Thu, Feb 5, 2026
[Archive]
Volume 23, Issue 9 (September 2025)
IJRM 2025, 23(9): 671-688 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tjahyadi D, Sudono Riyadi A, Dewi Nugrahani A, Amarullah Ritonga M, Rachmawati A, Husnitawati Madjid T. Potency of oral gonadotropin-releasing hormone antagonist as endometriosis-associated pain novel treatments: An updated meta-analysis of randomized controlled trials. IJRM 2025; 23 (9) :671-688
URL: http://ijrm.ir/article-1-3513-en.html
URL: http://ijrm.ir/article-1-3513-en.html
Dian Tjahyadi1 

 , Anthony Sudono Riyadi *2
, Anthony Sudono Riyadi *2 

 , Annisa Dewi Nugrahani1
, Annisa Dewi Nugrahani1 

 , Mulyanusa Amarullah Ritonga1
, Mulyanusa Amarullah Ritonga1 

 , Anita Rachmawati1
, Anita Rachmawati1 

 , Tita Husnitawati Madjid1
, Tita Husnitawati Madjid1 




 , Anthony Sudono Riyadi *2
, Anthony Sudono Riyadi *2 

 , Annisa Dewi Nugrahani1
, Annisa Dewi Nugrahani1 

 , Mulyanusa Amarullah Ritonga1
, Mulyanusa Amarullah Ritonga1 

 , Anita Rachmawati1
, Anita Rachmawati1 

 , Tita Husnitawati Madjid1
, Tita Husnitawati Madjid1 


1- Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia.
2- Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia. ,Anthony180187@yahoo.com
2- Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia. ,
Abstract: (573 Views)
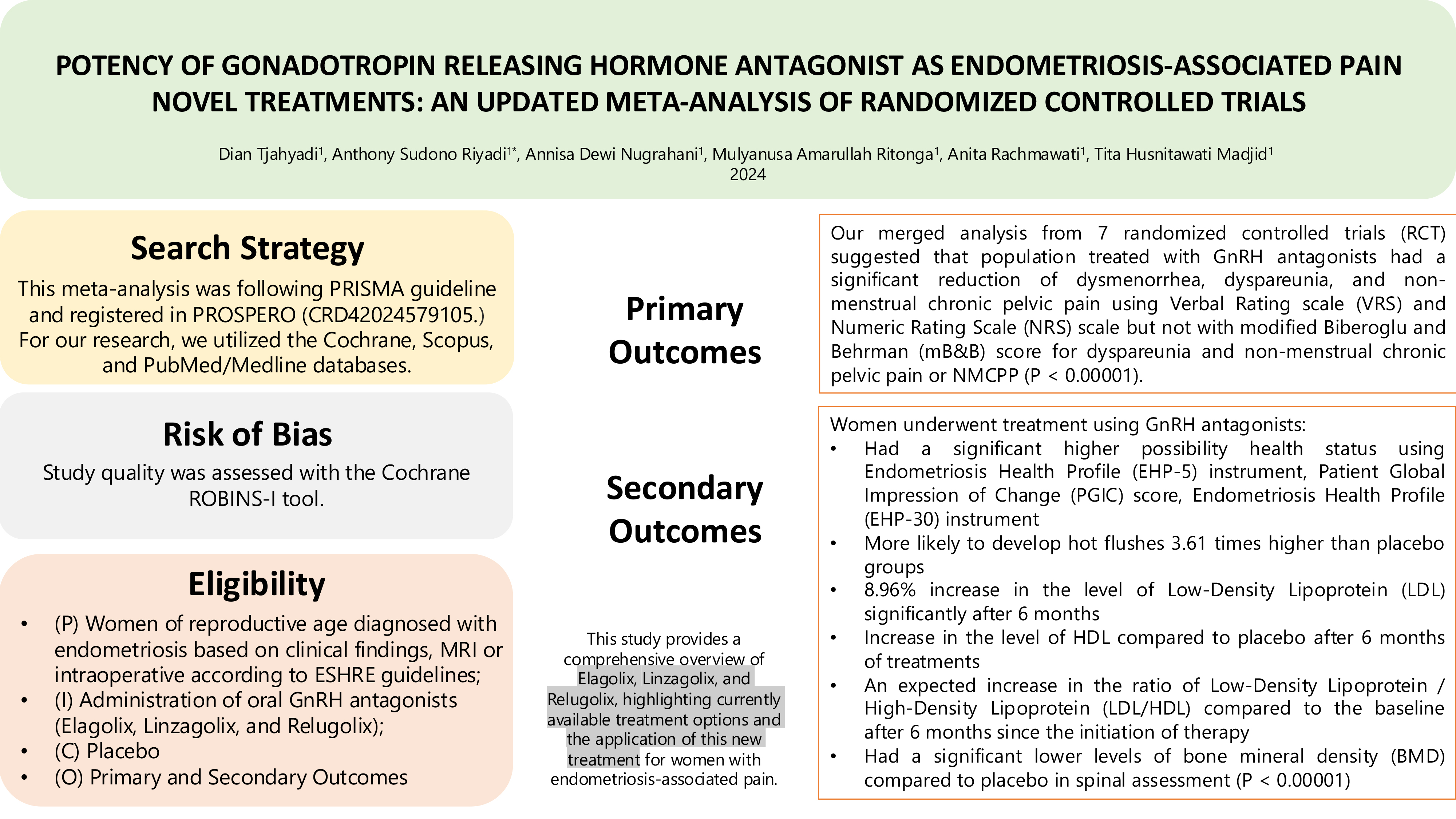
Background: Elagolix, Linzagolix, and Relugolix, as oral gonadotropin-releasing hormone antagonists, have emerged as promising treatments for endometriosis-associated pain.
Objective: To evaluate pain intensity reduction as the primary outcome and assess side effects and quality of life as secondary outcomes through an updated meta-analysis.
Materials and Methods: A systematic search was conducted in Cochrane, Scopus, and PubMed/Medline. Study quality was assessed using the Cochrane risk of bias in non-randomized studies of interventions tool. The pooled standard mean difference and p-value were calculated using a random-effects model (DerSimonian-Laird method), and the inconsistency index was applied to evaluate heterogeneity.
Results: 7 randomized controlled trials were included. Gonadotropin-releasing hormone antagonists significantly reduced dysmenorrhea, dyspareunia, and non-menstrual chronic pelvic pain when measured with the verbal and numeric rating scales, but not with the modified Biberoglu and Behrman score. Secondary outcomes showed significant improvements in health status (endometriosis health profile and patient global impression of change). However, treatment was associated with increased hot flushes (3.61-fold higher), an 8.96% increase in low-density lipoprotein after 6 months, and a rise in high-density lipoprotein compared with placebo. Bone mineral density in the spine was significantly lower in the treatment group (p < 0.001).
Conclusion: This meta-analysis provides updated evidence on Elagolix, Linzagolix, and Relugolix, confirming their effectiveness in managing endometriosis-associated pain, while highlighting important considerations regarding metabolic and bone health.
Objective: To evaluate pain intensity reduction as the primary outcome and assess side effects and quality of life as secondary outcomes through an updated meta-analysis.
Materials and Methods: A systematic search was conducted in Cochrane, Scopus, and PubMed/Medline. Study quality was assessed using the Cochrane risk of bias in non-randomized studies of interventions tool. The pooled standard mean difference and p-value were calculated using a random-effects model (DerSimonian-Laird method), and the inconsistency index was applied to evaluate heterogeneity.
Results: 7 randomized controlled trials were included. Gonadotropin-releasing hormone antagonists significantly reduced dysmenorrhea, dyspareunia, and non-menstrual chronic pelvic pain when measured with the verbal and numeric rating scales, but not with the modified Biberoglu and Behrman score. Secondary outcomes showed significant improvements in health status (endometriosis health profile and patient global impression of change). However, treatment was associated with increased hot flushes (3.61-fold higher), an 8.96% increase in low-density lipoprotein after 6 months, and a rise in high-density lipoprotein compared with placebo. Bone mineral density in the spine was significantly lower in the treatment group (p < 0.001).
Conclusion: This meta-analysis provides updated evidence on Elagolix, Linzagolix, and Relugolix, confirming their effectiveness in managing endometriosis-associated pain, while highlighting important considerations regarding metabolic and bone health.
Type of Study: Review Article |
Subject:
Reproductive Endocrinology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |