with the inner surface of the zona pellucida, a vertical line was drawn to the tangent line of the outer surface of the zona pellucida. The length of the vertical line (orthogonal intercept) was measured by Motic Images software. An average of 120 orthogonal intercepts (oi1, oi2, oi3, …., oi119, oi120) were measured for each ovary. Finally, the mean thickness of the zona pellucida (ZP) was estimated using the following formula: Where ‘N’ represents the total number of measurements, and ‘oi’ shows the length of each vertical line from the inner surface to the outer surface of the zona pellucida (18) (π = 3.14).
2.4.4. Estimation of the volume of the oocyte and its nucleus
According to the nucleator method, images of 20-micron sections were randomly selected using an optical microscope at ×1000 magnifications (BX41, Olympus, Japan), equipped with a camera (DP12, Olympus, Japan) and an unbiased counting frame. In brief, probing in the depth of sections, the oocyte's nucleus in maximum focus was selected (if the nucleus is not apparent, the center of the nucleus was considered as the virtual center of the nucleus) (18). 2 straight lines were drawn in different directions from the nucleus's center to the nuclear membrane (to estimate the volume of the nucleus) and to the oocyte membrane (to estimate the volume of the oocyte) (Figure 1d, e). Finally, the following formula was used, where ‘Vn’ represents the volume of the oocyte or nucleus and ‘Ln’ displays the distance from the nucleus's center to the nucleus membrane or from the nucleus's center to the oocyte membrane. The results were reported as µm3 (π = 3.14).
2.5. Lipid peroxidation assay
Lipid peroxidation in the damaged cell membrane increases the production of malondialdehyde (MDA). Therefore, MDA was measured as an index of lipid peroxidation. MDA assay was performed via the Aust and Buege method. Briefly, a reagent solution, including 0.375% thiobarbituric acid (Merck, Germany), 15% trichloroacetic acid (Merck, Germany), and 0.25 N hydrochloric acid. 200 μl of reagent and 100 μl of serum were mixed and placed in a bain-marie at 95°C for 15 min, then cooled and centrifuged (10 min, 1000 rpm). Lastly, the supernatant solution obtained was measured at 532 nm by spectrophotometer (PG Instruments Ltd, UK). Finally, the MDA level was calculated using the extinction coefficient of 1.56 × 105 M-1 cm-1 (5).
2.6. Ferric reducing antioxidant power (FRAP) assay
The FRAP method was applied to measure the total antioxidant capacity (TAC) (FRAP method presented by Benzie and Strain). Briefly, the FRAP reagent was prepared in a 1:1:10 ratio of 20 mM ferric chloride, 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ; Sigma-Aldrich, USA), and 300 mM sodium acetate buffer (pH 3.6). Also, different concentrations of FeSO4.7H2O were prepared. 1.5 ml of FRAP reagent was added to 50 μl of serum sample and FeSO4.7H2O. It was then incubated for 4 min at 37°C bain-marie, and absorbance at a wavelength of 593 nm by a spectrophotometer (PG Instruments Ltd, UK) was measured. The standard curve of different concentrations of FeSO4.7H2O was drawn using GraphPad Prism software. Finally, TAC levels were calculated in serum samples (5).
2.7. Hormonal assay
The levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the experimental groups were measured by the enzyme-linked immunosorbent assay (ELISA, Pishtazteb, Iran). Based on the kit protocol, hormonal levels were measured by sandwich ELISA method and using the monoclonal antibodies. Finally, the concentration of hormones was reported as IU/L.
Figure 1. A) The cavalieri technique to estimate the volume. B) The unbiased counting frame to estimate the follicular count. C) The harmonic method to estimate the thickness of zona pellucida. D, E) The nucleator method to estimate the volume of the oocyte and its nucleus. a/p and Δx, Δy represent the area and distance between 2 points in the x-y axis, respectively.
2.8. Ethical considerations
According to the principles approved by the Research Ethics Committee of Arak University, Arak, Iran (Code: IR.ARAKU.REC.1400.003), laboratory mice were employed in this study. All procedures were carried out following the ethical and ARRIVE guidelines.
2.9. Statistical analysis
Data were analyzed using the SPSS statistics software (Version 26, IBM Inc., USA). Shapiro-Wilk and Kolmogorov-Smirnov statistical tests were used to evaluate the normality of the data. Finally, considering the significance level p < 0.05 and 95% confidence interval, data were analyzed using one-way analysis of variance (ANOVA) and Tukey's post hoc test.
3. Results
In this experimental study, all 24 healthy 5-6 wk-old NMRI female mice (Pasteur Institute, Tehran, Iran) were treated in 4 groups until the end of the treatment period for 35 days. Hence, all participants data were reported.
3.1. Body and right ovary weight
No significant changes were observed in the body and ovary weight in the AgNPs group compared to the control group. Also, no difference was observed between the experimental groups. The detailed data are shown in table I.
3.2. Histological findings
Based on microscopic studies (Figure 2), folliculogenesis, the number of follicles, and corpus luteum in control and TQ groups were normal. In contrast, fewer follicles were observed in the AgNPs group. Meanwhile, the different types of follicles were observed in the co-administration group (TQ+AgNPs), similar to the control group.
3.3. Volume of the ovary, medulla, cortex, and corpus luteum
No differences were observed in the mean total volume of the ovary, medulla, and cortex in the AgNPs group compared to the control group. In contrast, an increase in the mean total volume of the ovary, medulla, and cortex in the TQ group compared to the AgNPs group, were observed (Table II). However, AgNPs caused a decrease in the volume of the corpus luteum in the AgNPs group compared to the control group. On the other hand, the volume of corpus luteum in the co-administration group (TQ+AgNPs) was improved compared to the AgNPs group, so it was observed at the level of the control group. Also, an increase in corpus luteum volume was observed in the TQ group compared to the control group (Table II).
3.4. Volume of the oocyte and its nucleus, and thickness of zona pellucida
Compared to the control group, no differences were observed in the mean volume of oocytes in primary, primordial, preantral, and antral follicles in the AgNPs group (Table II). Compared to the control group, no differences were observed in the mean volume of the oocyte nucleus in primary, primordial, preantral, and antral follicles in the AgNPs group (Table III). Also, no difference was observed in the mean thickness of the zona pellucida in preantral and antral follicles in the AgNPs group when compared with the control group (Table III).
3.5. Follicular count
A decrease in the number of primordial, primary, preantral, and antral follicles was seen in the AgNPs group compared to the control group. In the TQ+AgNPs group, an increase in the number of primary, preantral, and antral follicles was observed compared to the AgNPs group. Although the number of primordial follicles in the TQ+AgNPs group improved compared to the AgNPs group, this increase was insignificant. In addition, an increase in the number of primary, preantral, and antral follicles was observed in the TQ group compared to the control group (Table IV).
3.6. FRAP and lipid peroxidation
As shown in figure 3, the results of oxidative stress indices showed that the level of MDA increased in the AgNPs group. However, it was decreased in the co-administration group (TQ + AgNPs) compared to the AgNPs group. In addition, a decrease in MDA was observed in the TQ group compared to the control group. Also, the results of FRAP showed a decrease in TAC in the AgNPs group compared to the control group. In contrast, the level of TAC in the TQ + AgNPs group was increased compared to the AgNPs group.
3.7. The sexual hormones levels: LH and FSH
As shown in figure 3, compared to the control group, the levels of LH and FSH hormones were decreased in the AgNPs group. In parallel, in the TQ + AgNPs group, improvement of LH and FSH were observed compared to the AgNPs group. Also, TQ increased the concentration of LH and FSH compared to the control group.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Bala R, Singh V, Rajender S, Singh K. Environment, lifestyle, and female infertility. Reprod Sci 2021; 28: 617-638. [DOI:10.1007/s43032-020-00279-3] [PMID]
2. Liao Ch, Li Y, Tjong SC. Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci 2019; 20: 449. [DOI:10.3390/ijms20020449] [PMID] [PMCID]
3. Burdușel A-C, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018; 8: 681. [DOI:10.3390/nano8090681] [PMID] [PMCID]
4. Li Y, Cummins E. Hazard characterization of silver nanoparticles for human exposure routes. J Environment Sci Health Part A 2020; 55: 704-725. [DOI:10.1080/10934529.2020.1735852] [PMID]
5. Moghanlo H, Shariatzadeh SMA. Beneficial effects of Spirulina platensis on mice testis damaged by silver nanoparticles. Andrologia 2022; 54: e14606. [DOI:10.1111/and.14606] [PMID]
6. Zhang J, Wang F, Yalamarty SSK, Filipczak N, Jin Y, Li X. Nano silver-induced toxicity and associated mechanisms. Int J Nanomed 2022; 17: 1851-1864. [DOI:10.2147/IJN.S355131] [PMID] [PMCID]
7. Wang R, Song B, Wu J, Zhang Y, Chen A, Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomed 2018; 13: 8487-8506. [DOI:10.2147/IJN.S170723] [PMID] [PMCID]
8. Santacruz-Márquez R, Gonzalez-De los Santos M, Hernandez-Ochoa I. Ovarian toxicity of nanoparticles. Reprod Toxicol 2021; 103: 79-95. [DOI:10.1016/j.reprotox.2021.06.002] [PMID]
9. Zhang J, Liu S, Han J, Wang Z, Zhang S. On the developmental toxicity of silver nanoparticles. Mater Des 2021; 203: 109611. [DOI:10.1016/j.matdes.2021.109611]
10. Han JW, Jeong J-K, Gurunathan S, Choi Y-J, Das J, Kwon D-N, et al. Male-and female-derived somatic and germ cell-specific toxicity of silver nanoparticles in mouse. Nanotoxicology 2016; 10: 361-373. [DOI:10.3109/17435390.2015.1073396] [PMID]
11. Zhang D, Yu F, Li H, Wang Q, Wang M, Qian H, et al. AgNPs reduce reproductive capability of female mouse for their toxic effects on mouse early embryo development. Hum Exp Toxicol 2022; 41: 09603271221080235. [DOI:10.1177/09603271221080235] [PMID]
12. Mirzaei M, Razi M, Sadrkhanlou R. Nanosilver particles increase follicular atresia: Correlation with oxidative stress and aromatization. Environment Toxicol 2017; 32: 2244-2255. [DOI:10.1002/tox.22440] [PMID]
13. Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021; 13: 1784. [DOI:10.3390/nu13061784] [PMID] [PMCID]
14. Sadeghi E, Imenshahidi M, Hosseinzadeh H. Molecular mechanisms and signaling pathways of black cumin (Nigella sativa) and its active constituent, thymoquinone: A review. Mol Biol Rep 2023; 50: 5439-5454. [DOI:10.1007/s11033-023-08363-y] [PMID]
15. Sarkar C, Jamaddar S, Islam T, Mondal M, Islam MT, Mubarak MS. Therapeutic perspectives of the black cumin component thymoquinone: A review. Food Function 2021; 12: 6167-6213. [DOI:10.1039/D1FO00401H] [PMID]
16. Darakhshan S, Pour AB, Colagar AH, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res 2015; 95: 138-158. [DOI:10.1016/j.phrs.2015.03.011] [PMID]
17. Alaee S, Mirani M, Derakhshan Z, Koohpeyma F, Bakhtari A. Thymoquinone improves folliculogenesis, sexual hormones, gene expression of apoptotic markers and antioxidant enzymes in polycystic ovary syndrome rat model. Vet Med Sci 2023; 9: 290-300. [DOI:10.1002/vms3.958] [PMID] [PMCID]
18. Sanamiri K, Mehranjani MS, Shahhoseini M, Shariatzadeh MA. L-carnitine improves follicular survival and function in ovarian grafts in the mouse. Reprod Fertil Dev 2022; 34: 713-721. [DOI:10.1071/RD21287] [PMID]
19. Rezvani E, Rafferty A, McGuinness C, Kennedy J. Adverse effects of nanosilver on human health and the environment. Acta Biomater 2019; 94: 145-159. [DOI:10.1016/j.actbio.2019.05.042] [PMID]
20. Mohamed Y, El Ghareeb A-W, Attaby FA, Abd El-Rahman HA. Estimation of silver nanoparticles effect on the reproductive health of female Wistar rats. Egypt J Basic Appl Sci 2022; 9: 340-358. [DOI:10.1080/2314808X.2022.2092821]
21. Pourali P, Nouri M, Ameri F, Heidari T, Kheirkhahan N, Arabzadeh S, et al. Histopathological study of the maternal exposure to the biologically produced silver nanoparticles on different organs of the offspring. Naunyn Schmiedebergs Arch Pharmacol 2020; 393: 867-878. [DOI:10.1007/s00210-019-01796-y] [PMID]
22. Hou C-C, Zhu J-Q. Nanoparticles and female reproductive system: How do nanoparticles affect oogenesis and embryonic development. Oncotarget 2017; 8: 109799. [DOI:10.18632/oncotarget.19087] [PMID] [PMCID]
23. AL-ghamdi M, Huwait E, Elsawi N, Ali SS, Sayed A. Thymoquinone ameliorates acrylamide-induced reproductive toxicity in female rats: An experimental study. Int J Reprod BioMed 2023; 21: 61-70. [DOI:10.18502/ijrm.v21i1.12668] [PMID] [PMCID]
24. Saha P, Kumar S, Datta K, Tyagi RK. Upsurge in autophagy, associated with mifepristone-treated polycystic ovarian condition, is reversed upon thymoquinone treatment. J Steroid Biochem Mol Biol 2021; 208: 105823. [DOI:10.1016/j.jsbmb.2021.105823] [PMID]
25. Eini F, Bidadkosh A, Nazarian H, Piryaei A, Ghaffari Novin M, Joharchi K. Thymoquinone reduces intracytoplasmic oxidative stress and improves epigenetic modification in polycystic ovary syndrome mice oocytes, during in‐vitro maturation. Mol Reprod Dev 2019; 86: 1053-1066. [DOI:10.1002/mrd.23222] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |