Fri, Jan 30, 2026
[Archive]
Volume 22, Issue 12 (December 2024)
IJRM 2024, 22(12): 1003-1014 |
Back to browse issues page
Ethics code: IR.MUBABOL.REC.1401.050
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nazari Hagh Y, Ahmadifard M, Esmaelzadeh S, abbaszadeh S, Shokrzadeh N. Decreased expression of miRNA-200a and miRNA-223-3p in endometriosis cases during the secretory phase of the menstrual cycle: insights from a comprehensive case-control study with analysis of molecular biomarkers and their potential role in disease-induced infertility.. IJRM 2024; 22 (12) :1003-1014
URL: http://ijrm.ir/article-1-3306-en.html
URL: http://ijrm.ir/article-1-3306-en.html
Yasaman Nazari Hagh1 

 , Mohamadreza Ahmadifard2
, Mohamadreza Ahmadifard2 

 , Sedigheh Esmaelzadeh3
, Sedigheh Esmaelzadeh3 

 , Soheila Abbaszadeh3
, Soheila Abbaszadeh3 

 , Naser Shokrzadeh *4
, Naser Shokrzadeh *4 




 , Mohamadreza Ahmadifard2
, Mohamadreza Ahmadifard2 

 , Sedigheh Esmaelzadeh3
, Sedigheh Esmaelzadeh3 

 , Soheila Abbaszadeh3
, Soheila Abbaszadeh3 

 , Naser Shokrzadeh *4
, Naser Shokrzadeh *4 


1- School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran.
2- Department of Medical Genetics, School of Medicine, Babol University of Medical Sciences, Babol, Iran.
3- Infertility and Reproductive Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
4- Reproductive Health Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, Iran. ,shokrzadeh.n@umsu.ac.ir
2- Department of Medical Genetics, School of Medicine, Babol University of Medical Sciences, Babol, Iran.
3- Infertility and Reproductive Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
4- Reproductive Health Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, Iran. ,
Keywords: Endometriosis, miRNA, Implantation, Signaling pathways, Biomarkers, Infertility, Menstrual cycle.
Full-Text [PDF 5010 kb]
(567 Downloads)
| Abstract (HTML) (955 Views)
Full-Text: (173 Views)
1. Introduction
Endometriosis (EM) is a chronic estrogen-dependent inflammatory disorder that occurs when the endometrium develops beyond the uterine cavity, and it results in pain and infertility. This tissue may manifest on the surface of the ovary, beneath the uterus, inside the pelvic cavity, and on the intestinal wall (1-3). EM affects approximately 25-50% of infertile women, and about 30-50% of women with EM are infertile (4, 5). The disease disrupts progesterone and estrogen signaling pathways, resulting in progesterone resistance and estrogen dominance (6, 7). This hormonal imbalance induces inflammatory reaction and pelvic discomfort in the afflicted individual diminishes the endometrium's acceptability for embryo implantation and may jeopardize implantation via endometrial malfunction (8, 9). The embryo quality and receptivity of the endometrium are 2 critical components of implantation success (10, 11). Thus, the interplay between the expression levels of various molecules involved in endometrial receptivity and ovarian hormonal concentrations is crucial during embryo implantation (12).
For instance, it has recently been proven that microRNAs (miRNAs) play an essential role in embryo implantation and endometrial receptivity (13). The pierces communicating between the endometrium and blastocyst are essential for the implantation stage, and miRNAs are released from both the endometrium and blastocyst, and the gene's signaling undergoes a shift (14, 15). Therefore, it can be said that miRNAs play a key role in implantation, and considering the impacts of these small molecules in the pathophysiology of EM, there is a strong correlation between EM-related infertility and miRNAs (16, 17).
Hence, it is crucial to investigate these miRNAs in individuals, whose implantation process is disrupted, including EM group. By adjusting inflammation, proliferation, and angiogenesis will enable the implantation of endometrial cells in benign areas, where they play a role in the etiology and development of EM. Thus, it is possible to evaluate certain miRNAs as noninvasive indicators in the molecular diagnosis of this disease (18, 19).
Because there have been numerous studies conducted on genes associated with EM. This study investigates the level of expression of miR-223-3p and miR-200a in the endometrial tissue in the secretory phase of the menstrual cycle in women with EM, compared with healthy women. However, there is a lack of studies focusing on molecular markers during the specific phase of the uterine cycle in relation to EM and these markers were first detected in EM and the implantation stage.
2. Materials and Methods
2.1. Collection of samples and grouping
This case-control study was conducted with 36 women referred to the Center for Research on Reproductive Health and Infertility of Babol University of Medical Sciences and Fatemeh Al-Zahra Infertility Specialized Treatment Center in Babol, Iran between June 2022 and July 2023.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows, women aged below 38 yr, had a regular menstrual cycle (between 24 and 35 days), clarity about the last menstrual period, and willing to participate. Women with autoimmune diseases, endometrial hyperplasia, polyps, polycystic ovary syndrome, uterine anomalies, chronic anovulation, bilateral fallopian tube obstruction, a history of fibroids, and unexplained infertility were excluded.
They were divided into 2 groups (n = 18/each), women with (case) and without EM (control). Women in the EM group were diagnosed by surgery, laparoscopy, or laparotomy and histological sample or ultrasound (Esaote mylab40, Esaote, Italy) provided by the consultant during the follicular period of the menstrual cycle. Almost all women with EM were in stages 3 and 4, and none of the individuals used hormonal medications for at least 3 months before the study.
Endometrial samples were obtained using a pipelle between the 17th and 24th days of the cycle, related to the mid-secretory phase. After washing, the samples were separated into 2 separate groups. The formalin-preserved piece was examined histologically to confirm the endometrium secretory phase. The remaining samples were placed in Trizol (Sigma Aldrich, USA) and stored at -80°C.
2.3. Sample size
Based on similar studies and using the above formula, with a 95% confidence interval and 80% test power, the required sample size was calculated to be 18. Consequently, 18 women with EM (case) and 18 women without EM (control) were included in the study.
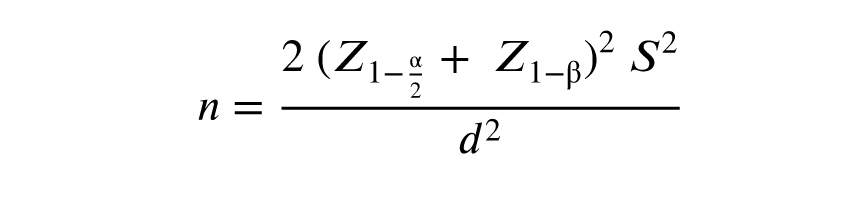
2.4. RNA extraction
Total RNA was extracted from 30 mg of endometrial tissue using Trizol (Sigma-Aldrich, USA). The tissue was homogenized with Trizol, followed by the addition of 300 µl of chloroform and centrifugation at 12,000 rpm for 10 min at 4°C. The supernatant was collected, washed with isopropanol, and centrifuged. Subsequently, 500 µl of sterile 75% ethanol was added to the pellet, which was stirred for 5 min and then centrifuged twice at 7500 rpm at 4°C. The RNA pellet was dissolved in 50 µl of nuclease-free H2O and heated at 65°C for 3 min. RNA concentration was quantified using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, USA), with an OD260 of 1.8-2.0 indicating high purity. The isolated RNA samples were stored at -80°C for future analysis.
2.5. cDNA synthesis and real-time polymerase chain reaction (RT-PCR)
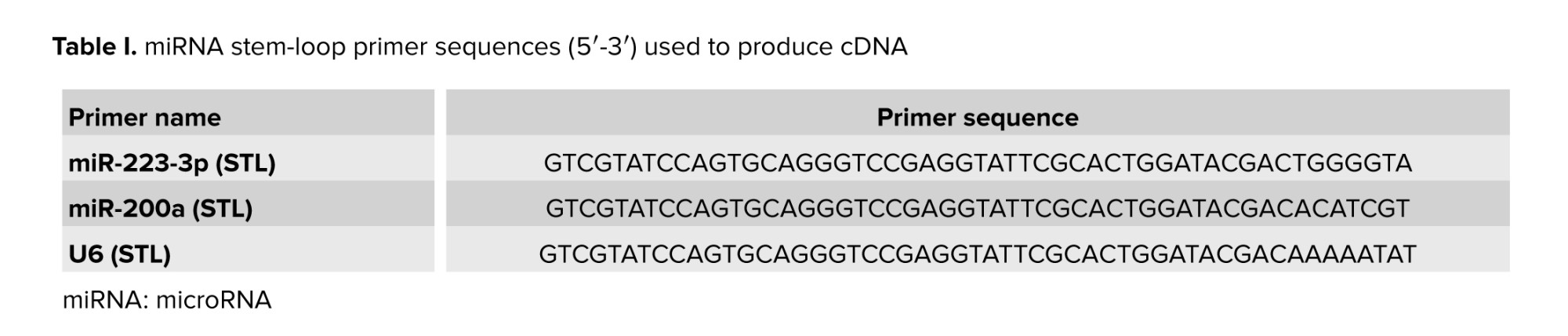
cDNA was synthesized from total RNA using a Biofact kit (Cat No.BR631_O96, Biofact, Korea) and stem-loop primers as per the manufacturer's protocol. The reaction mixture included 2xRTpermix, RNase-free dH2O, and miRNA-specific stem-loop primers. Reverse transcription was conducted at 37°C for 5 min, followed by 50°C for 30 min and 95°C for 5 min. The synthesized cDNA was stored at -20°C for subsequent PCR use, as detailed in table I.
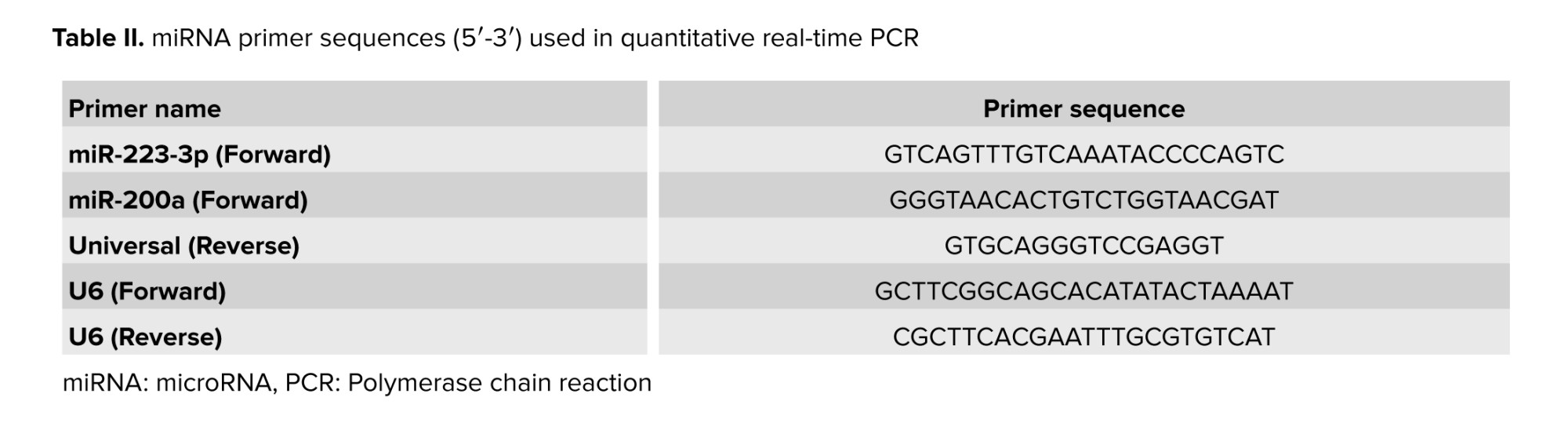
Real-time PCR was performed using the ABI StepOnePlus system (ABI Co., USA) with SYBR Green PCR Master Mix (Cat No. DQ383_10h, Biofact, Korea), specific forward primers (Table II), and U6 gene as an internal control. All reactions were executed in duplicate. 2 sets of primers were employed: one for cDNA synthesis and another for quantitative real-time PCR based on the stem-loop method (20). The primer design involved creating specific forward primers for each miRNA, such as miR-200a and miR-223-3p, and a universal reverse primer applicable to all miRNAs except U6. Key design considerations for the universal primer included minimizing binding site overlaps with the genomes of GM crops, ensuring high GC content, and aiming for a melting temperature (Tm) around 60°C. Specific primers used for gene amplification were derived from corresponding sequences, ensuring specificity in Universal Primer Multiplex Real-Time PCR.
2.6. Histological procedures
The samples of uterine endometrium were used to produce slides for histological examinations. The endometrial tissues were fixed in 10% formalin. The samples were embedded in paraffin then using a microtome, tissue templates were sliced into 5-µ-thick sections. Next, the pieces were then put on slides and dried. Finally, the tissues were stained with hematoxylin and eosin (H&E) and periodic acid schiff (PAS) staining according to standard histological techniques.
2.7. Ethical Considerations
This research was approved by the Ethical Committee of Babol University of Medical Sciences, Babol, Iran (Code: IR.MUBABOL.REC.1401.050). Prior to participation, all participants completed a consent form.
2.8. Statistical Analysis
For quantitative variables, t tests (U-Mann-Whitney) and the Chi-square test were used to analyze the data. In all tests, the confidence level was 95% and the level of significance was < 5% (p < 0.05); the software equipment used for data analysis was SPSS 25 (IBM, International Business Machines Corp., New Orchard Road Armonk, New York). The demographic features of the groups were shown using mean ± SD. The Kolmogorov-Smirnov test assessed the normality distribution of quantitative data. Using a one-way ANOVA followed by post hoc tests for multiple comparisons, mean difference between groups were evaluated.
3. Results
3.1. Demographic data
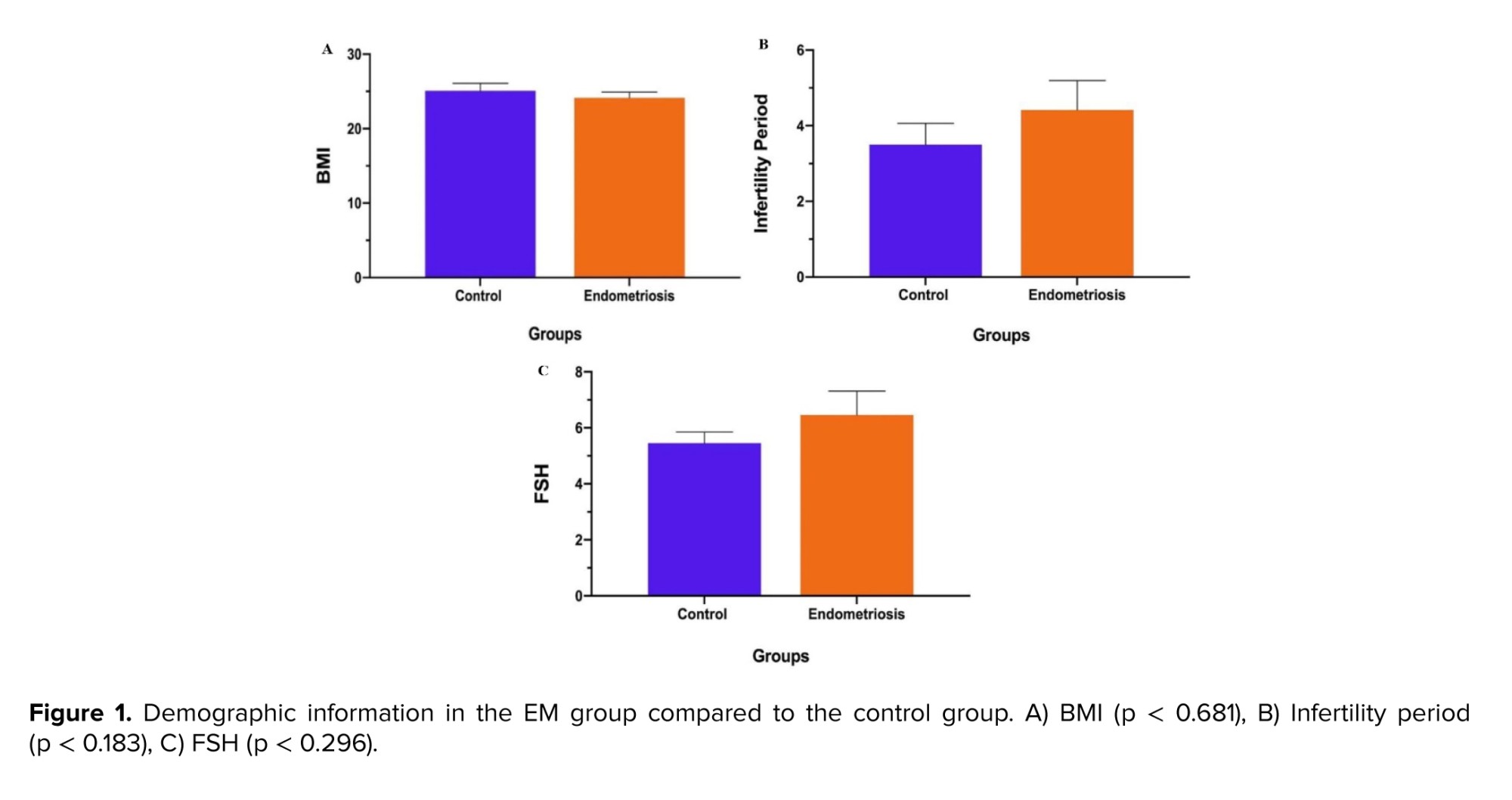
The evaluation of demographic data revealed that the body mass index (BMI) in EM group declined slightly, compared to the control group (p = 0.681) (Figure 1).
In contrast, the infertility period and follicle-stimulating hormone (FSH) levels in the EM group enhanced highly, compared to the control group (p = 0.183, 0.296, respectively); however, the difference of variables in both groups was not statistically significant (Table III) (Figure 1).
3.2. miR-200a and miR-223-3p expressions
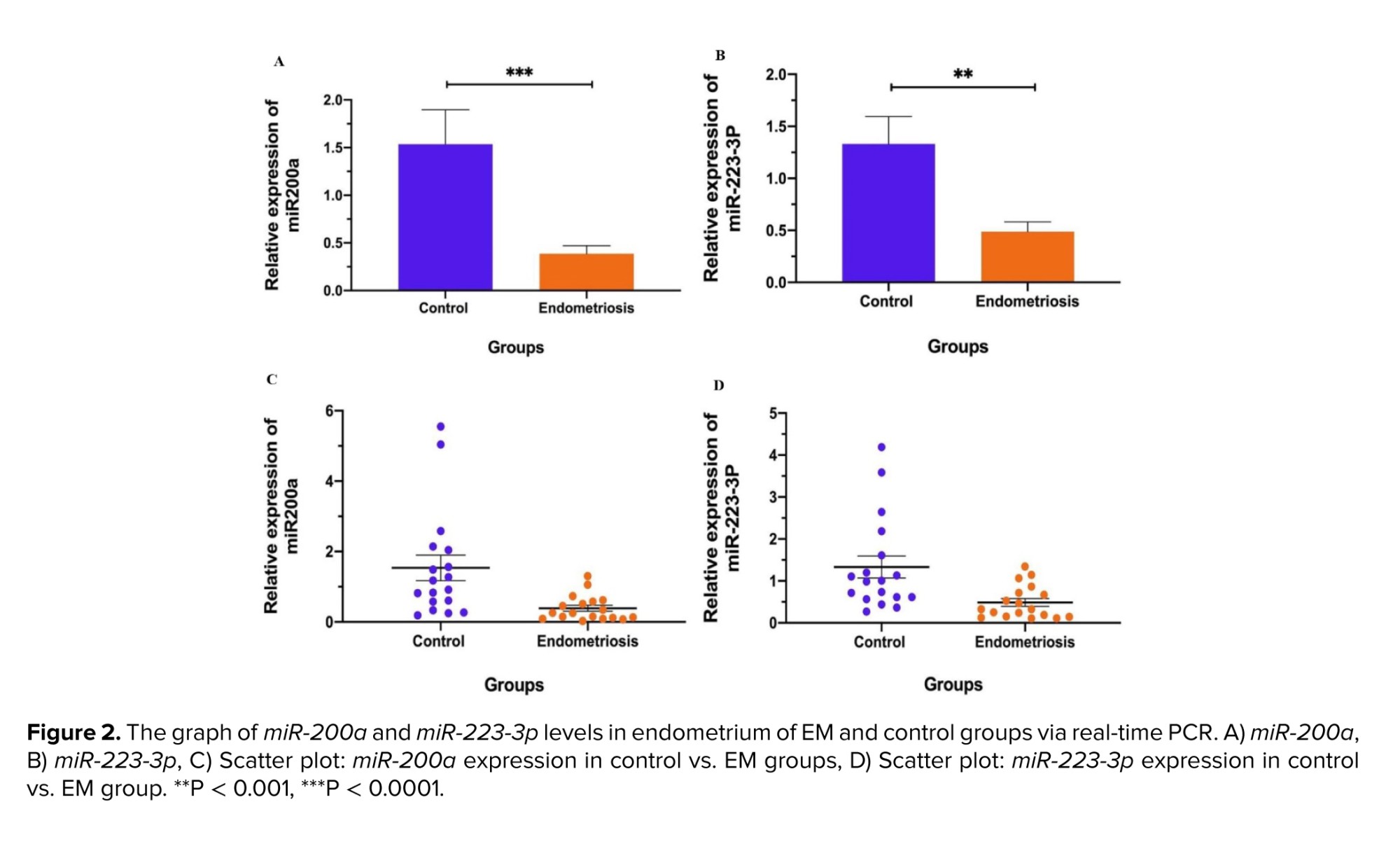
According to the statistical data, the expression level of miR-200a (mean ± SD) in the secretory period of the menstrual cycle in the eutopic endometrium of the EM group was significantly reduced than the control group (p < 0.01). Likewise, the expression of miR-223-3p was decreased dramatically in the EM group in comparison to the control group (p < 0.01) (Figure 2).
3.3. Histological analysis in the endometrium during the secretory phase
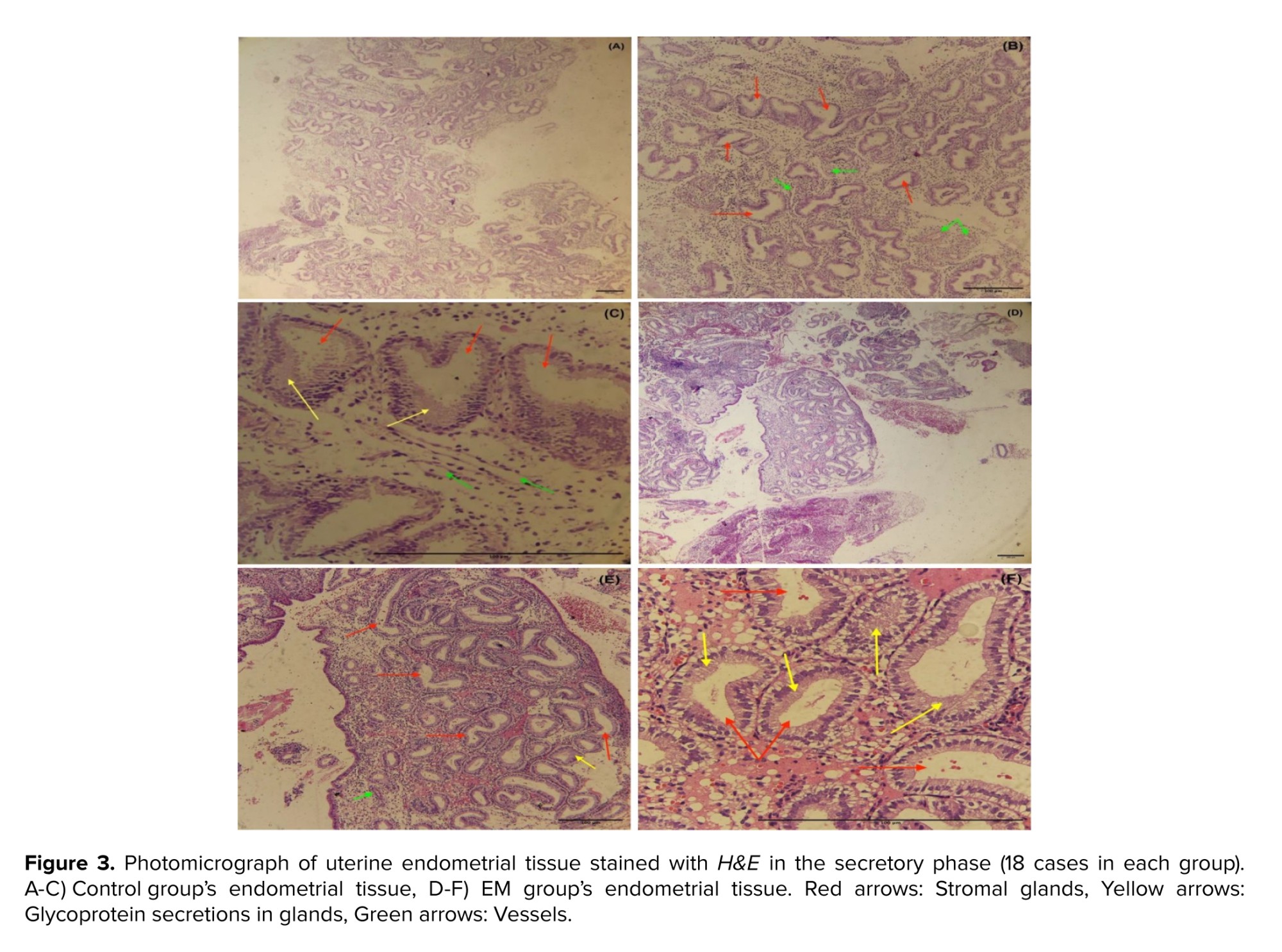
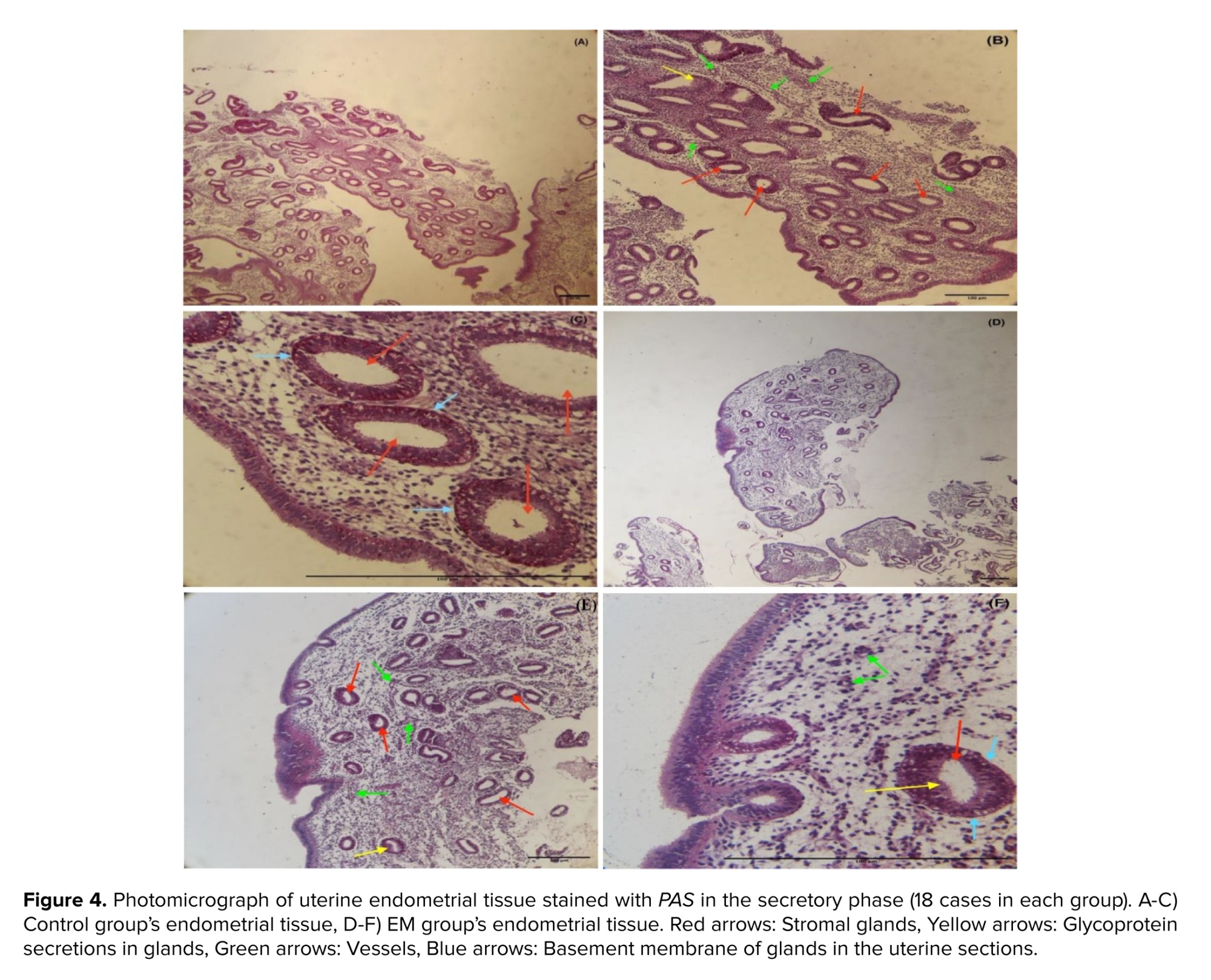
For the histological investigation, microscopic pictures of the endometrium in the secretory phase of the uterus were captured at x40 magnification. The results of the histological examination in the control and EM group showed that the secretory phase in the menstrual cycle causes changes in the endometrial tissue of the uterus. This includes the stromal glands with glycoprotein secretions, dilated and characterized by cuboidal epithelium. The basement membrane was seen beneath the epithelial cells with low thickness and the number of blood vessels was markedly increased in stroma, verified in the secretory phase of the menstruation (Figures 3, 4).
4. Discussion
EM is a complex condition with various factors contributing to its development. Researchers are exploring potential biomarkers, such as miRNAs, for early detection. miRNAs play a significant role in the implantation process and may impact infertility associated with EM. Additionally, during implantation, miRNAs are released into the bloodstream from the blastocyst and the endometrium. They act on signaling to adjust gene expression. Considering these small molecules' impact on EM's pathogenesis, we can seek an active collaboration between infertility caused by EM and miRNAs. As implantation occurs during the secretory phase of the menstrual cycle, the current study's specimens were likewise collected during this period, and histological methods were used to corroborate the sampling time. The results of histological examination during the secretory phase of the menstrual cycle confirmed sampling time alignment in the study.
The findings of analyzing the expression level of miR-200a in the current research indicated that this miRNA had a significant drop in expression in the eutopic endometrial tissue of women with EM compared to the control group (p < 0.000). Research published on the endometrial tissue of EM group also corroborates the reduction of miR-200a expression. A survey investigated the expression level of miR-200a in EM group -who were in the proliferative and secretory phase of the menstrual cycle and had not received any treatment before sampling- showed decreased expression in ectopic endometrial tissue compared to the eutopic endometrial in these cases (21). Another study investigated the expression levels of miR-200a in ectopic and eutopic endometrial of 16 cases with EM. It showed that miR-200a had a downregulation in the ectopic endometrial of these cases compared to their eutopic. The women who were in stages 3 and 4 of EM, aged between 24 and 48 yr, and did not receive any hormone treatment 3 months before sampling (22). According to research on the expression profiles of the miR-200 family throughout the implantation process in mice, the expression level of miR-200a was diminished during the preimplantation stage. The miR-200a were found to be elevated during decidualization, impacting its target Zeb1 gene, and Zeb2 facilitating implantation success (23). Research into the mechanism of miRNAs' action on the target tissue revealed that by targeting the three prime untranslated region of the targeted gene, miRNAs enhance and/or inhibit the expression of such a gene in the organ, consequently influencing the disease process (24). Several potential genes, including Zeb1 and Zeb2 (zinc finger E-box binding homeobox 1, 2), were anticipated to have high scores based on target scan searches for miR-200a (25). miR-200a and its molecular targets, Zeb1 and Zeb2, are regarded as epithelial-mesenchymal transition regulators (EMT) (26, 27). The epithelial-to-mesenchymal transition is a procedure in which epithelial cells lose their polarity and adhesion and obtain invasive and migratory capabilities. Also, they may convert into mesenchymal stem cells, which are capable of transforming into many cell types, in such a manner that they contribute to the beginning and metastasis of cancer development (28). In the substantial percentage of disorders, the expression levels of miR-200a and its target genes, especially Zeb1 and Zeb2, had declined, consequently raising EMT activity. In this context, the link between miR-200a and its target genes (Zeb1 and Zeb2) in rats with peritoneal fibrosis related to peritoneal dialysis was investigated. miR-200a exhibited a drop in expression in these rats, followed by an increase in Zeb1 and Zeb2 expression (29). Furthermore, it was shown in a study that increased miR-200a expression leads to implantation failure in mice. This investigation was done on female mice aged 6 to 8 wk. Following fertilization, endometrial samples were analyzed. This study discovered that the level of mmu-miR-200a expression has decreased at implantation sites. They demonstrated that an increase in mmu-miR-200a expression causes implantation failure and a decline in fertility (30). Therefore, the results of the past research compared with results obtained from the present study, it can be concluded that the lowering in the expression of miR-200a probably acts by influencing its target genes (Zeb1 and Zeb2). Hence, the upsurge in EMT activity leads to the migration and proliferation of epithelial cells. Since it is essential to suppress the expression of this miRNA for successful implantation, it may be deduced that additional genes and signaling pathways are likely implicated in the failure of implantation in these individuals.
Based on our findings, the level of miR-223-3p expression in eutopic endometrial tissue of EM group was considerably lower than in the control group (p < 0.0001). Our study was in line with the findings of research that investigated the degree of miR-233 expression on stromal cells of ectopic and eutopic endometrial in women with EM. The examined women were aged between 23 and 55 yr, had normal menstrual cycles, and had not been given hormones for a few months before sampling. This study demonstrated a reduction in the expression of miR-223 in the endometrial stromal cells of individuals with EM; the researchers indicated that the upregulation of miR-223 hindered the production of EMT-related molecules, therefore reducing cell migration and proliferation (31). Furthermore, in a study on the implantation process in female mice, it was shown that increasing the expression of miR-223-3p affects the leukemia inhibitory factor receptor signaling pathway. Suppressing LIF signaling causes a decrease in fertility; therefore, it can be concluded that the increase in the expression of miR-223-3p negatively affects the fertility process. This miRNA is connected to the three prime untranslated region of the LIF gene and prevents the expression of this gene (32). Moreover, in research on uterine receptivity in animal cases, it was shown that calcitonin can promote uterine receptivity by raising the expression of LIF and lowering the expression of miR223-3p via the ERK1/2-mTOR signaling pathway (33). Dexamethasone lowered uterine receptivity during the implantation phase by boosting the expression of miR223-3p and diminishing the expression of LIF in the endometrial sample of mice (34). Moreover investigating the relationship between electroacupuncture and the expression level of miR223-3p on the rate of uterine receptivity in rats, showed that the treatment with electroacupuncture in the uterus of rats by reducing the expression level of miR223-3p caused a slight increase in the implantation of blastocytes, resulting in, enhanced uterine receptivity and the occurrence of implantation procedure (35). According to the available evidence, miR-223 suppresses the metastasis of cervical cancer cells, preventing the process of EMT by augmenting E-cadherin and α-cadherin and reducing the mesenchymal marker vimentin. This shows that miR-223 might become a novel EM-targeting approach. miR-223 suppresses the metastasis of human cervical cancer cells by altering EMT (36). The difference in miR-223-3p expression level between our study and prior research may be attributed to a variety of factors, such as variations in the cases and control population, climate, race, sampling time, the stage of EM, and the type of sample.
According to the findings of this study, the expression level of miR-223-3p and miR-200a in the eutopic endometrial tissue of women with EM was significantly lower than in the control group (p < 0.0001).
5. Conclusion
Compared to the control group, women with EM exhibited significantly reduced expression levels of miR-223-3p and miR-200a in the eutopic endometrial tissue. Our findings suggest that these miRNAs, especially miR-200a and miR-223-3p, are implicated in the disease's pathogenesis, while it is probable that other genes and signaling pathways contribute to the implantation failure associated with this condition. Consequently, by conducting additional research, these miRNAs may be utilized as diagnostic biomarkers for EM.
Data Availability
The data supporting the findings of this study are available upon reasonable request from the corresponding author, Naser Shokrzadeh, as they contain information that could compromise the privacy of research participants.
Author Contributions
N. Shokrzadeh served as the executive for the experiments and acted as the primary supervisor and study designer. M. Ahmadifard fulfilled the role of second supervisor. Y. Nazari Hagh conducted the experiments and performed the analysis of the study results. S. Abbaszadeh and S. Esmaelzadeh were responsible for examining cases and collecting samples. All authors have approved the final manuscript and assume responsibility for the integrity of the data.
Acknowledgments
We are grateful to all the women who participated in the present study. This research was funded by the vice chancellor for research and technology of Babol University of Medical Sciences and Health Services, Babol, Iran (grant number: 140114324). Also, we have not used artificial intelligence in any way (translation, revision, grammar check, etc.).
Conflict of Interest
The authors declare that there is no conflict of interest.
Endometriosis (EM) is a chronic estrogen-dependent inflammatory disorder that occurs when the endometrium develops beyond the uterine cavity, and it results in pain and infertility. This tissue may manifest on the surface of the ovary, beneath the uterus, inside the pelvic cavity, and on the intestinal wall (1-3). EM affects approximately 25-50% of infertile women, and about 30-50% of women with EM are infertile (4, 5). The disease disrupts progesterone and estrogen signaling pathways, resulting in progesterone resistance and estrogen dominance (6, 7). This hormonal imbalance induces inflammatory reaction and pelvic discomfort in the afflicted individual diminishes the endometrium's acceptability for embryo implantation and may jeopardize implantation via endometrial malfunction (8, 9). The embryo quality and receptivity of the endometrium are 2 critical components of implantation success (10, 11). Thus, the interplay between the expression levels of various molecules involved in endometrial receptivity and ovarian hormonal concentrations is crucial during embryo implantation (12).
For instance, it has recently been proven that microRNAs (miRNAs) play an essential role in embryo implantation and endometrial receptivity (13). The pierces communicating between the endometrium and blastocyst are essential for the implantation stage, and miRNAs are released from both the endometrium and blastocyst, and the gene's signaling undergoes a shift (14, 15). Therefore, it can be said that miRNAs play a key role in implantation, and considering the impacts of these small molecules in the pathophysiology of EM, there is a strong correlation between EM-related infertility and miRNAs (16, 17).
Hence, it is crucial to investigate these miRNAs in individuals, whose implantation process is disrupted, including EM group. By adjusting inflammation, proliferation, and angiogenesis will enable the implantation of endometrial cells in benign areas, where they play a role in the etiology and development of EM. Thus, it is possible to evaluate certain miRNAs as noninvasive indicators in the molecular diagnosis of this disease (18, 19).
Because there have been numerous studies conducted on genes associated with EM. This study investigates the level of expression of miR-223-3p and miR-200a in the endometrial tissue in the secretory phase of the menstrual cycle in women with EM, compared with healthy women. However, there is a lack of studies focusing on molecular markers during the specific phase of the uterine cycle in relation to EM and these markers were first detected in EM and the implantation stage.
2. Materials and Methods
2.1. Collection of samples and grouping
This case-control study was conducted with 36 women referred to the Center for Research on Reproductive Health and Infertility of Babol University of Medical Sciences and Fatemeh Al-Zahra Infertility Specialized Treatment Center in Babol, Iran between June 2022 and July 2023.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows, women aged below 38 yr, had a regular menstrual cycle (between 24 and 35 days), clarity about the last menstrual period, and willing to participate. Women with autoimmune diseases, endometrial hyperplasia, polyps, polycystic ovary syndrome, uterine anomalies, chronic anovulation, bilateral fallopian tube obstruction, a history of fibroids, and unexplained infertility were excluded.
They were divided into 2 groups (n = 18/each), women with (case) and without EM (control). Women in the EM group were diagnosed by surgery, laparoscopy, or laparotomy and histological sample or ultrasound (Esaote mylab40, Esaote, Italy) provided by the consultant during the follicular period of the menstrual cycle. Almost all women with EM were in stages 3 and 4, and none of the individuals used hormonal medications for at least 3 months before the study.
Endometrial samples were obtained using a pipelle between the 17th and 24th days of the cycle, related to the mid-secretory phase. After washing, the samples were separated into 2 separate groups. The formalin-preserved piece was examined histologically to confirm the endometrium secretory phase. The remaining samples were placed in Trizol (Sigma Aldrich, USA) and stored at -80°C.
2.3. Sample size
Based on similar studies and using the above formula, with a 95% confidence interval and 80% test power, the required sample size was calculated to be 18. Consequently, 18 women with EM (case) and 18 women without EM (control) were included in the study.
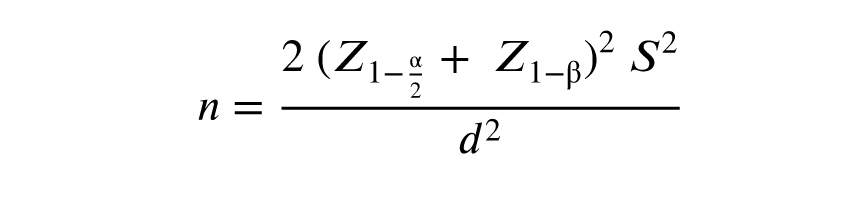
2.4. RNA extraction
Total RNA was extracted from 30 mg of endometrial tissue using Trizol (Sigma-Aldrich, USA). The tissue was homogenized with Trizol, followed by the addition of 300 µl of chloroform and centrifugation at 12,000 rpm for 10 min at 4°C. The supernatant was collected, washed with isopropanol, and centrifuged. Subsequently, 500 µl of sterile 75% ethanol was added to the pellet, which was stirred for 5 min and then centrifuged twice at 7500 rpm at 4°C. The RNA pellet was dissolved in 50 µl of nuclease-free H2O and heated at 65°C for 3 min. RNA concentration was quantified using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, USA), with an OD260 of 1.8-2.0 indicating high purity. The isolated RNA samples were stored at -80°C for future analysis.
2.5. cDNA synthesis and real-time polymerase chain reaction (RT-PCR)
cDNA was synthesized from total RNA using a Biofact kit (Cat No.BR631_O96, Biofact, Korea) and stem-loop primers as per the manufacturer's protocol. The reaction mixture included 2xRTpermix, RNase-free dH2O, and miRNA-specific stem-loop primers. Reverse transcription was conducted at 37°C for 5 min, followed by 50°C for 30 min and 95°C for 5 min. The synthesized cDNA was stored at -20°C for subsequent PCR use, as detailed in table I.
Real-time PCR was performed using the ABI StepOnePlus system (ABI Co., USA) with SYBR Green PCR Master Mix (Cat No. DQ383_10h, Biofact, Korea), specific forward primers (Table II), and U6 gene as an internal control. All reactions were executed in duplicate. 2 sets of primers were employed: one for cDNA synthesis and another for quantitative real-time PCR based on the stem-loop method (20). The primer design involved creating specific forward primers for each miRNA, such as miR-200a and miR-223-3p, and a universal reverse primer applicable to all miRNAs except U6. Key design considerations for the universal primer included minimizing binding site overlaps with the genomes of GM crops, ensuring high GC content, and aiming for a melting temperature (Tm) around 60°C. Specific primers used for gene amplification were derived from corresponding sequences, ensuring specificity in Universal Primer Multiplex Real-Time PCR.
2.6. Histological procedures
The samples of uterine endometrium were used to produce slides for histological examinations. The endometrial tissues were fixed in 10% formalin. The samples were embedded in paraffin then using a microtome, tissue templates were sliced into 5-µ-thick sections. Next, the pieces were then put on slides and dried. Finally, the tissues were stained with hematoxylin and eosin (H&E) and periodic acid schiff (PAS) staining according to standard histological techniques.


2.7. Ethical Considerations
This research was approved by the Ethical Committee of Babol University of Medical Sciences, Babol, Iran (Code: IR.MUBABOL.REC.1401.050). Prior to participation, all participants completed a consent form.
2.8. Statistical Analysis
For quantitative variables, t tests (U-Mann-Whitney) and the Chi-square test were used to analyze the data. In all tests, the confidence level was 95% and the level of significance was < 5% (p < 0.05); the software equipment used for data analysis was SPSS 25 (IBM, International Business Machines Corp., New Orchard Road Armonk, New York). The demographic features of the groups were shown using mean ± SD. The Kolmogorov-Smirnov test assessed the normality distribution of quantitative data. Using a one-way ANOVA followed by post hoc tests for multiple comparisons, mean difference between groups were evaluated.
3. Results
3.1. Demographic data
The evaluation of demographic data revealed that the body mass index (BMI) in EM group declined slightly, compared to the control group (p = 0.681) (Figure 1).
In contrast, the infertility period and follicle-stimulating hormone (FSH) levels in the EM group enhanced highly, compared to the control group (p = 0.183, 0.296, respectively); however, the difference of variables in both groups was not statistically significant (Table III) (Figure 1).
3.2. miR-200a and miR-223-3p expressions
According to the statistical data, the expression level of miR-200a (mean ± SD) in the secretory period of the menstrual cycle in the eutopic endometrium of the EM group was significantly reduced than the control group (p < 0.01). Likewise, the expression of miR-223-3p was decreased dramatically in the EM group in comparison to the control group (p < 0.01) (Figure 2).
3.3. Histological analysis in the endometrium during the secretory phase
For the histological investigation, microscopic pictures of the endometrium in the secretory phase of the uterus were captured at x40 magnification. The results of the histological examination in the control and EM group showed that the secretory phase in the menstrual cycle causes changes in the endometrial tissue of the uterus. This includes the stromal glands with glycoprotein secretions, dilated and characterized by cuboidal epithelium. The basement membrane was seen beneath the epithelial cells with low thickness and the number of blood vessels was markedly increased in stroma, verified in the secretory phase of the menstruation (Figures 3, 4).





4. Discussion
EM is a complex condition with various factors contributing to its development. Researchers are exploring potential biomarkers, such as miRNAs, for early detection. miRNAs play a significant role in the implantation process and may impact infertility associated with EM. Additionally, during implantation, miRNAs are released into the bloodstream from the blastocyst and the endometrium. They act on signaling to adjust gene expression. Considering these small molecules' impact on EM's pathogenesis, we can seek an active collaboration between infertility caused by EM and miRNAs. As implantation occurs during the secretory phase of the menstrual cycle, the current study's specimens were likewise collected during this period, and histological methods were used to corroborate the sampling time. The results of histological examination during the secretory phase of the menstrual cycle confirmed sampling time alignment in the study.
The findings of analyzing the expression level of miR-200a in the current research indicated that this miRNA had a significant drop in expression in the eutopic endometrial tissue of women with EM compared to the control group (p < 0.000). Research published on the endometrial tissue of EM group also corroborates the reduction of miR-200a expression. A survey investigated the expression level of miR-200a in EM group -who were in the proliferative and secretory phase of the menstrual cycle and had not received any treatment before sampling- showed decreased expression in ectopic endometrial tissue compared to the eutopic endometrial in these cases (21). Another study investigated the expression levels of miR-200a in ectopic and eutopic endometrial of 16 cases with EM. It showed that miR-200a had a downregulation in the ectopic endometrial of these cases compared to their eutopic. The women who were in stages 3 and 4 of EM, aged between 24 and 48 yr, and did not receive any hormone treatment 3 months before sampling (22). According to research on the expression profiles of the miR-200 family throughout the implantation process in mice, the expression level of miR-200a was diminished during the preimplantation stage. The miR-200a were found to be elevated during decidualization, impacting its target Zeb1 gene, and Zeb2 facilitating implantation success (23). Research into the mechanism of miRNAs' action on the target tissue revealed that by targeting the three prime untranslated region of the targeted gene, miRNAs enhance and/or inhibit the expression of such a gene in the organ, consequently influencing the disease process (24). Several potential genes, including Zeb1 and Zeb2 (zinc finger E-box binding homeobox 1, 2), were anticipated to have high scores based on target scan searches for miR-200a (25). miR-200a and its molecular targets, Zeb1 and Zeb2, are regarded as epithelial-mesenchymal transition regulators (EMT) (26, 27). The epithelial-to-mesenchymal transition is a procedure in which epithelial cells lose their polarity and adhesion and obtain invasive and migratory capabilities. Also, they may convert into mesenchymal stem cells, which are capable of transforming into many cell types, in such a manner that they contribute to the beginning and metastasis of cancer development (28). In the substantial percentage of disorders, the expression levels of miR-200a and its target genes, especially Zeb1 and Zeb2, had declined, consequently raising EMT activity. In this context, the link between miR-200a and its target genes (Zeb1 and Zeb2) in rats with peritoneal fibrosis related to peritoneal dialysis was investigated. miR-200a exhibited a drop in expression in these rats, followed by an increase in Zeb1 and Zeb2 expression (29). Furthermore, it was shown in a study that increased miR-200a expression leads to implantation failure in mice. This investigation was done on female mice aged 6 to 8 wk. Following fertilization, endometrial samples were analyzed. This study discovered that the level of mmu-miR-200a expression has decreased at implantation sites. They demonstrated that an increase in mmu-miR-200a expression causes implantation failure and a decline in fertility (30). Therefore, the results of the past research compared with results obtained from the present study, it can be concluded that the lowering in the expression of miR-200a probably acts by influencing its target genes (Zeb1 and Zeb2). Hence, the upsurge in EMT activity leads to the migration and proliferation of epithelial cells. Since it is essential to suppress the expression of this miRNA for successful implantation, it may be deduced that additional genes and signaling pathways are likely implicated in the failure of implantation in these individuals.
Based on our findings, the level of miR-223-3p expression in eutopic endometrial tissue of EM group was considerably lower than in the control group (p < 0.0001). Our study was in line with the findings of research that investigated the degree of miR-233 expression on stromal cells of ectopic and eutopic endometrial in women with EM. The examined women were aged between 23 and 55 yr, had normal menstrual cycles, and had not been given hormones for a few months before sampling. This study demonstrated a reduction in the expression of miR-223 in the endometrial stromal cells of individuals with EM; the researchers indicated that the upregulation of miR-223 hindered the production of EMT-related molecules, therefore reducing cell migration and proliferation (31). Furthermore, in a study on the implantation process in female mice, it was shown that increasing the expression of miR-223-3p affects the leukemia inhibitory factor receptor signaling pathway. Suppressing LIF signaling causes a decrease in fertility; therefore, it can be concluded that the increase in the expression of miR-223-3p negatively affects the fertility process. This miRNA is connected to the three prime untranslated region of the LIF gene and prevents the expression of this gene (32). Moreover, in research on uterine receptivity in animal cases, it was shown that calcitonin can promote uterine receptivity by raising the expression of LIF and lowering the expression of miR223-3p via the ERK1/2-mTOR signaling pathway (33). Dexamethasone lowered uterine receptivity during the implantation phase by boosting the expression of miR223-3p and diminishing the expression of LIF in the endometrial sample of mice (34). Moreover investigating the relationship between electroacupuncture and the expression level of miR223-3p on the rate of uterine receptivity in rats, showed that the treatment with electroacupuncture in the uterus of rats by reducing the expression level of miR223-3p caused a slight increase in the implantation of blastocytes, resulting in, enhanced uterine receptivity and the occurrence of implantation procedure (35). According to the available evidence, miR-223 suppresses the metastasis of cervical cancer cells, preventing the process of EMT by augmenting E-cadherin and α-cadherin and reducing the mesenchymal marker vimentin. This shows that miR-223 might become a novel EM-targeting approach. miR-223 suppresses the metastasis of human cervical cancer cells by altering EMT (36). The difference in miR-223-3p expression level between our study and prior research may be attributed to a variety of factors, such as variations in the cases and control population, climate, race, sampling time, the stage of EM, and the type of sample.
According to the findings of this study, the expression level of miR-223-3p and miR-200a in the eutopic endometrial tissue of women with EM was significantly lower than in the control group (p < 0.0001).
5. Conclusion
Compared to the control group, women with EM exhibited significantly reduced expression levels of miR-223-3p and miR-200a in the eutopic endometrial tissue. Our findings suggest that these miRNAs, especially miR-200a and miR-223-3p, are implicated in the disease's pathogenesis, while it is probable that other genes and signaling pathways contribute to the implantation failure associated with this condition. Consequently, by conducting additional research, these miRNAs may be utilized as diagnostic biomarkers for EM.
Data Availability
The data supporting the findings of this study are available upon reasonable request from the corresponding author, Naser Shokrzadeh, as they contain information that could compromise the privacy of research participants.
Author Contributions
N. Shokrzadeh served as the executive for the experiments and acted as the primary supervisor and study designer. M. Ahmadifard fulfilled the role of second supervisor. Y. Nazari Hagh conducted the experiments and performed the analysis of the study results. S. Abbaszadeh and S. Esmaelzadeh were responsible for examining cases and collecting samples. All authors have approved the final manuscript and assume responsibility for the integrity of the data.
Acknowledgments
We are grateful to all the women who participated in the present study. This research was funded by the vice chancellor for research and technology of Babol University of Medical Sciences and Health Services, Babol, Iran (grant number: 140114324). Also, we have not used artificial intelligence in any way (translation, revision, grammar check, etc.).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Cellular and Molecular Biology of Reproduction
References
1. Cousins FL, McKinnon BD, Mortlock S, Fitzgerald HC, Zhang C, Montgomery GW, et al. New concepts on the etiology of endometriosis. J Obstet Gynaecol Res 2023; 49: 1090-1105. [DOI:10.1111/jog.15549] [PMID] [PMCID]
2. Marquardt RM, Tran DN, Lessey BA, Rahman MS, Jeong J-W. Epigenetic dysregulation in endometriosis: Implications for pathophysiology and therapeutics. Endoc Rev 2023; 44: 1074-1095. [DOI:10.1210/endrev/bnad020] [PMID] [PMCID]
3. Balalau DO, Ciupitu IA, Bogheanu D-M, Ghiocel-Zariosu A-I, Balalau C, Ples L, et al. Management of pelvic pain caused by endometriosis. J Mind Med Sci 2023; 10: 79-84. [DOI:10.22543/2392-7674.1390]
4. Da Broi MG, Ferriani RA, Navarro PA. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist Reprod 2019; 23: 273-280. [DOI:10.5935/1518-0557.20190029] [PMID] [PMCID]
5. Lee D, Kim SK, Lee JR, Jee BC. Management of endometriosis-related infertility: Considerations and treatment options. Clin Exp Reprod Med 2020; 47: 1-11.
https://doi.org/10.5653/cerm.2020.47.1.1.e1 [DOI:10.5653/cerm.2019.02971]
6. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96: 623-632. [DOI:10.1111/aogs.13156] [PMID]
7. Zhang P, Wang G. Progesterone resistance in endometriosis: Current evidence and putative mechanisms. Int J Mol Sci 2023; 24: 6992. [DOI:10.3390/ijms24086992] [PMID] [PMCID]
8. Marquardt RM, Kim TH, Shin J-H, Jeong J-W. Progesterone and estrogen signaling in the endometrium: What goes wrong in endometriosis? Int J Mol Sci 2019; 20: 3822. [DOI:10.3390/ijms20153822] [PMID] [PMCID]
9. Niknafs B, Shokrzadeh N, Alivand MR, Hesam Shariati MB. The effect of dexamethasone on uterine receptivity, mediated by the ERK1/2-mTOR pathway, and the implantation window: An experimental study. Int J Reprod BioMed 2022; 20: 47-58. [DOI:10.18502/ijrm.v20i1.10408] [PMID] [PMCID]
10. Shokrzadeh N, Alivand MR, Abedelahi A, Hessam Shariati MB, Niknafs B. Calcitonin administration improves endometrial receptivity via regulation of LIF, Muc‐1 and microRNA Let‐7a in mice. J Cell Physiol 2019; 234: 12989-13000. [DOI:10.1002/jcp.27969] [PMID]
11. Alves AR, Dias MF, Silvestre M. Endometrial fluid biomarkers and their potential as predictors of successful embryo implantation. BioMedicine 2023; 13: 1-8. [DOI:10.37796/2211-8039.1413] [PMID] [PMCID]
12. Shokrzadeh N, Alivand MR, Abedelahi A, Hessam Shariati MB, Niknafs B. Upregulation of HB‐EGF, Msx. 1, and miRNA Let‐7a by administration of calcitonin through mTOR and ERK1/2 pathways during a window of implantation in mice. Mol Reprod Dev 2018; 85: 790-801. [DOI:10.1002/mrd.23061] [PMID]
13. Zarei R, Nikpour P, Rashidi B, Eskandari N, Aboutorabi R. Evaluation of Muc1 gene expression at the time of implantation in diabetic rat models treated with insulin, metformin and pioglitazone in the normal cycle and ovulation induction cycle. Int J Fertil Steril 2020; 14: 218.
14. Paul AB, Sadek ST, Mahesan AM. The role of microRNAs in human embryo implantation: A review. J Assist Reprod Genet 2019; 36: 179-187. [DOI:10.1007/s10815-018-1326-y] [PMID] [PMCID]
15. Zhou W, Dimitriadis E. Secreted MicroRNA to predict embryo implantation outcome: From research to clinical diagnostic application. Front Cell Dev Biol 2020; 8: 586510. [DOI:10.3389/fcell.2020.586510] [PMID] [PMCID]
16. Azam INA, Wahab NA, Mokhtar MH, Shafiee MN, Mokhtar NM. Roles of microRNAs in regulating apoptosis in the pathogenesis of endometriosis. Life 2022; 12: 1321. [DOI:10.3390/life12091321] [PMID] [PMCID]
17. Cai H, Zhu X-X, Li Z-F, Zhu Y-P, Lang J-H. MicroRNA dysregulation and steroid hormone receptor expression in uterine tissues of rats with endometriosis during the implantation window. Chin Med J 2018; 131: 2193-2204. [DOI:10.4103/0366-6999.240808] [PMID] [PMCID]
18. Wilczynski M, Danielska J, Dzieniecka M, Szymanska B, Wojciechowski M, Malinowski A. Prognostic and clinical significance of miRNA-205 in endometrioid endometrial cancer. PLoS One 2016; 11: e0164687. [DOI:10.1371/journal.pone.0164687] [PMID] [PMCID]
19. Liu Y, Chen J, Zhu X, Tang L, Luo X, Shi Y. Role of miR 449b 3p in endometriosis via effects on endometrial stromal cell proliferation and angiogenesis. Mol Med Rep 2018; 18: 3359-3365. [DOI:10.3892/mmr.2018.9341] [PMID] [PMCID]
20. Abel Y, Rederstorff M. Stem-Loop qRT-PCR-based quantification of miRNAs. Methods Mol Biol 2021; 2300: 59-64. [DOI:10.1007/978-1-0716-1386-3_6] [PMID]
21. Ohlsson Teague EMC, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, et al. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 2009; 23: 265-275. [DOI:10.1210/me.2008-0387] [PMID] [PMCID]
22. Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, et al. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010; 2010: 369549. [DOI:10.1155/2010/369549] [PMID] [PMCID]
23. Jimenez PT, Mainigi MA, Word RA, Kraus WL, Mendelson CR. miR-200 regulates endometrial development during early pregnancy. Mol Endocrinol 2016; 30: 977-987. [DOI:10.1210/me.2016-1050] [PMID] [PMCID]
24. Gulyaeva LF, Kushlinskiy NE. Regulatory mechanisms of microRNA expression. J Transl Med 2016; 14: 143. [DOI:10.1186/s12967-016-0893-x] [PMID] [PMCID]
25. McGeary SE, Lin KS, Shi CY, Pham TM, Bisaria N, Kelley GM, et al. The biochemical basis of microRNA targeting efficacy. Science 2019; 366: eaav1741. [DOI:10.1126/science.aav1741] [PMID] [PMCID]
26. Górecki I, Rak B. The role of microRNAs in epithelial to mesenchymal transition and cancers; focusing on mir-200 family. Cancer Treat Res Commun 2021; 28: 100385. [DOI:10.1016/j.ctarc.2021.100385] [PMID]
27. Wu Y, Sun K, Tu Y, Li P, Hao D, Yu P, et al. miR‐200a‐3p regulates epithelial-mesenchymal transition and inflammation in chronic rhinosinusitis with nasal polyps by targeting ZEB1 via ERK/p38 pathway. Int Forum Allergy Rhinol 2024; 14: 41-56. [DOI:10.1002/alr.23215] [PMID]
28. Roche J. The epithelial-to-mesenchymal transition in cancer. Cancers 2018; 10: 52. [DOI:10.3390/cancers10020052] [PMID] [PMCID]
29. Guo R, Hao G, Bao Y, Xiao J, Zhan X, Shi X, et al. MiR-200a negatively regulates TGF-β1-induced epithelial-mesenchymal transition of peritoneal mesothelial cells by targeting ZEB1/2 expression. Am J Physiol Renal Physiol 2018; 314: F1087-F1095. [DOI:10.1152/ajprenal.00566.2016] [PMID]
30. Shen L-J, He J-L, Yang D-H, Ding Y-B, Chen X-M, Geng Y-Q, et al. Mmu-microRNA-200a overexpression leads to implantation defect by targeting phosphatase and tensin homolog in mouse uterus. Reprod Sci 2013; 20: 1518-1528. [DOI:10.1177/1933719113488453] [PMID] [PMCID]
31. Xue Y, Lin X, Shi T, Tian Y. miRNA-223 expression in patient-derived eutopic and ectopic endometrial stromal cells and its effect on epithelial-to-mesenchymal transition in endometriosis. Clinics 2022; 77: 100112. [DOI:10.1016/j.clinsp.2022.100112] [PMID] [PMCID]
32. Dong X, Sui C, Huang K, Wang L, Hu D, Xiong T, et al. MicroRNA-223-3p suppresses leukemia inhibitory factor expression and pinopodes formation during embryo implantation in mice. Am J Transl Res 2016; 8: 1155.
33. Niknafs B, Hesam Shariati MB, Shokrzadeh N. miR223‐3p, HAND2, and LIF expression regulated by calcitonin in the ERK1/2‐mTOR pathway during the implantation window in the endometrium of mice. Am J Reprod Immunol 2021; 85: e13333. [DOI:10.1111/aji.13333] [PMID]
34. Shariati MBH, Niknafs B, Seghinsara AM, Shokrzadeh N, Alivand MR. Administration of dexamethasone disrupts endometrial receptivity by alteration of expression of miRNA 223, 200a, LIF, Muc1, SGK1, and ENaC via the ERK1/2‐mTOR pathway. J Cell Physiol 2019; 234: 19629-19639. [DOI:10.1002/jcp.28562] [PMID]
35. You F, Du X, Zhang T, Wang Y, Lv Y, Zeng L. Electroacupuncture improves endometrial receptivity through miRNA-223-3p-mediated regulation of leukemia inhibitory factor/signal transducer and activator of transcription 3 signaling pathway. Bioengineered 2022; 13: 10298-10312. [DOI:10.1080/21655979.2022.2062524] [PMID] [PMCID]
36. Tang Y, Wang Y, Chen Q, Qiu N, Zhao Y, You X. MiR-223 inhibited cell metastasis of human cervical cancer by modulating epithelial-mesenchymal transition. Int J Clin Exp Pathol 2015; 8: 11224.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |