Thu, Jan 29, 2026
[Archive]
Volume 23, Issue 6 (June 2025)
IJRM 2025, 23(6): 485-492 |
Back to browse issues page
Ethics code: IR.DUMS.REC.1400.027
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Poormoosavi S M, Behmanesh M A, Pourmotahari F, Tavvalapour K, Janati S. Impact of COVID-19 infection on placental histopathology and maternal-perinatal outcomes: A cross-sectional study. IJRM 2025; 23 (6) :485-492
URL: http://ijrm.ir/article-1-3464-en.html
URL: http://ijrm.ir/article-1-3464-en.html
Seyedeh Mahsa Poormoosavi1 

 , Mohammad Amin Behmanesh2
, Mohammad Amin Behmanesh2 

 , Fatemeh Pourmotahari3
, Fatemeh Pourmotahari3 

 , Kosar Tavvalapour4
, Kosar Tavvalapour4 

 , Sima Janati *5
, Sima Janati *5 




 , Mohammad Amin Behmanesh2
, Mohammad Amin Behmanesh2 

 , Fatemeh Pourmotahari3
, Fatemeh Pourmotahari3 

 , Kosar Tavvalapour4
, Kosar Tavvalapour4 

 , Sima Janati *5
, Sima Janati *5 


1- Department of Histology, School of Medicine, Research and Clinical Center for Infertility, Dezful University of Medical Sciences, Dezful, Iran.
2- Department of Histology, School of Medicine, Dezful University of Medical Sciences, Dezful, Iran.
3- Department of Biostatistics, School of Medicine, Dezful University of Medical Sciences, Dezful, Iran.
4- Student Research Committee, Dezful University of Medical Sciences, Dezful, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Research and Clinical Center for Infertility, Dezful University of Medical Sciences, Dezful, Iran. ,janati.s@dums.ac.ir; sjanati@ymail.com
2- Department of Histology, School of Medicine, Dezful University of Medical Sciences, Dezful, Iran.
3- Department of Biostatistics, School of Medicine, Dezful University of Medical Sciences, Dezful, Iran.
4- Student Research Committee, Dezful University of Medical Sciences, Dezful, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Research and Clinical Center for Infertility, Dezful University of Medical Sciences, Dezful, Iran. ,
Keywords: Pathology, Placenta, Pregnancy, Severe acute respiratory syndrome coronavirus 2, COVID-19.
Full-Text [PDF 1158 kb]
(322 Downloads)
| Abstract (HTML) (417 Views)
5. Conclusion
Our study reveals that placental tissue in pregnant women infected with COVID-19 during the third trimester exhibits histopathological changes, potentially impairing maternal-fetal exchange. Strengths of this study include its case-control design, standardized histopathological analysis, and clinical correlation with fetal outcomes. However, limitations such as the small sample size and single-center setting should be acknowledged. Further multicenter studies with larger cohorts and long-term follow-up are needed to assess the clinical significance of these findings.
Data Availability
Data are available from the corresponding author upon reasonable request and with the permission of the ethics committee.
Author Contributions
SM. Poormoosavi: Concept, design, and writing. MA. Behmanesh: Acquisition and histopathological examination. F. Pourmotahari: Statistical analysis. K. Tavvalapour: Providing technical assistance. S. Janati has complete access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design authors had a critical revision of the manuscript for important intellectual content.
Acknowledgments
This study was financially supported by the Dezful University of Medical Sciences, Dezful, Iran (grant number: MED-400032-1399). We did not use artificial intelligence for any part of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (91 Views)
1. Introduction
Coronaviruses are a large family of viruses affecting humans and animals, with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East respiratory syndrome coronavirus causing significant mortality in recent years. These RNA viruses primarily spread through contact with the eyes, nose, or mouth (1), accounting for about 35% of upper respiratory infections. Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, emerged in late 2019 in China and rapidly became a global pandemic (2). Its clinical presentation varies from asymptomatic to severe and life-threatening cases (3). Pregnant women face more significant risks from COVID-19 compared to non-pregnant individuals. While extensive research has focused on non-pregnant adults, uncertainties remain regarding the clinical characteristics and potential vertical transmission of the virus from mother to fetus (4-6).
The placenta plays a crucial role in maintaining, supporting, and facilitating fetal growth by connecting the mother and fetus. The large surface area of the placental membrane enables the bidirectional transfer of substances between the placenta and maternal blood through 4 mechanisms: simple diffusion, facilitated diffusion, active transport, and pinocytosis. It is well established that certain viral species can cause congenital abnormalities (7), and the route of viral transmission from mother to fetus depends on the specific virus. Various viruses can infect multiple fetal components, including syncytiotrophoblasts, cytotrophoblasts, endothelial cells, hematopoietic cells, and fetal membranes. In contrast, the placenta provides physical and immunological protection for the fetus through natural killer cells, macrophages, and T lymphocytes. However, if the placenta is compromised as a defensive barrier, pathogens can infiltrate and cause harm to the fetus (8).
A significant concern is whether SARS-CoV-2 can be vertically transmitted to the fetus, leading to infection and congenital contamination, thereby posing risks to both the fetus and mother. Therefore, histopathological examination of placental tissue can provide significant insights into maternal and fetal health. This study aimed to investigate the histological changes in the placental tissue of mothers with COVID-19.
2. Materials and Methods
2.1. Study design and participants
This cross-sectional study was conducted on 40 pregnant women referred to Ganjavian, Dezful, Iran, for delivery (vaginal delivery or cesarean section) between June and October 2021. Participants were categorized into 2 groups based on their COVID-19 infection status: women who tested positive for COVID-19 via polymerase chain reaction (PCR) during their third trimester or on the day of delivery (n = 30), and women who were not infected (n = 10). Women who had a history of trophoblastic disease, reproductive system abnormalities, infertility, or had undergone assisted reproductive techniques were excluded. Additionally, those with pre-existing hypertension or diabetes, maternal or fetal complications during the current pregnancy (such as preeclampsia, chorioamnionitis, third-trimester bleeding, meconium-stained amniotic fluid, or umbilical cord abnormalities), a history of recurrent miscarriages, premature delivery, or premature rupture of membranes were not considered for participation. The presence of hematoma or macroscopic placental lesions also served as exclusion criteria.
Placental samples were collected immediately after delivery and stored at 4°C before being fixed in a 10% formalin buffer. The placentas were subsequently weighed, trimmed, and sectioned to include membranes, the umbilical cord, the maternal surface, total thickness, and any potential lesions. Macroscopic features such as size, maternal and fetal surfaces, lobulation, membrane transparency, and cord length were evaluated before the samples were processed for standard histological examination. Tissue sections of 5-6 µm thickness were prepared, with 4 slides obtained from each tissue block. Hematoxylin and eosin (H&E) staining was performed, and microscopic characteristics, including trophoblastic cells, maternal and fetal villi, fibrin deposition, localized inflammation, fibrosis, basal plate necrosis, vascular alterations, and microcalcification, were analyzed. Additionally, maternal characteristics (including age [yr], gestational age at delivery [wk], number of prior deliveries and pregnancies, and placental weight [gr]) were recorded. Maternal outcomes (such as oxygen administration, mode of delivery [vaginal/cesarean]) and fetal outcomes (including 1- and 5-min Apgar scores and newborn throat swab results) were documented and compared between groups.
2.2. Sample size
This study included all pregnant women who met the eligibility criteria and were admitted to Ganjavian hospital, Dezful, Iran, for delivery between June and October 2021 (n = 40).
2.3. Ethical Considerations
The study protocol was reviewed and approved by the Ethics Committee of Dezful University of Medical Sciences, Dezful, Iran (Code: IR.DUMS.REC.1400.027). Written informed consent was obtained from all participants prior to enrollment, ensuring compliance with ethical guidelines.
2.4. Statistical Analysis
Statistical analyses were conducted using SPSS software version 26.0 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Illinois, USA) and Microsoft Excel 2021. Continuous variables were presented as mean ± standard deviation (SD) or as a range (median-interquartile range), depending on the data distribution. Categorical variables were summarized as frequencies and percentages. Fisher’s exact test was employed to compare categorical variables between 2 groups. The Shapiro-Wilk test was used to assess the normality of data distribution. For comparisons of continuous variables between groups, either the independent t test or the Mann-Whitney U test was applied, as appropriate. P < 0.05 was considered statistically significant.
3. Results
Initially, a total of 46 pregnant women were deemed eligible for participation in the study. However, 6 women were excluded for the following reasons: one woman experienced a miscarriage, one woman had a premature rupture of membranes, one woman had undergone in vitro fertilization, and 3 women declined to participate or did not provide consent. Consequently, the final study population comprised 40 pregnant women, who were stratified into 2 groups based on their PCR test results: 30 women diagnosed with COVID-19 (group I) and 10 women without COVID-19 (group II).
Within group I, 19 women (63.3%) exhibited clinical symptoms, while 11 women (36.7%) remained asymptomatic. The mean duration from symptom onset to delivery among women diagnosed with COVID-19 was 22.13 ± 17.49 days (6-70 days), whereas the mean interval from COVID-19 diagnosis to delivery was 15.97 ± 17.68 days. Among the 30 neonates born in group I, 3 (10%) tested positive for COVID-19 (Table I).
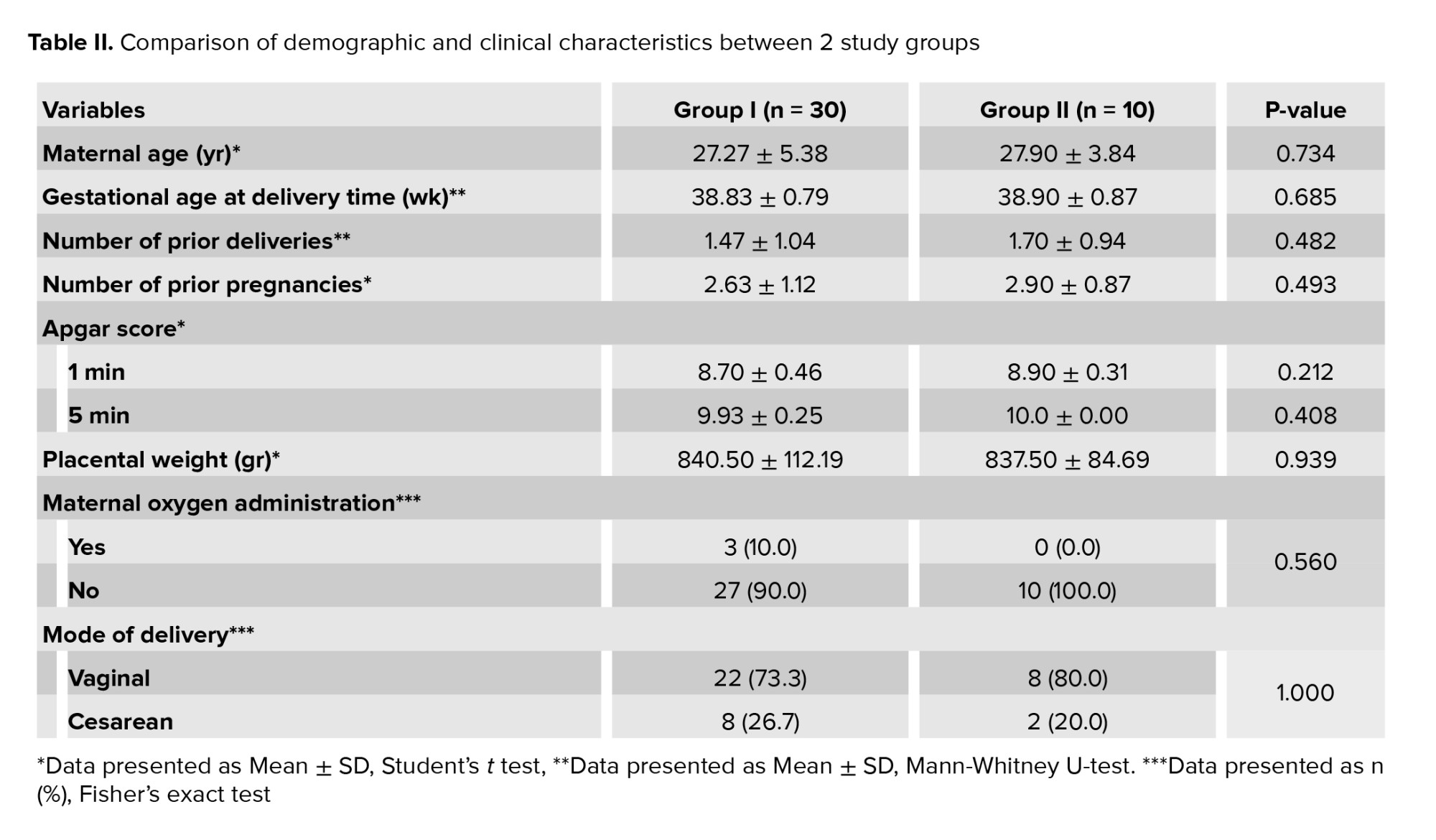
A comparative analysis between the 2 groups revealed no statistically significant differences in maternal age, the number of prior deliveries and pregnancies, gestational age, 1- and 5-min Apgar scores, placental weight, maternal oxygen administration, or mode of delivery (Table II).
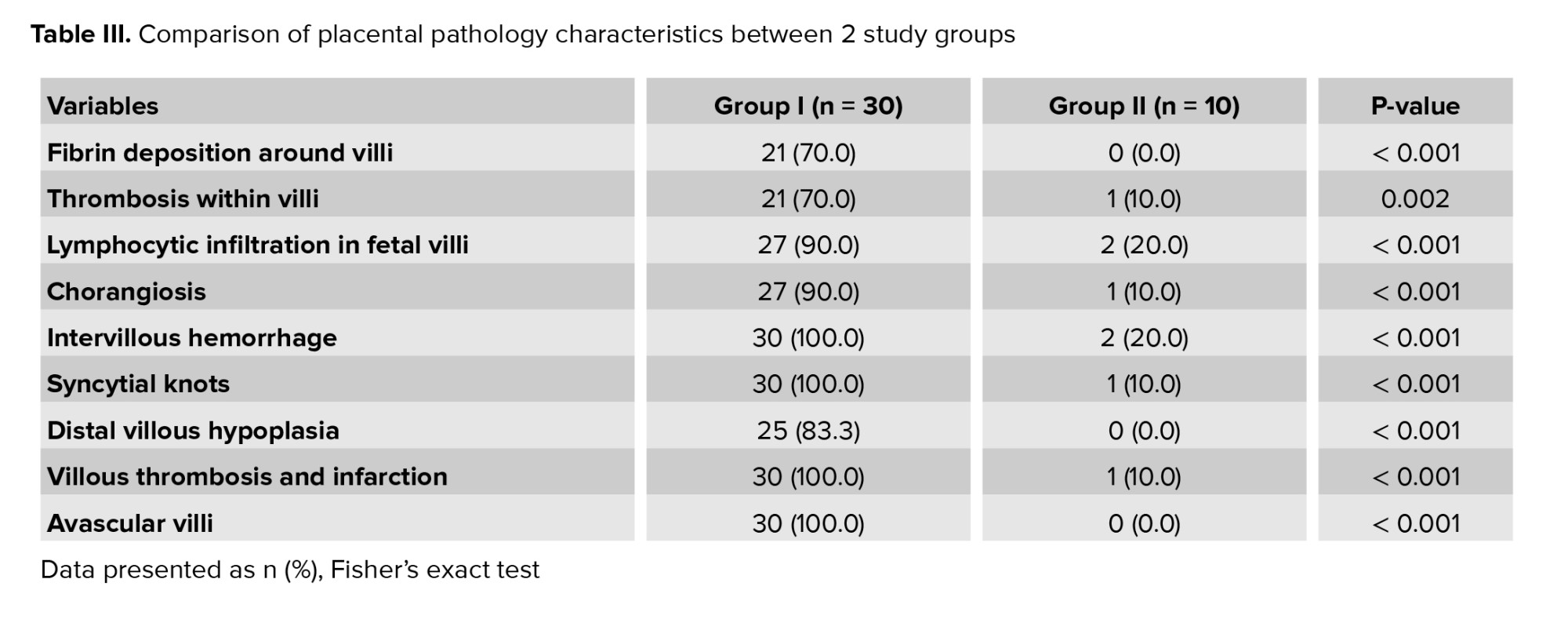
As presented in table III, a statistically significant difference was observed between 2 groups in terms of placental histopathological changes. Notable findings included increased fibrin deposition surrounding the villi, thrombosis within the villous structures, lymphocytic infiltration in fetal villi, chorangiosis, edema, intervillous hemorrhage, the presence of syncytial knots, distal villous hypoplasia, villous thrombosis and infarction, as well as avascular villi (Table III, Figure 1).
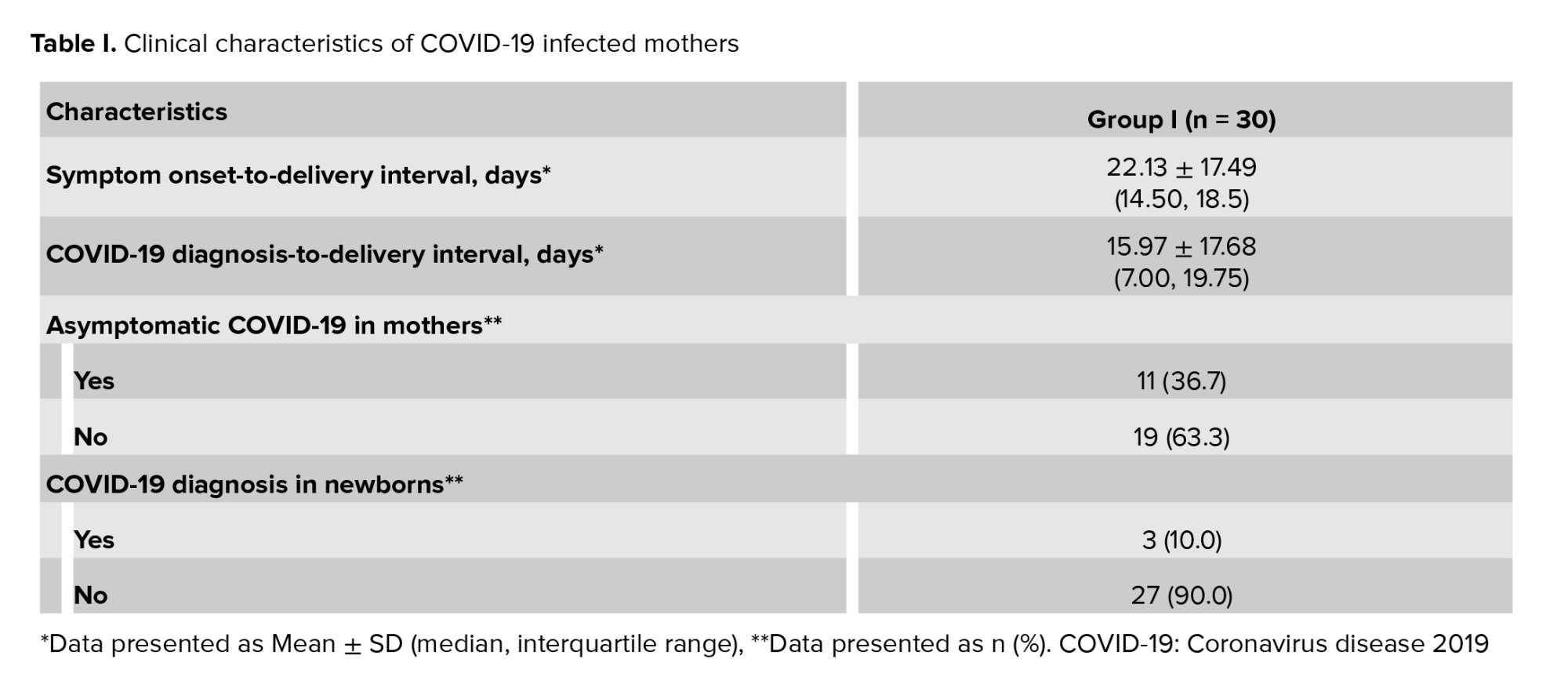
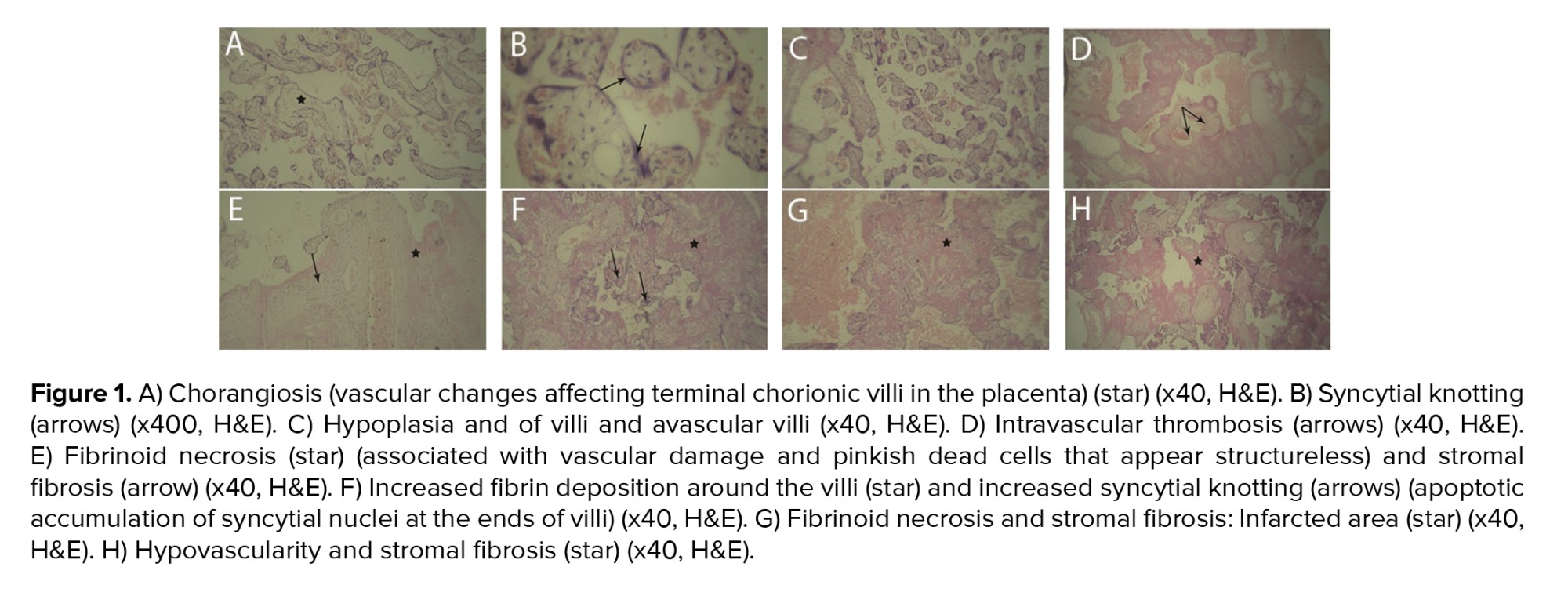
4. Discussion
The findings of this study revealed a statistically significant difference in placental changes between the 2 study groups. These alterations included fibrin deposition around the villi, thrombosis within the villous structures, lymphocytic infiltration into the fetal villi, chorangioma, edema, intervillous hemorrhage, syncytial knot formation, distal villous hypoplasia, as well as thrombosis and hemorrhage in avascular villi. Among these changes, intervillous hemorrhage, syncytial knots, thrombosis, and avascular villi were most frequently observed abnormalities among the participants.
A systematic review in 2022 examined the pathological changes in the placentas of pregnant women with COVID-19. The majority of studies included in this review reported significant histopathological alterations, such as villitis, placental vascular insufficiency, villous thrombosis, and chorangioma. However, the authors also noted that some studies did not observe substantial pathological changes or reported only minimal alterations, which may be attributed to small sample sizes or the absence of a control group. The conclusions of this review align with the findings of the present study, further supporting the presence of significant pathological changes in placental tissue associated with COVID-19 (9).
In another study, which analyzed the presence of viral particles and RNA in the placentas of 5 pregnant women with COVID-19, the primary histopathological finding was thrombus formation in large vessels. Additionally, they observed avascular villi and fibrin deposition within the stromal vessels of the villi, findings that align with those of the present study (10).
Similarly, research by Shanes et al. identified vascular injury in the decidua and thrombus formation within the villi as the most prominent pathological features. Their study also reported villous necrosis, infarction, impaired blood supply to the villi, and villous agglutination. While villous necrosis and agglutination were not observed in our study, the remaining findings were consistent with our results (4).
Argueta et al. examined the clinical and pathological characteristics of 20 pregnant individuals who tested positive for COVID-19. Their study found that all neonates had negative reverse transcription PCR (RT-PCR) test results. Placental histopathological analysis revealed vascular insufficiency and villous thrombosis in 9 cases, fibrin deposition in 7 cases, and villous inflammation in 4 cases (11). In contrast, our study identified 3 neonates with positive RT-PCR test results at birth, which may be attributable to differences in sample size. Nevertheless, the histopathological findings of their study were largely consistent with our results.
Blasco et al. investigated placental pathology in 29 pregnant women infected with COVID-19 during the third trimester. Their findings indicated that none of the neonates contracted the infection, and RT-PCR testing of the placenta yielded negative results, an aspect not assessed in our study. Their study also included a control group of 58 pregnant individuals, some of whom had diabetes or experienced prolonged membrane rupture (> 12 hr), both of which were exclusion criteria in our study. Notably, chorioamnionitis was reported in 5 cases within the COVID-19 group and in 23 cases within the control group, demonstrating a statistically significant difference. However, since chorioamnionitis was also an exclusion criterion in our study, these findings cannot be directly compared. Additionally, 6 cases of villous inflammation and 19 cases of chorangiosis were identified, but these did not exhibit a statistically significant difference from the control group, potentially due to confounding clinical conditions unrelated to COVID-19 (12). Overall, the findings of this study diverge from those of our investigation.
Another study in 2022 on 7 mothers infected with COVID-19 found that although all the infants tested negative for COVID-19, placental samples were positive for the virus. Histopathological examination revealed varying degrees of fibrin deposition around the villi and trophoblastic necrosis (13). These findings are consistent with our study, with the exception that our study identified 3 neonates who tested positive for COVID-19 at birth. This discrepancy may be attributed to the larger sample size in our study. One more study examined the effects of COVID-19 on the placenta in pregnant individuals with a distinct focus and showed a high prevalence of asymptomatic cases and an increased incidence of preterm deliveries in 21 participants with COVID-19. Notably, all placentas exhibited abnormalities related to blood flow and inflammation. In contrast, our study compared 30 pregnant individuals with COVID-19 to 10 without COVID-19 and identified cases of neonatal COVID-19 infection (14). Furthermore, our findings highlighted specific placental changes, such as increased blood clot formation and fibrin deposition, with significant differences observed between the 2 groups. Despite methodological differences, both studies reinforce the notion that COVID-19 can adversely affect placental function and compromise maternal-fetal exchange. Similarly, Singh and others investigated the impact of COVID-19 on placental health in 2021, underscoring the relevance of this topic during the pandemic. Their findings align with the consensus that, in most cases, the effects of COVID-19 on mothers and neonates were mild. However, they emphasize the need for further research to deepen our understanding of the relationship between COVID-19 and placental pathology (15). Finally, based on our findings and other studies, the following recommendations are proposed:
Coronaviruses are a large family of viruses affecting humans and animals, with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East respiratory syndrome coronavirus causing significant mortality in recent years. These RNA viruses primarily spread through contact with the eyes, nose, or mouth (1), accounting for about 35% of upper respiratory infections. Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, emerged in late 2019 in China and rapidly became a global pandemic (2). Its clinical presentation varies from asymptomatic to severe and life-threatening cases (3). Pregnant women face more significant risks from COVID-19 compared to non-pregnant individuals. While extensive research has focused on non-pregnant adults, uncertainties remain regarding the clinical characteristics and potential vertical transmission of the virus from mother to fetus (4-6).
The placenta plays a crucial role in maintaining, supporting, and facilitating fetal growth by connecting the mother and fetus. The large surface area of the placental membrane enables the bidirectional transfer of substances between the placenta and maternal blood through 4 mechanisms: simple diffusion, facilitated diffusion, active transport, and pinocytosis. It is well established that certain viral species can cause congenital abnormalities (7), and the route of viral transmission from mother to fetus depends on the specific virus. Various viruses can infect multiple fetal components, including syncytiotrophoblasts, cytotrophoblasts, endothelial cells, hematopoietic cells, and fetal membranes. In contrast, the placenta provides physical and immunological protection for the fetus through natural killer cells, macrophages, and T lymphocytes. However, if the placenta is compromised as a defensive barrier, pathogens can infiltrate and cause harm to the fetus (8).
A significant concern is whether SARS-CoV-2 can be vertically transmitted to the fetus, leading to infection and congenital contamination, thereby posing risks to both the fetus and mother. Therefore, histopathological examination of placental tissue can provide significant insights into maternal and fetal health. This study aimed to investigate the histological changes in the placental tissue of mothers with COVID-19.
2. Materials and Methods
2.1. Study design and participants
This cross-sectional study was conducted on 40 pregnant women referred to Ganjavian, Dezful, Iran, for delivery (vaginal delivery or cesarean section) between June and October 2021. Participants were categorized into 2 groups based on their COVID-19 infection status: women who tested positive for COVID-19 via polymerase chain reaction (PCR) during their third trimester or on the day of delivery (n = 30), and women who were not infected (n = 10). Women who had a history of trophoblastic disease, reproductive system abnormalities, infertility, or had undergone assisted reproductive techniques were excluded. Additionally, those with pre-existing hypertension or diabetes, maternal or fetal complications during the current pregnancy (such as preeclampsia, chorioamnionitis, third-trimester bleeding, meconium-stained amniotic fluid, or umbilical cord abnormalities), a history of recurrent miscarriages, premature delivery, or premature rupture of membranes were not considered for participation. The presence of hematoma or macroscopic placental lesions also served as exclusion criteria.
Placental samples were collected immediately after delivery and stored at 4°C before being fixed in a 10% formalin buffer. The placentas were subsequently weighed, trimmed, and sectioned to include membranes, the umbilical cord, the maternal surface, total thickness, and any potential lesions. Macroscopic features such as size, maternal and fetal surfaces, lobulation, membrane transparency, and cord length were evaluated before the samples were processed for standard histological examination. Tissue sections of 5-6 µm thickness were prepared, with 4 slides obtained from each tissue block. Hematoxylin and eosin (H&E) staining was performed, and microscopic characteristics, including trophoblastic cells, maternal and fetal villi, fibrin deposition, localized inflammation, fibrosis, basal plate necrosis, vascular alterations, and microcalcification, were analyzed. Additionally, maternal characteristics (including age [yr], gestational age at delivery [wk], number of prior deliveries and pregnancies, and placental weight [gr]) were recorded. Maternal outcomes (such as oxygen administration, mode of delivery [vaginal/cesarean]) and fetal outcomes (including 1- and 5-min Apgar scores and newborn throat swab results) were documented and compared between groups.
2.2. Sample size
This study included all pregnant women who met the eligibility criteria and were admitted to Ganjavian hospital, Dezful, Iran, for delivery between June and October 2021 (n = 40).
2.3. Ethical Considerations
The study protocol was reviewed and approved by the Ethics Committee of Dezful University of Medical Sciences, Dezful, Iran (Code: IR.DUMS.REC.1400.027). Written informed consent was obtained from all participants prior to enrollment, ensuring compliance with ethical guidelines.
2.4. Statistical Analysis
Statistical analyses were conducted using SPSS software version 26.0 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Illinois, USA) and Microsoft Excel 2021. Continuous variables were presented as mean ± standard deviation (SD) or as a range (median-interquartile range), depending on the data distribution. Categorical variables were summarized as frequencies and percentages. Fisher’s exact test was employed to compare categorical variables between 2 groups. The Shapiro-Wilk test was used to assess the normality of data distribution. For comparisons of continuous variables between groups, either the independent t test or the Mann-Whitney U test was applied, as appropriate. P < 0.05 was considered statistically significant.
3. Results
Initially, a total of 46 pregnant women were deemed eligible for participation in the study. However, 6 women were excluded for the following reasons: one woman experienced a miscarriage, one woman had a premature rupture of membranes, one woman had undergone in vitro fertilization, and 3 women declined to participate or did not provide consent. Consequently, the final study population comprised 40 pregnant women, who were stratified into 2 groups based on their PCR test results: 30 women diagnosed with COVID-19 (group I) and 10 women without COVID-19 (group II).
Within group I, 19 women (63.3%) exhibited clinical symptoms, while 11 women (36.7%) remained asymptomatic. The mean duration from symptom onset to delivery among women diagnosed with COVID-19 was 22.13 ± 17.49 days (6-70 days), whereas the mean interval from COVID-19 diagnosis to delivery was 15.97 ± 17.68 days. Among the 30 neonates born in group I, 3 (10%) tested positive for COVID-19 (Table I).
A comparative analysis between the 2 groups revealed no statistically significant differences in maternal age, the number of prior deliveries and pregnancies, gestational age, 1- and 5-min Apgar scores, placental weight, maternal oxygen administration, or mode of delivery (Table II).
As presented in table III, a statistically significant difference was observed between 2 groups in terms of placental histopathological changes. Notable findings included increased fibrin deposition surrounding the villi, thrombosis within the villous structures, lymphocytic infiltration in fetal villi, chorangiosis, edema, intervillous hemorrhage, the presence of syncytial knots, distal villous hypoplasia, villous thrombosis and infarction, as well as avascular villi (Table III, Figure 1).




4. Discussion
The findings of this study revealed a statistically significant difference in placental changes between the 2 study groups. These alterations included fibrin deposition around the villi, thrombosis within the villous structures, lymphocytic infiltration into the fetal villi, chorangioma, edema, intervillous hemorrhage, syncytial knot formation, distal villous hypoplasia, as well as thrombosis and hemorrhage in avascular villi. Among these changes, intervillous hemorrhage, syncytial knots, thrombosis, and avascular villi were most frequently observed abnormalities among the participants.
A systematic review in 2022 examined the pathological changes in the placentas of pregnant women with COVID-19. The majority of studies included in this review reported significant histopathological alterations, such as villitis, placental vascular insufficiency, villous thrombosis, and chorangioma. However, the authors also noted that some studies did not observe substantial pathological changes or reported only minimal alterations, which may be attributed to small sample sizes or the absence of a control group. The conclusions of this review align with the findings of the present study, further supporting the presence of significant pathological changes in placental tissue associated with COVID-19 (9).
In another study, which analyzed the presence of viral particles and RNA in the placentas of 5 pregnant women with COVID-19, the primary histopathological finding was thrombus formation in large vessels. Additionally, they observed avascular villi and fibrin deposition within the stromal vessels of the villi, findings that align with those of the present study (10).
Similarly, research by Shanes et al. identified vascular injury in the decidua and thrombus formation within the villi as the most prominent pathological features. Their study also reported villous necrosis, infarction, impaired blood supply to the villi, and villous agglutination. While villous necrosis and agglutination were not observed in our study, the remaining findings were consistent with our results (4).
Argueta et al. examined the clinical and pathological characteristics of 20 pregnant individuals who tested positive for COVID-19. Their study found that all neonates had negative reverse transcription PCR (RT-PCR) test results. Placental histopathological analysis revealed vascular insufficiency and villous thrombosis in 9 cases, fibrin deposition in 7 cases, and villous inflammation in 4 cases (11). In contrast, our study identified 3 neonates with positive RT-PCR test results at birth, which may be attributable to differences in sample size. Nevertheless, the histopathological findings of their study were largely consistent with our results.
Blasco et al. investigated placental pathology in 29 pregnant women infected with COVID-19 during the third trimester. Their findings indicated that none of the neonates contracted the infection, and RT-PCR testing of the placenta yielded negative results, an aspect not assessed in our study. Their study also included a control group of 58 pregnant individuals, some of whom had diabetes or experienced prolonged membrane rupture (> 12 hr), both of which were exclusion criteria in our study. Notably, chorioamnionitis was reported in 5 cases within the COVID-19 group and in 23 cases within the control group, demonstrating a statistically significant difference. However, since chorioamnionitis was also an exclusion criterion in our study, these findings cannot be directly compared. Additionally, 6 cases of villous inflammation and 19 cases of chorangiosis were identified, but these did not exhibit a statistically significant difference from the control group, potentially due to confounding clinical conditions unrelated to COVID-19 (12). Overall, the findings of this study diverge from those of our investigation.
Another study in 2022 on 7 mothers infected with COVID-19 found that although all the infants tested negative for COVID-19, placental samples were positive for the virus. Histopathological examination revealed varying degrees of fibrin deposition around the villi and trophoblastic necrosis (13). These findings are consistent with our study, with the exception that our study identified 3 neonates who tested positive for COVID-19 at birth. This discrepancy may be attributed to the larger sample size in our study. One more study examined the effects of COVID-19 on the placenta in pregnant individuals with a distinct focus and showed a high prevalence of asymptomatic cases and an increased incidence of preterm deliveries in 21 participants with COVID-19. Notably, all placentas exhibited abnormalities related to blood flow and inflammation. In contrast, our study compared 30 pregnant individuals with COVID-19 to 10 without COVID-19 and identified cases of neonatal COVID-19 infection (14). Furthermore, our findings highlighted specific placental changes, such as increased blood clot formation and fibrin deposition, with significant differences observed between the 2 groups. Despite methodological differences, both studies reinforce the notion that COVID-19 can adversely affect placental function and compromise maternal-fetal exchange. Similarly, Singh and others investigated the impact of COVID-19 on placental health in 2021, underscoring the relevance of this topic during the pandemic. Their findings align with the consensus that, in most cases, the effects of COVID-19 on mothers and neonates were mild. However, they emphasize the need for further research to deepen our understanding of the relationship between COVID-19 and placental pathology (15). Finally, based on our findings and other studies, the following recommendations are proposed:
- Investigation of placental changes in pregnant individuals with COVID-19 or other highly virulent viruses during the first and second trimesters.
- Analysis of placental swabs to confirm the presence of the virus within placental tissue.
- Examination of amniotic fluid to assess the potential for intrauterine viral transmission.
5. Conclusion
Our study reveals that placental tissue in pregnant women infected with COVID-19 during the third trimester exhibits histopathological changes, potentially impairing maternal-fetal exchange. Strengths of this study include its case-control design, standardized histopathological analysis, and clinical correlation with fetal outcomes. However, limitations such as the small sample size and single-center setting should be acknowledged. Further multicenter studies with larger cohorts and long-term follow-up are needed to assess the clinical significance of these findings.
Data Availability
Data are available from the corresponding author upon reasonable request and with the permission of the ethics committee.
Author Contributions
SM. Poormoosavi: Concept, design, and writing. MA. Behmanesh: Acquisition and histopathological examination. F. Pourmotahari: Statistical analysis. K. Tavvalapour: Providing technical assistance. S. Janati has complete access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design authors had a critical revision of the manuscript for important intellectual content.
Acknowledgments
This study was financially supported by the Dezful University of Medical Sciences, Dezful, Iran (grant number: MED-400032-1399). We did not use artificial intelligence for any part of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Pattology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




