Tue, Feb 24, 2026
[Archive]
Volume 23, Issue 8 (August 2025)
IJRM 2025, 23(8): 647-658 |
Back to browse issues page
Ethics code: IR.SBMU.ENDOCRINE.REC.1403.038
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sadeghian Bakhi E, Hayati Roodbari N, Anvari M, Ramezani Tehrani F. Prenatal exposure to a single dose of testosterone adversely affects the oocyte and embryo quality in rats during adulthood: An experimental study. IJRM 2025; 23 (8) :647-658
URL: http://ijrm.ir/article-1-3526-en.html
URL: http://ijrm.ir/article-1-3526-en.html
1- Department of Biology, School of Basic Science, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Reproductive Endocrinology Research Center, Research Institute for Endocrine Molecular Biology, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. & Foundation for Research and Education Excellence, Vestavia Hills, AL, USA. ,ramezani@endocrine.ac.ir; fah.tehrani@gmail.com; framezan@postharvard.edu
2- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Reproductive Endocrinology Research Center, Research Institute for Endocrine Molecular Biology, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. & Foundation for Research and Education Excellence, Vestavia Hills, AL, USA. ,
Full-Text [PDF 449 kb]
(575 Downloads)
| Abstract (HTML) (574 Views)
Full-Text: (81 Views)
1. Introduction
Previous studies have demonstrated that women experiencing gestational hyperandrogenemia (androgen excess), such as those with polycystic ovary syndrome (PCOS), have elevated androgen levels in cord blood; consequently, the fetus is also exposed to high levels of androgens during prenatal life (1-4). Exposure to elevated androgen levels during prenatal life can adversely affect the hypothalamic-pituitary-ovarian axis in the developing fetus, potentially resulting in long-term reproductive system dysfunction, such as polyfollicular ovarian morphology, hyperandrogenemia, and luteinizing hormone hypersecretion in offspring in their later life (5, 6).
In both animal and human studies, androgen exposure has been associated with impaired mitochondrial function and structure in the oocyte, reduced oocyte and embryo quality, impaired development of oocytes and blastocysts, and reduced fertility outcomes (7-13). However, only a limited number of studies have examined the effects of prenatal androgen exposure (PAE) on oocyte and embryo quality. Moreover, the mechanisms underlying the decline in oocyte and embryo quality following early-life androgen exposure remain poorly understood.
Growth differentiation factor-9 (GDF-9), an oocyte-derived gene, plays a pivotal role in follicular development as well as the proliferation and differentiation of granulosa cells (GCs), making it as a potential biomarker of oocyte quality (14, 15). However, previous studies have reported conflicting effects of androgen exposure on GDF-9 expression during folliculogenesis, with some indicating decrease in the GDF-9 expression (16, 17) and others suggesting higher GDF-9 levels (18) both of which may disrupt normal ovarian function. Moreover, the critical role of GDF-9 in fertility and reproductive function has been well established (19).
Due to ethical and practical constraints in human research, animals provide a controlled and feasible approach for studying the effects of PAE on oocyte and embryo quality, as well as the expression of related genes in the ovaries. Rats are considered the most appropriate model because of their well-defined genetic background, significant genomic similarity to humans, and practical advantages including small body size, ease of handling, rapid growth, and short reproductive cycles.
To the best of our knowledge, no previous study has comprehensively examined the effects of a single dose of testosterone administered during a critical window of fetal development on both oocyte and embryo quality, as well as the expression of key ovarian genes during adulthood. Although several studies have investigated the impact of prolonged or postnatal androgen exposure on reproductive outcomes (5-13), the current study aimed to investigate whether prenatal exposure to a single dose of testosterone could affect the reproductive system, especially oocyte and embryo quality in rats during adulthood.
2. Materials and Methods
2.1. Animals
10 female Wistar rats (12-13 wk, 185 ± 10 gr) were obtained from the Animal Center of Shahid Sadoughi University of Medical Sciences (Yazd, Iran). Each female rat was housed overnight with a male rat in one polypropylene cage under standard animal housing conditions (12-hr light/dark cycle, 22 ± 3oC temperature, and 45-55% relative humidity), with ad libitum access to food and water. The first day of pregnancy was confirmed by the presence of a vaginal plug.
2.2. PAE, maintenance, and studyʼs groups
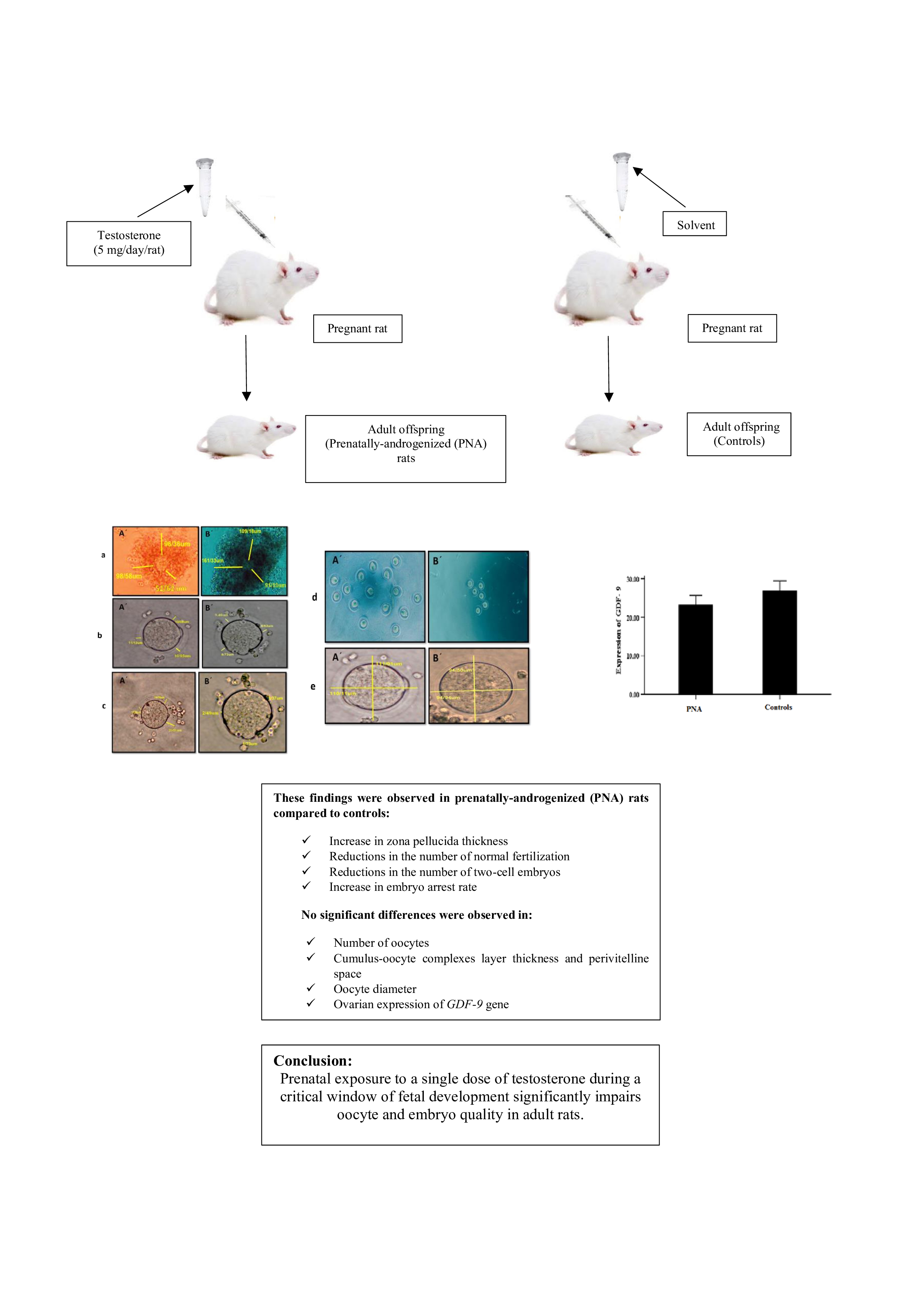
The present study utilized the methodology previously established for the induction of a rat model of PCOS (5). In summary, pregnant rats were randomly assigned to either an experimental or a control group (n = 5/group). On gestational day 20, rats in the experimental group received a subcutaneous injection of 5 mg free testosterone (T1500; Sigma, Steinheim, Germany) dissolved in 500 µl of a vehicle composed of sesame oil (S3547; Sigma) and benzyl benzoate (B6630; Sigma). In contrast, control group rats were administered 500 µl of the vehicle solution alone, consisting of sesame oil and benzyl benzoate in a 4:1 ratio. After weaning period, female offspring of 2 groups, including prenatally-androgenized (PNA) rats (n = 8) and non-exposed rats (controls) (n = 8), were kept in cages in groups of 3 or 4 in standard animal housing conditions (12-hr light/dark cycle, a controlled temperature of 22 ± 3oC, and a relative humidity of 45-55%) with free access to food and water. PNA rats and their controls were followed until 70-85 days of age (adulthood) and studied in terms of oocyte and embryo quality as well as ovarian expression of the GDF-9 gene (Figure 1).
2.3. Preparation of cultivation environment
One day prior to in vitro fertilization (IVF), the culture media were carefully prepared under sterile conditions within a laminar flow hood. HamsF-10 medium was used for washing both sperm and oocytes. For oocyte-specific washing with G-MOPs medium (Vitrolife, Gothenburg, Sweden), a 60 mm dish was prepared with around 10 (50-µL) drops, subsequently covered with mineral oil (Lifeglobal, USA). IVF medium (G-IVF) (Vitrolife, Gothenburg, Sweden): A 60 mm dish was used, and about 9 drops (1 drop in the middle and 8 drops around) were placed in the dish. Following IVF, G1 medium (Vitrolife, Gothenburg, Sweden) was placed in a 30 mm dish, where 9 (20-µL) drops were added. All media were covered with mineral oil (Lifeglobal, USA) and incubated in an environment of 5% CO2 and 95% air with high humidity (Memmert, Germany), except for the G-MOPs medium, which was maintained in a standard incubator.
2.4. Preparation of sperm suspension
Sperms were collected from the caudal epididymis of mature Wistar male rats. On the day of IVF, the caudal epididymis was carefully removed and placed in HamsF-10 medium. The sperms were then incubated at 37oC for 40-60 min (20, 21).
2.5. Ovarian stimulation and collection of oocytes
Superovulation was induced in 10-12-wk-old PNA rats and their controls weighing 170-180 gr by intraperitoneal injection (i.p) of 37 IU Menopur (Ferring GmbH, Wittland11, D-24109 Kiel, Germany). After 48 hr, 50 IU human chorionic gonadotropin (Netherlands) was injected (i.p). 15-16 hr after human chorionic gonadotropin injection, the animals were sacrificed with cervical dislocation. The oviduct was immediately removed and transferred to HamsF-10 medium. Oocytes were collected by the flushing method under a stereomicroscope (Olympus.SZX16, Japan), the cumulus-oocyte complexes (COCs) were then quickly transferred to a dish containing the G-MOPS medium. Following 4-5 washes in G-MOPS medium, the COCs were transferred to IVF medium (G-IVF), washed with several drops of this medium, and placed in the central drop for fertilization. The dish was incubated in an incubator at 37oC with 5% CO2 and 95% air with high humidity (21, 22).
2.6. IVF and embryo development
After 40 min, sperms at a concentration of 1×10⁶ sperm/mL were carefully removed and returned to the incubator. Following 5 hr of incubation with the sperms, the oocytes were separated, washed, and subsequently cultured in G1 medium (21, 22).
2.7. COCs, zona pellucida (ZP) thickness, perivitelline space (PVS), and oocyte diameter measurement
To evaluate COCs, they were washed under a stereomicroscope using HamsF-10 medium, followed by G-MOPS medium, and subsequently transferred to G-IVF medium. Each sample was carefully positioned under an inverted microscope (Olympus, Japan) at 40X magnification for imaging. COCs thickness (μm) was measured at 3 different points using ImageJ software. Finally, mean values were calculated for each group (n = 8/group).
To assess ZP thickness and the PVS, the cumulus layer was enzymatically removed. In brief, cumulus masses were transferred under a stereomicroscope to a 100-μL drop of hyaluronidase (Irvine Scientific, USA) and covered with mineral oil. Pipetting was performed several times to facilitate the removal of the cumulus. The cumulus layer was removed, and ZP and PVS became visible. Samples were then imaged using an inverted microscope at 100X magnification. Subsequently, ZP thickness (μm) and PVS (μm) were measured at 3 different points using ImageJ software. Oocyte diameter was also measured using ImageJ software. Finally, mean values were calculated for each group (n = 8/group) (21, 23).
2.8. Oocytes count
After transferring the oviducts to the washing medium (HamsF-10) and releasing the COCs using the flushing method, the COCs were removed, and the number of oocytes was counted (n = 8/group) (23).
2.9. Embryonic development process
5 hr after preparation of sperm suspension and transferring of oocytes to G1 medium, embryonic development was monitored and the number of embryos was counted under an inverted microscope every 24 hr for 4 days (n = 8/group) (21). Embryo arrest was defined as the cessation of embryonic development for at least 24 hr, typically observed during the cleavage stage before blastocyst formation (24).
2.10. RNA isolation, complementary DNA (cDNA) synthesis, and quantitative real-time polymerase chain reaction (qRT-PCR)
After killing all rats, one ovary from each rat was removed, snap-frozen in liquid nitrogen, and stored at -80oC for subsequent molecular assessment.
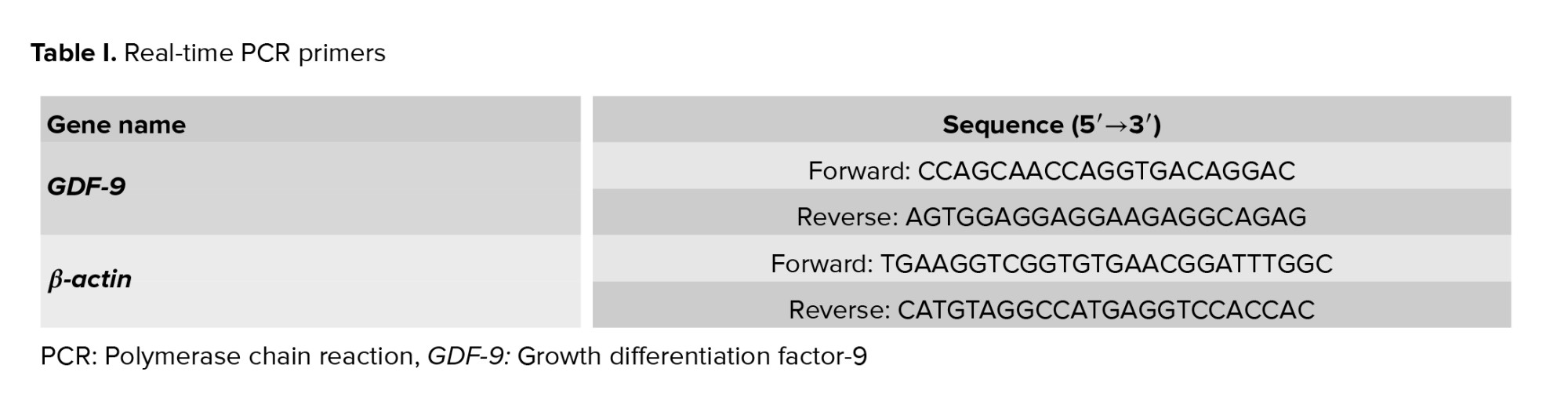
Total RNA was isolated from the ovarian tissue using the TRIzol method. RNA quantity and purity were assessed by measuring the absorbance ratio at 280/260 nm with a NanoDrop spectrophotometer (Thermo Fisher). To synthesize cDNA, the total RNA was treated with DNase I (Thermo Fisher Scientific, USA) to prevent genomic DNA contamination. Subsequently, the total RNA was converted into cDNA using the cDNA synthesis kit (4500 YT, Yekta Tajhiz, Iran) via reverse transcription using random hexamer and initial oligo-dT reaction according to the manufacturerʼs instructions. To assay the expression of the GDF-9 gene in ovarian tissue, qRT-PCR was performed using SYBR-Green and the primers (sequences are listed in table I). Gene duplication process was done using applied biosystem and SYBR-green master mix (Ampliqon). The melting curve is used to ensure the lack of non-specific product proliferation and primer dimer. Melting curve analysis did not show any primer dimer and non-specific products in the assay. The cycle threshold was determined. The gene expression level was calculated using the 2-ΔΔCt method and using β-actin as a reference gene. The analysis of gene expression was performed using the Livak method as relative fold change.
PCR was conducted using the Rotor-Gene 6000 real-time PCR system (Corbett Research, Sydney, Australia) under a 40-cycle protocol. The thermal cycling conditions included an initial denaturation step at 95oC for 10 min, followed by a 2-step amplification cycle consisting of denaturation at 95oC for 10 sec, annealing at 58oC for 10 sec, and extension at 70oC for 20 sec. Fluorescence signals were continuously monitored throughout the reaction to enable real-time quantification of gene expression.
2.11. Ethical Considerations
This experimental study was conducted in 2024 in the biotechnology department of Research and Clinical Center for Infertility, Yazd, Iran. Animal care and handling were performed in accordance with established principles of laboratory animal welfare. Ethics Committee of Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, (Tehran, Iran) approved this study (Code: IR.SBMU.ENDOCRINE.REC.1403.038).
2.12. Statistical Analysis
Continuous variables were assessed for normality using the one-sample Kolmogorov-Smirnov test. The Mann-Whitney U test was used to compare the differences between 2 study groups. Data were presented as median and interquartile range (Q1-Q3). Statistical analysis was conducted using SPSS software (version 24.0; SPSS Inc., Chicago). The p ≤ 0.05 was considered statistically significant.
3. Results
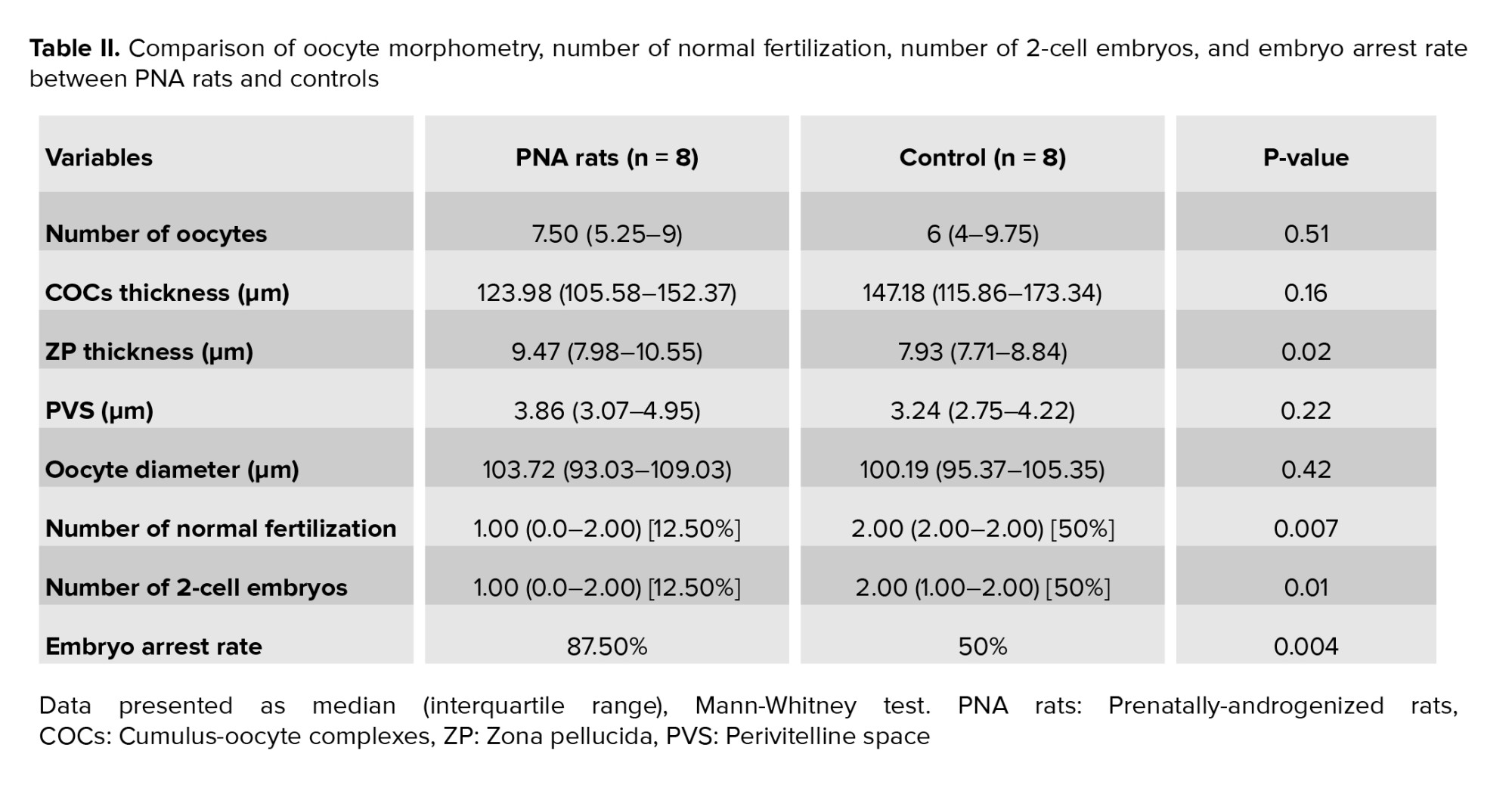
No significant differences were observed in the number of oocytes, COCs layer thickness, PVS, and oocyte diameter in PNA rats compared to controls (Table II). A significant increase was observed in the ZP thickness in PNA rats compared to controls (Table II). Qualitatively, the stability of GCs in PNA rats was lower than control rats. Number of normal fertilization and 2-cell embryos significantly decreased in PNA rats compared to controls (Table II). Embryo arrest rate in PNA rats significantly increased compared to controls (Table II).
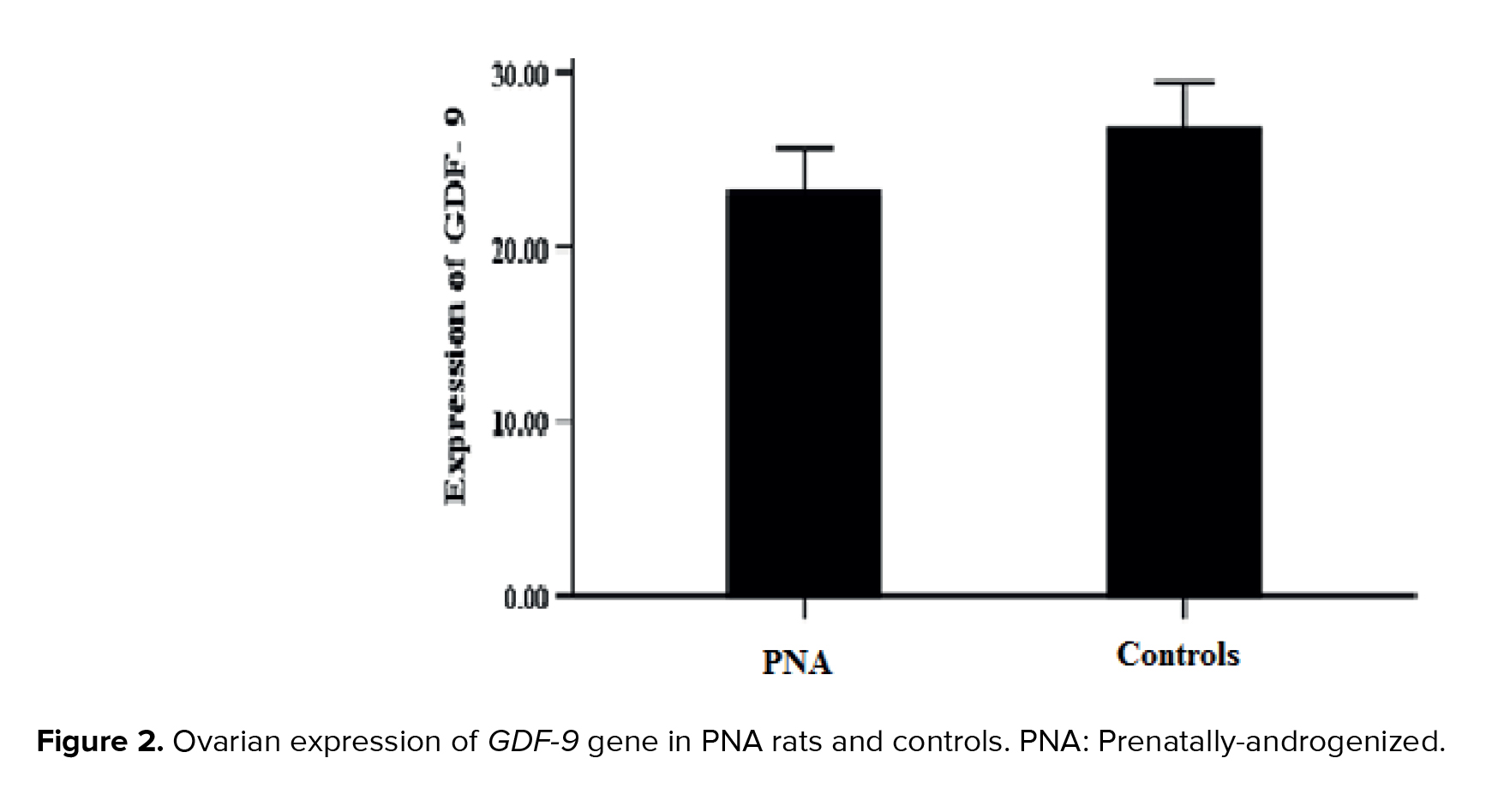
No statistically significant difference was observed in the ovarian expression of the GDF-9 gene between 2 study groups (PNA rats and controls) (p > 0.05) (Figure 2).
4. Discussion
In the present study, we demonstrated that prenatal exposure to a single dose of testosterone during the late gestational period led to increased ZP thickness, a higher rate of embryo arrest, reduced number of normal fertilization and 2-cell embryos, and also alterations in the density and stability of GCs, in rats in their later life (during adulthood). However, no significant differences were observed in the number of oocytes, COCs layer thickness, PVS, oocyte diameter, and the ovarian expression of the GDF-9 gene between 2 groups of study (PNA rats and controls).
It is well documented that the fetus is highly sensitive to exposure to sex steroid hormones during early development. PAE significantly affects fetal development and can lead to long-term consequences, such as reproductive system dysfunction in offspring (5, 6). Hormonal imbalances in the intrauterine environment can alter molecular pathways and gene expression levels, as well as the structure and function of developing fetal organs, potentially leading to reproductive and endocrine disorders in later life.
Previous studies have demonstrated that PAE can significantly disrupt the hypothalamic-pituitary-ovarian axis, resulting in luteinizing hormone hypersecretion, hyperandrogenism, polycystic ovarian morphology, and impaired fertility outcomes (5, 6, 25). Additionally, PAE has been associated with a reduced number of oocytes and primordial follicles, and also diminished ovarian reserve in adult females (5, 6, 8, 12, 13, 25, 26).
Consistent with our findings, both human and animal studies have shown that androgen exposure during the pre or postnatal period adversely affects oocyte and embryo quality (7, 8, 11). For example, mice implanted subdermally with dihydrotestosterone exhibited impaired mitochondrial structure and function in the oocyte, resulting in reduced oocyte quality (7). Similarly, the lean PCOS mouse model showed compromised oocyte quality, which was associated with mitochondrial ultrastructural and functional defects (8). Mouse models exposed to prenatal androgen excess demonstrated impaired oocyte and blastocyst development, and also abnormal fertilization, highlighting the detrimental effects of androgen excess on early embryonic development (10). In vitro studies have further demonstrated that testosterone propionate negatively affects oocyte maturation, as evidenced by significantly decreased maturation rates and delayed progression from germinal vesicle breakdown to metaphase II in hyperandrogenic mice (9, 27). A mouse model of PCOS, induced by PAE, exhibited poor oocyte quality, impaired embryonic development, and reduced fertility (10). Clinically, patients with PCOS showed higher miscarriage rates, suggesting that a hyperandrogenic ovarian microenvironment may adversely influence fertility and pregnancy outcomes (11). Additionally, hyperandrogenemia has been shown to impair preimplantation embryonic development, with a notable reduction in blastocyst formation rate in these hyperandrogenic patients (10). Some other studies have also shown that poor oocyte quality, reduced embryonic development, and infertility may also arise from an abnormal intrauterine environment during critical periods of fetal life (27-29). Women with PCOS and hyperandrogenism exhibit significantly lower oocyte and embryo quality, along with reduced fertilization rate (30). Furthermore, supplementation with dehydroepiandrosterone has been reported to adversely affect ovulation and oocyte quality (31).
Androgen exposure may adversely affect oocyte and embryo quality through several mechanisms. Hyperandrogenism is associated with increased accumulation of advanced glycation end products in GCs (32). Elevated advanced glycation end products lead to protein cross-linking and aggregation, disrupting cell signaling and function, ultimately leading to cell damage and death (27). Additionally, exposure to androgen excess can elevate reactive oxygen species (ROS) levels in both oocytes and GCs (33). The accumulation of intracytoplasmic ROS in these cells is associated with impaired glutathione production, which may compromise cytoplasmic maturation in oocytes (34).
Excess androgen exposure has also been shown to elevate oxidative stress levels in oocytes, increase the expression of Forkhead box protein O1 (FOXO1), and adversely affect mitochondrial energy metabolism and DNA integrity, ultimately leading to oocyte apoptosis. In oocytes from PCOS mice, expression levels of ROS, FOXO1, Caspase-3, and a marked elevation in γ-H2AX levels was observed, accompanied by a significant reduction in mitochondrial membrane potential. These findings indicated that excess androgen exposure induced oxidative stress, which subsequently induced higher expression of FOXO1, leading to increased DNA damage and apoptosis in oocytes. Furthermore, hyperandrogenic conditions in women adversely affect the metabolic status of GCs within ovarian follicles, thereby disrupting follicular growth and oocyte maturation. A study conducted on mice demonstrated that hyperandrogenism induces epigenetic changes that impair oocyte quality (27). Additionally, elevated concentrations of testosterone in follicular fluid have been implicated in compromising oocyte and embryo quality, as well as lowering fertilization rates. These effects may be mediated through alterations in the transcriptional levels of aryl hydrocarbon receptor signaling downstream genes. Moreover, studies have shown that fatty acid and cholesterol synthesis may be inhibited in the ovarian GCs of women with hyperandrogenism, potentially disrupting oocyte maturation (30).
Insulin resistance (IR) may represent an additional pathway that contributes to the reduced quality of oocytes and embryos associated with hyperandrogenism. It is well-documented that women with hyperandrogenism, particularly those with PCOS, commonly exhibit IR (35). The presence of IR and decreased glucose concentrations in follicular fluid impair glucose transfer and energy metabolism in oocytes and follicular cells, ultimately resulting in reduced oocyte and embryo quality (36). Consequently, IR may be regarded as an indicator of poor oocyte maturation, suboptimal embryo quality, and prolonged stimulation duration. It has been reported that women with IR require higher doses of gonadotropins and a longer stimulation period to achieve follicular maturation (37).
In this study, we observed that the expression of GDF-9 gene in the ovaries of PNA rats did not differ significantly to that of control group. GDF-9, a paracrine factor secreted by ovarian follicles, plays a critical role in follicular development, GCs proliferation and differentiation, and the overall process of folliculogenesis in mammals (38). However, a lack of consensus was observed regarding GDF-9 expression in studies investigating the effects of androgen exposure. While one study found that follicles cultured with dehydroepiandrosterone showed reduced expression of genes related to oocyte maturation, such as GDF-9 and bone morphogenetic protein 15, possibly due to impaired steroid hormone synthesis and lipid metabolism, which inhibited follicular development and ovulation (31), others suggested higher GDF-9 levels (18).
4.1. Strengths and limitations
The main strength of our study was its focus on PAE, specifically the administration of a single dose of testosterone during a critical window of fetal development. Although previous research has documented the adverse effects of androgens on oocyte and embryo quality, only a limited number have investigated the effects of PAE. In contrast to postnatal models that rely on prolonged high-dose androgen administration, prenatal exposure models typically induce reproductive disorders following a shorter exposure period. To the best of our knowledge, this is the first study to evaluate the long-term effects of prenatal exposure to a single dose of testosterone on oocyte and embryo quality in adult rats, compared to age-matched controls. Our model, involving a single dose of testosterone exposure on the 20th day of fetal life, offers distinct advantages over models employing prolonged androgen exposure. This approach not only reduces animal stress, enhancing ethical acceptability, but also better mimics critical windows of fetal programming. Understanding how PAE affects oocyte and embryo quality may provide valuable insights into the developmental origins of female reproductive disorders and aid in identifying potential therapeutic targets.
However, several limitations should be acknowledged. A lack of assessment was observed in the quality of 2-cell embryos. Additionally, the study did not include morphological evaluations and embryo grading, and assessment of oxidative stress markers. Moreover, the expressions of other potentially related genes, such as FOXO1 and Caspase-3 were not examined. Addressing these limitations in future research may elucidate the mechanisms underlying PAE-induced reproductive dysfunction.
5. Conclusion
Prenatal exposure to a single dose of testosterone during a critical window of fetal development significantly impairs oocyte and embryo quality in adult rats. These findings indicate that PAE may be a potential risk factor for infertility by compromising reproductive competence later in life. Nevertheless, further studies are needed to validate these findings and to elucidate the underlying molecular and physiological mechanisms.
Data Availability
The datasets utilized and/or analyzed in this study can be obtained from the corresponding author upon reasonable request.
Author Contributions
E. Sadeghian Bakhi and F. Ramezani Tehrani contributed to the study conception and design. Material preparation, data collection, and analysis were performed by E. Sadeghian Bakhi and F. Ramezani Tehrani. E. Sadeghian Bakhi, N. Hayati Roodbari, M. Anvari, and F. Ramezani Tehrani were involved in reviewing the manuscript and critical discussion. The first draft of the manuscript was written by E. Sadeghian Bakhi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank the laboratory personnel of Yazd Reproductive Sciences Institute, Yazd, Iran for their help in performing all the experiments. This study was funded by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant number: 1-43010709). No artificial intelligence (AI) tools were used in the preparation, translation, or revision of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Previous studies have demonstrated that women experiencing gestational hyperandrogenemia (androgen excess), such as those with polycystic ovary syndrome (PCOS), have elevated androgen levels in cord blood; consequently, the fetus is also exposed to high levels of androgens during prenatal life (1-4). Exposure to elevated androgen levels during prenatal life can adversely affect the hypothalamic-pituitary-ovarian axis in the developing fetus, potentially resulting in long-term reproductive system dysfunction, such as polyfollicular ovarian morphology, hyperandrogenemia, and luteinizing hormone hypersecretion in offspring in their later life (5, 6).
In both animal and human studies, androgen exposure has been associated with impaired mitochondrial function and structure in the oocyte, reduced oocyte and embryo quality, impaired development of oocytes and blastocysts, and reduced fertility outcomes (7-13). However, only a limited number of studies have examined the effects of prenatal androgen exposure (PAE) on oocyte and embryo quality. Moreover, the mechanisms underlying the decline in oocyte and embryo quality following early-life androgen exposure remain poorly understood.
Growth differentiation factor-9 (GDF-9), an oocyte-derived gene, plays a pivotal role in follicular development as well as the proliferation and differentiation of granulosa cells (GCs), making it as a potential biomarker of oocyte quality (14, 15). However, previous studies have reported conflicting effects of androgen exposure on GDF-9 expression during folliculogenesis, with some indicating decrease in the GDF-9 expression (16, 17) and others suggesting higher GDF-9 levels (18) both of which may disrupt normal ovarian function. Moreover, the critical role of GDF-9 in fertility and reproductive function has been well established (19).
Due to ethical and practical constraints in human research, animals provide a controlled and feasible approach for studying the effects of PAE on oocyte and embryo quality, as well as the expression of related genes in the ovaries. Rats are considered the most appropriate model because of their well-defined genetic background, significant genomic similarity to humans, and practical advantages including small body size, ease of handling, rapid growth, and short reproductive cycles.
To the best of our knowledge, no previous study has comprehensively examined the effects of a single dose of testosterone administered during a critical window of fetal development on both oocyte and embryo quality, as well as the expression of key ovarian genes during adulthood. Although several studies have investigated the impact of prolonged or postnatal androgen exposure on reproductive outcomes (5-13), the current study aimed to investigate whether prenatal exposure to a single dose of testosterone could affect the reproductive system, especially oocyte and embryo quality in rats during adulthood.
2. Materials and Methods
2.1. Animals
10 female Wistar rats (12-13 wk, 185 ± 10 gr) were obtained from the Animal Center of Shahid Sadoughi University of Medical Sciences (Yazd, Iran). Each female rat was housed overnight with a male rat in one polypropylene cage under standard animal housing conditions (12-hr light/dark cycle, 22 ± 3oC temperature, and 45-55% relative humidity), with ad libitum access to food and water. The first day of pregnancy was confirmed by the presence of a vaginal plug.
2.2. PAE, maintenance, and studyʼs groups
The present study utilized the methodology previously established for the induction of a rat model of PCOS (5). In summary, pregnant rats were randomly assigned to either an experimental or a control group (n = 5/group). On gestational day 20, rats in the experimental group received a subcutaneous injection of 5 mg free testosterone (T1500; Sigma, Steinheim, Germany) dissolved in 500 µl of a vehicle composed of sesame oil (S3547; Sigma) and benzyl benzoate (B6630; Sigma). In contrast, control group rats were administered 500 µl of the vehicle solution alone, consisting of sesame oil and benzyl benzoate in a 4:1 ratio. After weaning period, female offspring of 2 groups, including prenatally-androgenized (PNA) rats (n = 8) and non-exposed rats (controls) (n = 8), were kept in cages in groups of 3 or 4 in standard animal housing conditions (12-hr light/dark cycle, a controlled temperature of 22 ± 3oC, and a relative humidity of 45-55%) with free access to food and water. PNA rats and their controls were followed until 70-85 days of age (adulthood) and studied in terms of oocyte and embryo quality as well as ovarian expression of the GDF-9 gene (Figure 1).
2.3. Preparation of cultivation environment
One day prior to in vitro fertilization (IVF), the culture media were carefully prepared under sterile conditions within a laminar flow hood. HamsF-10 medium was used for washing both sperm and oocytes. For oocyte-specific washing with G-MOPs medium (Vitrolife, Gothenburg, Sweden), a 60 mm dish was prepared with around 10 (50-µL) drops, subsequently covered with mineral oil (Lifeglobal, USA). IVF medium (G-IVF) (Vitrolife, Gothenburg, Sweden): A 60 mm dish was used, and about 9 drops (1 drop in the middle and 8 drops around) were placed in the dish. Following IVF, G1 medium (Vitrolife, Gothenburg, Sweden) was placed in a 30 mm dish, where 9 (20-µL) drops were added. All media were covered with mineral oil (Lifeglobal, USA) and incubated in an environment of 5% CO2 and 95% air with high humidity (Memmert, Germany), except for the G-MOPs medium, which was maintained in a standard incubator.
2.4. Preparation of sperm suspension
Sperms were collected from the caudal epididymis of mature Wistar male rats. On the day of IVF, the caudal epididymis was carefully removed and placed in HamsF-10 medium. The sperms were then incubated at 37oC for 40-60 min (20, 21).
2.5. Ovarian stimulation and collection of oocytes
Superovulation was induced in 10-12-wk-old PNA rats and their controls weighing 170-180 gr by intraperitoneal injection (i.p) of 37 IU Menopur (Ferring GmbH, Wittland11, D-24109 Kiel, Germany). After 48 hr, 50 IU human chorionic gonadotropin (Netherlands) was injected (i.p). 15-16 hr after human chorionic gonadotropin injection, the animals were sacrificed with cervical dislocation. The oviduct was immediately removed and transferred to HamsF-10 medium. Oocytes were collected by the flushing method under a stereomicroscope (Olympus.SZX16, Japan), the cumulus-oocyte complexes (COCs) were then quickly transferred to a dish containing the G-MOPS medium. Following 4-5 washes in G-MOPS medium, the COCs were transferred to IVF medium (G-IVF), washed with several drops of this medium, and placed in the central drop for fertilization. The dish was incubated in an incubator at 37oC with 5% CO2 and 95% air with high humidity (21, 22).
2.6. IVF and embryo development
After 40 min, sperms at a concentration of 1×10⁶ sperm/mL were carefully removed and returned to the incubator. Following 5 hr of incubation with the sperms, the oocytes were separated, washed, and subsequently cultured in G1 medium (21, 22).
2.7. COCs, zona pellucida (ZP) thickness, perivitelline space (PVS), and oocyte diameter measurement
To evaluate COCs, they were washed under a stereomicroscope using HamsF-10 medium, followed by G-MOPS medium, and subsequently transferred to G-IVF medium. Each sample was carefully positioned under an inverted microscope (Olympus, Japan) at 40X magnification for imaging. COCs thickness (μm) was measured at 3 different points using ImageJ software. Finally, mean values were calculated for each group (n = 8/group).
To assess ZP thickness and the PVS, the cumulus layer was enzymatically removed. In brief, cumulus masses were transferred under a stereomicroscope to a 100-μL drop of hyaluronidase (Irvine Scientific, USA) and covered with mineral oil. Pipetting was performed several times to facilitate the removal of the cumulus. The cumulus layer was removed, and ZP and PVS became visible. Samples were then imaged using an inverted microscope at 100X magnification. Subsequently, ZP thickness (μm) and PVS (μm) were measured at 3 different points using ImageJ software. Oocyte diameter was also measured using ImageJ software. Finally, mean values were calculated for each group (n = 8/group) (21, 23).
2.8. Oocytes count
After transferring the oviducts to the washing medium (HamsF-10) and releasing the COCs using the flushing method, the COCs were removed, and the number of oocytes was counted (n = 8/group) (23).
2.9. Embryonic development process
5 hr after preparation of sperm suspension and transferring of oocytes to G1 medium, embryonic development was monitored and the number of embryos was counted under an inverted microscope every 24 hr for 4 days (n = 8/group) (21). Embryo arrest was defined as the cessation of embryonic development for at least 24 hr, typically observed during the cleavage stage before blastocyst formation (24).
2.10. RNA isolation, complementary DNA (cDNA) synthesis, and quantitative real-time polymerase chain reaction (qRT-PCR)
After killing all rats, one ovary from each rat was removed, snap-frozen in liquid nitrogen, and stored at -80oC for subsequent molecular assessment.
Total RNA was isolated from the ovarian tissue using the TRIzol method. RNA quantity and purity were assessed by measuring the absorbance ratio at 280/260 nm with a NanoDrop spectrophotometer (Thermo Fisher). To synthesize cDNA, the total RNA was treated with DNase I (Thermo Fisher Scientific, USA) to prevent genomic DNA contamination. Subsequently, the total RNA was converted into cDNA using the cDNA synthesis kit (4500 YT, Yekta Tajhiz, Iran) via reverse transcription using random hexamer and initial oligo-dT reaction according to the manufacturerʼs instructions. To assay the expression of the GDF-9 gene in ovarian tissue, qRT-PCR was performed using SYBR-Green and the primers (sequences are listed in table I). Gene duplication process was done using applied biosystem and SYBR-green master mix (Ampliqon). The melting curve is used to ensure the lack of non-specific product proliferation and primer dimer. Melting curve analysis did not show any primer dimer and non-specific products in the assay. The cycle threshold was determined. The gene expression level was calculated using the 2-ΔΔCt method and using β-actin as a reference gene. The analysis of gene expression was performed using the Livak method as relative fold change.
PCR was conducted using the Rotor-Gene 6000 real-time PCR system (Corbett Research, Sydney, Australia) under a 40-cycle protocol. The thermal cycling conditions included an initial denaturation step at 95oC for 10 min, followed by a 2-step amplification cycle consisting of denaturation at 95oC for 10 sec, annealing at 58oC for 10 sec, and extension at 70oC for 20 sec. Fluorescence signals were continuously monitored throughout the reaction to enable real-time quantification of gene expression.


2.11. Ethical Considerations
This experimental study was conducted in 2024 in the biotechnology department of Research and Clinical Center for Infertility, Yazd, Iran. Animal care and handling were performed in accordance with established principles of laboratory animal welfare. Ethics Committee of Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, (Tehran, Iran) approved this study (Code: IR.SBMU.ENDOCRINE.REC.1403.038).
2.12. Statistical Analysis
Continuous variables were assessed for normality using the one-sample Kolmogorov-Smirnov test. The Mann-Whitney U test was used to compare the differences between 2 study groups. Data were presented as median and interquartile range (Q1-Q3). Statistical analysis was conducted using SPSS software (version 24.0; SPSS Inc., Chicago). The p ≤ 0.05 was considered statistically significant.
3. Results
No significant differences were observed in the number of oocytes, COCs layer thickness, PVS, and oocyte diameter in PNA rats compared to controls (Table II). A significant increase was observed in the ZP thickness in PNA rats compared to controls (Table II). Qualitatively, the stability of GCs in PNA rats was lower than control rats. Number of normal fertilization and 2-cell embryos significantly decreased in PNA rats compared to controls (Table II). Embryo arrest rate in PNA rats significantly increased compared to controls (Table II).
No statistically significant difference was observed in the ovarian expression of the GDF-9 gene between 2 study groups (PNA rats and controls) (p > 0.05) (Figure 2).


4. Discussion
In the present study, we demonstrated that prenatal exposure to a single dose of testosterone during the late gestational period led to increased ZP thickness, a higher rate of embryo arrest, reduced number of normal fertilization and 2-cell embryos, and also alterations in the density and stability of GCs, in rats in their later life (during adulthood). However, no significant differences were observed in the number of oocytes, COCs layer thickness, PVS, oocyte diameter, and the ovarian expression of the GDF-9 gene between 2 groups of study (PNA rats and controls).
It is well documented that the fetus is highly sensitive to exposure to sex steroid hormones during early development. PAE significantly affects fetal development and can lead to long-term consequences, such as reproductive system dysfunction in offspring (5, 6). Hormonal imbalances in the intrauterine environment can alter molecular pathways and gene expression levels, as well as the structure and function of developing fetal organs, potentially leading to reproductive and endocrine disorders in later life.
Previous studies have demonstrated that PAE can significantly disrupt the hypothalamic-pituitary-ovarian axis, resulting in luteinizing hormone hypersecretion, hyperandrogenism, polycystic ovarian morphology, and impaired fertility outcomes (5, 6, 25). Additionally, PAE has been associated with a reduced number of oocytes and primordial follicles, and also diminished ovarian reserve in adult females (5, 6, 8, 12, 13, 25, 26).
Consistent with our findings, both human and animal studies have shown that androgen exposure during the pre or postnatal period adversely affects oocyte and embryo quality (7, 8, 11). For example, mice implanted subdermally with dihydrotestosterone exhibited impaired mitochondrial structure and function in the oocyte, resulting in reduced oocyte quality (7). Similarly, the lean PCOS mouse model showed compromised oocyte quality, which was associated with mitochondrial ultrastructural and functional defects (8). Mouse models exposed to prenatal androgen excess demonstrated impaired oocyte and blastocyst development, and also abnormal fertilization, highlighting the detrimental effects of androgen excess on early embryonic development (10). In vitro studies have further demonstrated that testosterone propionate negatively affects oocyte maturation, as evidenced by significantly decreased maturation rates and delayed progression from germinal vesicle breakdown to metaphase II in hyperandrogenic mice (9, 27). A mouse model of PCOS, induced by PAE, exhibited poor oocyte quality, impaired embryonic development, and reduced fertility (10). Clinically, patients with PCOS showed higher miscarriage rates, suggesting that a hyperandrogenic ovarian microenvironment may adversely influence fertility and pregnancy outcomes (11). Additionally, hyperandrogenemia has been shown to impair preimplantation embryonic development, with a notable reduction in blastocyst formation rate in these hyperandrogenic patients (10). Some other studies have also shown that poor oocyte quality, reduced embryonic development, and infertility may also arise from an abnormal intrauterine environment during critical periods of fetal life (27-29). Women with PCOS and hyperandrogenism exhibit significantly lower oocyte and embryo quality, along with reduced fertilization rate (30). Furthermore, supplementation with dehydroepiandrosterone has been reported to adversely affect ovulation and oocyte quality (31).
Androgen exposure may adversely affect oocyte and embryo quality through several mechanisms. Hyperandrogenism is associated with increased accumulation of advanced glycation end products in GCs (32). Elevated advanced glycation end products lead to protein cross-linking and aggregation, disrupting cell signaling and function, ultimately leading to cell damage and death (27). Additionally, exposure to androgen excess can elevate reactive oxygen species (ROS) levels in both oocytes and GCs (33). The accumulation of intracytoplasmic ROS in these cells is associated with impaired glutathione production, which may compromise cytoplasmic maturation in oocytes (34).
Excess androgen exposure has also been shown to elevate oxidative stress levels in oocytes, increase the expression of Forkhead box protein O1 (FOXO1), and adversely affect mitochondrial energy metabolism and DNA integrity, ultimately leading to oocyte apoptosis. In oocytes from PCOS mice, expression levels of ROS, FOXO1, Caspase-3, and a marked elevation in γ-H2AX levels was observed, accompanied by a significant reduction in mitochondrial membrane potential. These findings indicated that excess androgen exposure induced oxidative stress, which subsequently induced higher expression of FOXO1, leading to increased DNA damage and apoptosis in oocytes. Furthermore, hyperandrogenic conditions in women adversely affect the metabolic status of GCs within ovarian follicles, thereby disrupting follicular growth and oocyte maturation. A study conducted on mice demonstrated that hyperandrogenism induces epigenetic changes that impair oocyte quality (27). Additionally, elevated concentrations of testosterone in follicular fluid have been implicated in compromising oocyte and embryo quality, as well as lowering fertilization rates. These effects may be mediated through alterations in the transcriptional levels of aryl hydrocarbon receptor signaling downstream genes. Moreover, studies have shown that fatty acid and cholesterol synthesis may be inhibited in the ovarian GCs of women with hyperandrogenism, potentially disrupting oocyte maturation (30).
Insulin resistance (IR) may represent an additional pathway that contributes to the reduced quality of oocytes and embryos associated with hyperandrogenism. It is well-documented that women with hyperandrogenism, particularly those with PCOS, commonly exhibit IR (35). The presence of IR and decreased glucose concentrations in follicular fluid impair glucose transfer and energy metabolism in oocytes and follicular cells, ultimately resulting in reduced oocyte and embryo quality (36). Consequently, IR may be regarded as an indicator of poor oocyte maturation, suboptimal embryo quality, and prolonged stimulation duration. It has been reported that women with IR require higher doses of gonadotropins and a longer stimulation period to achieve follicular maturation (37).
In this study, we observed that the expression of GDF-9 gene in the ovaries of PNA rats did not differ significantly to that of control group. GDF-9, a paracrine factor secreted by ovarian follicles, plays a critical role in follicular development, GCs proliferation and differentiation, and the overall process of folliculogenesis in mammals (38). However, a lack of consensus was observed regarding GDF-9 expression in studies investigating the effects of androgen exposure. While one study found that follicles cultured with dehydroepiandrosterone showed reduced expression of genes related to oocyte maturation, such as GDF-9 and bone morphogenetic protein 15, possibly due to impaired steroid hormone synthesis and lipid metabolism, which inhibited follicular development and ovulation (31), others suggested higher GDF-9 levels (18).
4.1. Strengths and limitations
The main strength of our study was its focus on PAE, specifically the administration of a single dose of testosterone during a critical window of fetal development. Although previous research has documented the adverse effects of androgens on oocyte and embryo quality, only a limited number have investigated the effects of PAE. In contrast to postnatal models that rely on prolonged high-dose androgen administration, prenatal exposure models typically induce reproductive disorders following a shorter exposure period. To the best of our knowledge, this is the first study to evaluate the long-term effects of prenatal exposure to a single dose of testosterone on oocyte and embryo quality in adult rats, compared to age-matched controls. Our model, involving a single dose of testosterone exposure on the 20th day of fetal life, offers distinct advantages over models employing prolonged androgen exposure. This approach not only reduces animal stress, enhancing ethical acceptability, but also better mimics critical windows of fetal programming. Understanding how PAE affects oocyte and embryo quality may provide valuable insights into the developmental origins of female reproductive disorders and aid in identifying potential therapeutic targets.
However, several limitations should be acknowledged. A lack of assessment was observed in the quality of 2-cell embryos. Additionally, the study did not include morphological evaluations and embryo grading, and assessment of oxidative stress markers. Moreover, the expressions of other potentially related genes, such as FOXO1 and Caspase-3 were not examined. Addressing these limitations in future research may elucidate the mechanisms underlying PAE-induced reproductive dysfunction.
5. Conclusion
Prenatal exposure to a single dose of testosterone during a critical window of fetal development significantly impairs oocyte and embryo quality in adult rats. These findings indicate that PAE may be a potential risk factor for infertility by compromising reproductive competence later in life. Nevertheless, further studies are needed to validate these findings and to elucidate the underlying molecular and physiological mechanisms.
Data Availability
The datasets utilized and/or analyzed in this study can be obtained from the corresponding author upon reasonable request.
Author Contributions
E. Sadeghian Bakhi and F. Ramezani Tehrani contributed to the study conception and design. Material preparation, data collection, and analysis were performed by E. Sadeghian Bakhi and F. Ramezani Tehrani. E. Sadeghian Bakhi, N. Hayati Roodbari, M. Anvari, and F. Ramezani Tehrani were involved in reviewing the manuscript and critical discussion. The first draft of the manuscript was written by E. Sadeghian Bakhi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank the laboratory personnel of Yazd Reproductive Sciences Institute, Yazd, Iran for their help in performing all the experiments. This study was funded by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant number: 1-43010709). No artificial intelligence (AI) tools were used in the preparation, translation, or revision of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |