Wed, Jan 28, 2026
[Archive]
Volume 23, Issue 10 (October 2025)
IJRM 2025, 23(10): 787-802 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fares K S, Tayeh G H A, Whaibeh E R, Jaalouk L Y, Matar Y M, Mrad M A. The influence of environmental and chemical exposures on fertility in the Eastern Mediterranean region: A narrative review. IJRM 2025; 23 (10) :787-802
URL: http://ijrm.ir/article-1-3548-en.html
URL: http://ijrm.ir/article-1-3548-en.html
Kassandra Said Fares1 

 , Georges Hani Abi Tayeh *2
, Georges Hani Abi Tayeh *2 

 , Emile Roger Whaibeh3
, Emile Roger Whaibeh3 

 , Lina Yasser Jaalouk4
, Lina Yasser Jaalouk4 

 , Yara Matar Matar4
, Yara Matar Matar4 

 , Myriam Andraos Mrad5
, Myriam Andraos Mrad5 




 , Georges Hani Abi Tayeh *2
, Georges Hani Abi Tayeh *2 

 , Emile Roger Whaibeh3
, Emile Roger Whaibeh3 

 , Lina Yasser Jaalouk4
, Lina Yasser Jaalouk4 

 , Yara Matar Matar4
, Yara Matar Matar4 

 , Myriam Andraos Mrad5
, Myriam Andraos Mrad5 


1- Higher Institute for Public Health, Saint Joseph University of Beirut, Beirut, Lebanon. & Saint Joseph Fertility Center, Beirut, Lebanon.
2- Saint Joseph Fertility Center, Beirut, Lebanon. & Health and Environment Response Agency, HERA, Lebanon. Faculty of Medicine, Saint Joseph University of Beirut, Beirut, Lebanon. Unit of Obstetrics and Gynecology and Fertility, Hotel-Dieu de France Hospital, Beirut, Lebanon. ,georges.abitayeh@hera-me.org
3- Higher Institute for Public Health, Saint Joseph University of Beirut, Beirut, Lebanon. & Health and Environment Response Agency, HERA, Lebanon. Public Health Department, Faculty of Health Sciences, University of Balamand, Beirut, Lebanon.
4- Public Health Department, Faculty of Health Sciences, University of Balamand, Beirut, Lebanon.
5- Health and Environment Response Agency, HERA, Lebanon. & Public Health Department, Faculty of Health Sciences, University of Balamand, Beirut, Lebanon.
2- Saint Joseph Fertility Center, Beirut, Lebanon. & Health and Environment Response Agency, HERA, Lebanon. Faculty of Medicine, Saint Joseph University of Beirut, Beirut, Lebanon. Unit of Obstetrics and Gynecology and Fertility, Hotel-Dieu de France Hospital, Beirut, Lebanon. ,
3- Higher Institute for Public Health, Saint Joseph University of Beirut, Beirut, Lebanon. & Health and Environment Response Agency, HERA, Lebanon. Public Health Department, Faculty of Health Sciences, University of Balamand, Beirut, Lebanon.
4- Public Health Department, Faculty of Health Sciences, University of Balamand, Beirut, Lebanon.
5- Health and Environment Response Agency, HERA, Lebanon. & Public Health Department, Faculty of Health Sciences, University of Balamand, Beirut, Lebanon.
Full-Text [PDF 435 kb]
(427 Downloads)
| Abstract (HTML) (286 Views)
Full-Text: (1 Views)
1. Introduction
Reports indicate that infertility, which affects more than 70 million couples around the globe, is a growing medical and social challenge (1). Moreover, this issue has been found to worsen over the years in the Eastern Mediterranean Region (EMR), comprising 22 countries with over 597 million people by 2013 (2, 3). For instance, in Lebanon, the fertility rate dropped from 3 in 1993 to 2.1 in 2020 (4). Similarly, a sample from Babol, Iran, showed that more than 120,000 new infertile couples are registered every year (5). In 2015, a meta-analysis of 13 papers involving 55,658 Iranian couples found a lifetime infertility frequency of 13.2%, which is markedly higher than the global rate of 10.5% (6). In the Kingdom of Saudi Arabia (KSA), 18.93% of couples in the reproductive age failed to achieve at least one live birth (7).
Consequently, centers that offer assisted reproduction technologies (ART), especially intracytoplasmic sperm injection have become widely available in the region (8). A Lebanese survey demonstrated that most infertile couples (87.5%) opt for ART, which are socially and religiously accepted (82.2% and 66.2%, respectively) (9).
Several environmental and occupational exposures have been suggested to interfere with reproduction. Few reviews have published data concerning these factors among infertile couples living in the EMR. For men, environmental, industrial, and air pollution can decrease sperm counts, while for women, ovarian dysfunction is a leading factor (10). A review of evidence among Qatari couples highlighted the impact of smoking, coffee and alcohol consumption, stress, body weight, and environmental exposures (11). Another review focused on the effect of multiple trace elements necessary for normal spermatogenesis, adequate levels of sex hormones, and satisfactory sperm characteristics (12). The available reviews describe the results of some EMR countries, or are limited to specific environmental exposures, or study either infertile men or infertile women separately. Since previous research published contains little data on their effect on the embryological and clinical outcomes of ART, a broader review, in scope, is crucial to identify literature gaps and guide research in this area. For that purpose, we focused on existing information concerning these risk factors in the EMR. Findings were divided into 2 reviews: the first one focused on the dietary habits and nutritional supplementation and their impact on fertility (13). The second discussed the influence of chemical, climatic, and other environmental exposures on the reproductive functions.
The literature search was conducted using PubMed and Scopus databases. Publications from January 2012-June 2024 were considered. A total of 63 studies were found eligible for the synthesis. Around 59% (n = 37) of the publications evaluated male factor infertility, while almost 36% (n = 23) examined the outcomes of infertile women. The search was constrained in scope and depended on specific databases and keywords, without the application of formal inclusion/exclusion criteria or quality assessment of studies. Consequently, certain references may have been involuntarily excluded. The findings should be regarded as a summary and synthesis of specific evidence rather than a systematic review.
2. Temperature
Climatic factors, including temperature, have a detrimental impact on fertility rates (14-17). A cross-sectional study conducted on 3670 women showed that seasonality affects reproduction without any particular pattern, mainly due to its temperature and photoperiod parameters (15).
For instance, in summer, sperm concentration and motility were lower than usual compared to the number of metaphase II oocytes, which was relatively higher (15, 18). A correlational study conducted on 51,065 women found that a global warming-induced temperature increase has negative effects on human fertility. On one hand, cool environmental conditions were found to have a positive impact on the quality and quantity of the spermatogenesis process in men; whereas, a lively lifestyle with jobs depending on agricultural productions led to efficient economic conditions, which in turn promoted better access to calorie sources, resulting in higher productivity potential for women (16). Cross-sectional studies conducted in Pakistan and KSA on 360 and 137 males, respectively, explain that the successful spermatogenesis process depends on scrotal temperature stability; any increase in scrotal temperature as compared to the normal core body temperature can lead to an impairment in sperm production and function (17, 19). Moreover, males exposed to high temperatures in their jobs, like bakers, textile industry workers, and ceramic oven operators, were more likely to have poor sperm qualities, a loss of libido, and high levels of stress, which in turn led to a disturbance in the nervous system and the hormonal equilibrium (19). Similarly, 2 cross-sectional studies found that in such jobs, exposure to harsh temperatures in addition to stressful work conditions, including financial, psychological, chemical, and physical ones, also lead to increased abortion rates, decreased sperm motility, and abnormal sperm morphology, with defects in the tail and head (17, 20). While other studies shed light on how occupational exposures, coupled with varicocele for infertile males, resulted in decreased semen parameters and ubiquitination median, which is used as a marker to measure the ubiquitin-proteasome system’s function. It was shown that a group of 90 infertile men exposed to high temperatures and a group of 51 infertile men with different grades of varicocele, had a significantly lower mean of sperm parameters when compared to a control group of 60 normospermic men who did not present varicocele and were not exposed to any chemicals (21).
3. Chemical exposures
3.1. Bisphenol A (BPA)
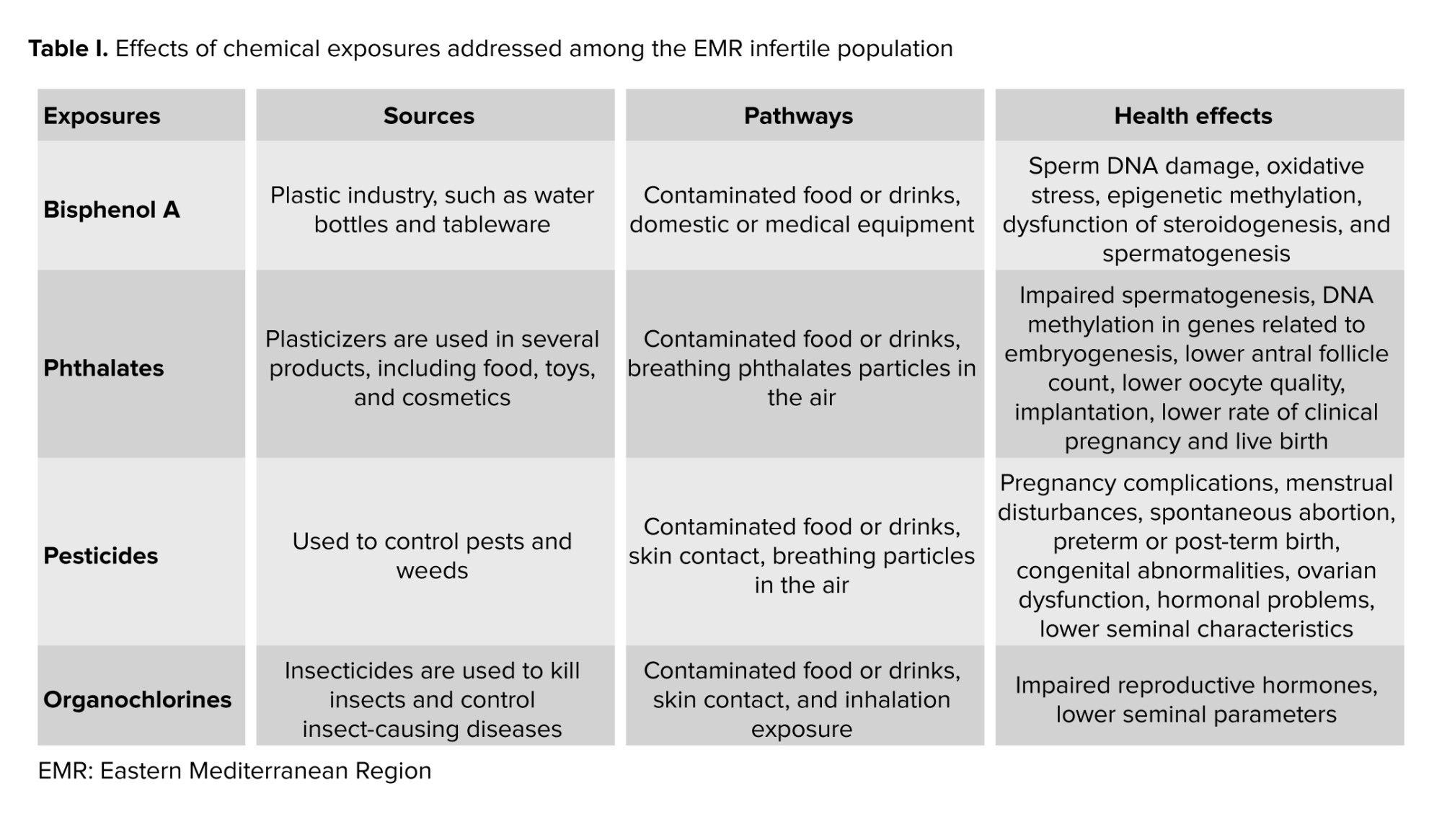
Exposure to chemicals, like BPA, a substance used in the plastic industry, which may enter the body through contaminated food or drinks, domestic or medical equipment, and occupational sources, also has adverse effects on fertility (Table I). For instance, BPA was positively linked to the deoxyribonucleic acid (DNA) damage and negatively correlated to semen quality and antioxidant levels. In fact, 2 case-control studies discussed the BPA’s severe effects among Egyptian groups. They explained that BPA tends to bind to estrogen receptors, thus inducing non-genomic steroid action. Consequently, because of the exposure to BPA, testicular testosterone levels decrease, and the risk for prostatic hyperplasia and predisposition to carcinoma increases. Moreover, it was shown that when taken in, BPA leads to errors in the hypothalamo-pituitary-gonadal axis and sperm functions through increased sperm DNA damage, oxidative stress, and epigenetic methylation, in addition to a potential dysfunction of steroidogenesis and spermatogenesis (22, 23). Also, all of the 50 infertile men had increased levels of urinary BPA as compared to the 50 fertile controls, and in turn exhibited notable distinctions in terms of sperm morphology, motility, count, and oxidative stress (22). High urine BPA also raised levels of sex hormone binding globulin and estradiol, while lowering levels of active androgens (24).
3.2. Phthalates
Phthalates are industrial chemicals, also known as plasticizers, used in several consumer products, including food, toys, and cosmetics (Table I). They are known to have reproductive and developmental health risks. In fact, in a cross-sectional study done in KSA, after exposure to phthalates during fetal and early development, alterations in testis development and function can happen, thus affecting a male’s fertility (25). Their findings showed that exposure to phthalates suppresses the level of Leydig cells, which play a crucial role in the production of growth factor 3 and testosterone, leading to certain aspects of testicular dysgenesis syndrome, like impaired spermatogenesis.
Mono(2-ethyl-5-oxohexyl) phthalate, one of the Di-(2-ethylhexyl) phthalate metabolites, has been measured in Jordanian females urine. Its exposure, stemming from microwave heat and cosmetic products, shows an association with infertility (26). Results of over 500 Saudi individuals, both males and females, pinpointed that being exposed to phthalates could also be associated with DNA methylation in genes related to embryogenesis, with lower egg count, egg quality, implantation, clinical pregnancy, and live birth rates, and with polycystic ovarian syndrome (PCOS) in humans (27-29).
Moreover, it was found that increased levels of certain phthalates (like the percentage of mono-2-ethylhexylphthalate being less than 29%) in women led to increased risks of biochemical pregnancy, in addition to failed clinical pregnancy and live birth. For instance, mono-2-ethylhexylphthalate exposure was positively associated with failed clinical pregnancies in 599 couples undergoing in vitro fertilization (IVF) (30).
3.3. Pesticides
Pesticides, substances used to control pests in agriculture, are also known to have adverse effects on a person’s reproductive health (Table I). In 2020, a study on Iranian women working in greenhouses found that reproductive disorders, including pregnancy complications, menstrual disturbances, reduced fertility, stillbirth, spontaneous abortion, preterm or post-term birth, congenital abnormalities, ovarian dysfunction, and hormonal problems, are all more common among women in agricultural-related industries. For example, the authors even showed that ovary or fallopian tube problems were 4 to 16 times higher among women who worked in agriculture than in those who worked in different fields. It was also shown that exposure to pesticides could decrease the number and motility of Y-bearing sperm, thus affecting the fetus’s gender, increasing the odds of getting a girl (31).
In Tunisia, one study on 2122 males showed how pesticides can impact the post-testicular sperm maturation, which involves the epididymis, prostate, and seminal vesicles, and eventually lead to an increase in pH, and a decrease in seminal volume, motility, percentage of normally shaped sperm, and seminal zinc (Zn) concentration (20). Furthermore, individuals who were exposed showed higher levels of DNA breakage and chromatin decondensation (32). They can also disrupt the sex hormonal activities, thus resulting in the secretory dysfunction of male accessory glands (20). In particular, organochlorine pollutants, including dichlorodiphenyltrichloroethanes (DDTs), polychlorinated biphenyls, and hexachlorobenzene essentially used for pest control in agriculture, are known to affect a male’s reproductive system negatively. For instance, DDTs and their respective metabolites have high androgenic and estrogenic activities, acting as hormonal receptor antagonists or agonists, thus hindering the work of reproductive hormones. Increased serum levels of polychlorinated biphenyls are shown to decrease seminal parameters, and hexachlorobenzene was seen to be associated with genital abnormalities such as hypospadias and with defects in sperm motility (33).
3.4. Trace elements
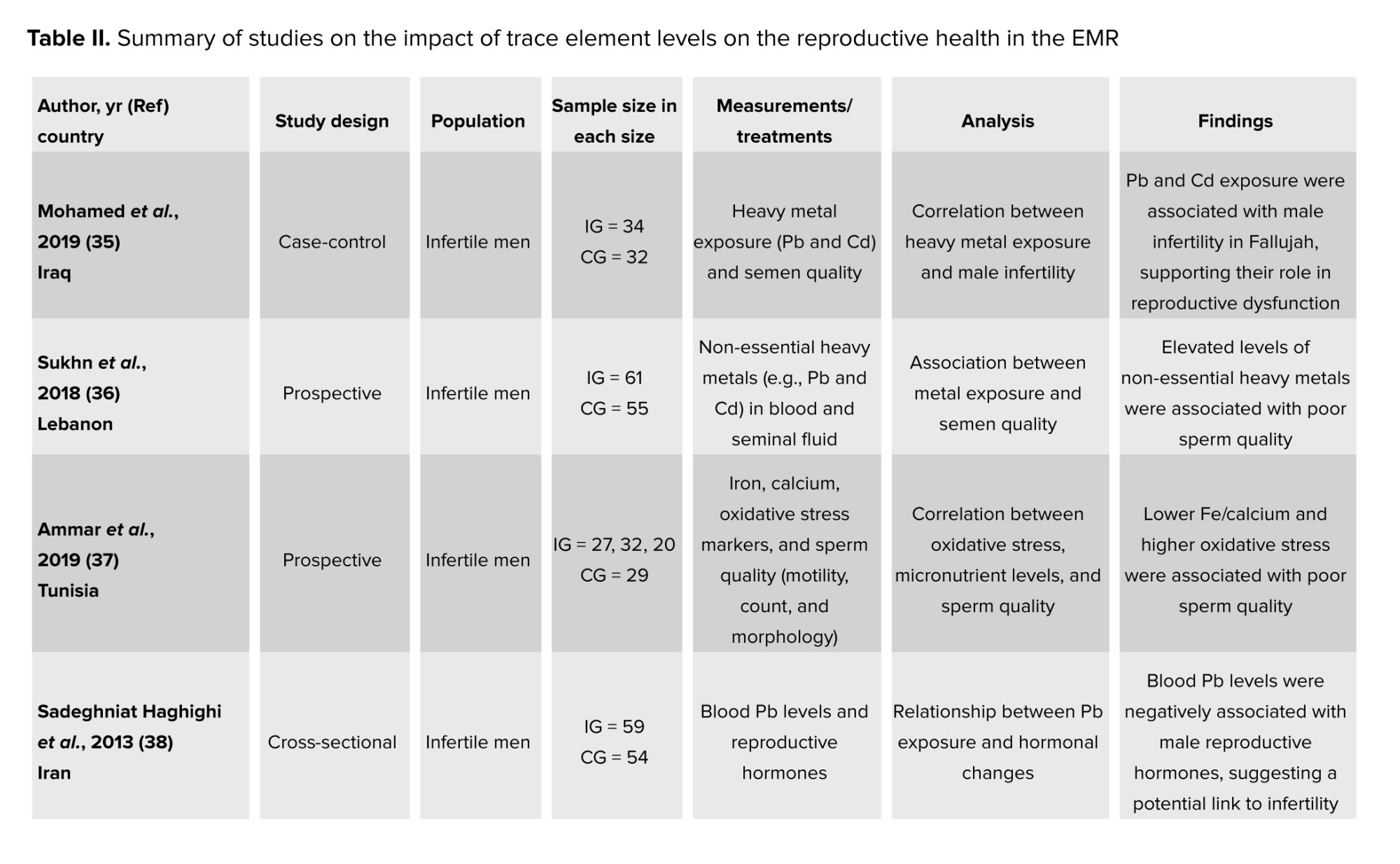
Trace elements, also known as trace metals, are minerals found in living organisms in minor quantities. If ingested in significant amounts over extended periods, any trace element can become harmful (34). Many publications focused on their impact on reproductive health in the EMR (Table II). In fact, these metals can affect human reproductive health in 2 ways: either directly, by targeting set reproductive organs, or indirectly, by working on the neuroendocrine system (35). Nonetheless, in general, these metals have been associated with a decline in fertility rates and were believed to negatively affect fine-tuning of biochemical reactions responsible for the regulation of the function of the male reproductive system (36, 37). In their case-control study, trace elements were found in higher levels in the 64 infertile males as compared to the 32 fertile men (35).
Lead (Pb), for instance, was also found to have adverse effects on different systems of the human body, especially the reproductive one (38). Constantly being subjected to Pb, such as painters, has the potential to impact the quality of semen, particularly affecting sperm movement (motility) and causing an elevation in testosterone levels. This also damages Leydig and Sertoli cells, thus impairing testosterone formation and the process of spermatogenesis, respectively, and limits the motility of sperm (35, 36, 39). Similarly, in a cross-sectional study, 85 welders (exposed to Pb fumes) had much higher blood Pb levels than the 80 administrative staff (not exposed), as well as a relatively lower sperm count and motility than the control group (40).
Pb was also shown to result in the absence of libido in males and issues, and spontaneous abortions in females (38). Moreover, Pb coupled with nickel was found to be in significantly higher amounts in the 70 women with PCOS than in the 50 control participants. This can be explained by the fact that these trace elements increase the reproductive hormone levels that relate to oxidative stress and hinder the physiological balance, allowing for deregulation of glucose via reactive oxygen species (ROS) pathways (41).
Cadmium (Cd) was also found to have negative effects on male fertility rates. The presence of Cd is linked to an increased amount of ROS that surpasses the antioxidant defense, in addition to a decreased number of sperm. In their case-control study, the authors observed augmented levels of Cd in the 64 infertile participants as compared to the 37 control individuals. This was linked to an increase and an imbalance in the levels of ROS, which led to oxidative stress, resulting in lipid peroxidation, alterations in DNA and protein structures, and sperm death. This result can be explained through Cd’s ability to compete with essential metals over protein-binding sites, leading to the release of Fe2+ and Cu2+ ions, which induce the production of ROS, and in turn, increase oxidative stress (42).
Iron (Fe) was thought to decrease the motility and the quality of sperm, by inducing lipid peroxidation and forming hydroxyl radicals on one hand, and to affect women’s infertility on the other (37, 43, 44). To better understand this mechanism, a prospective control study found a positive and significant association between Fe and the multiple anomalies index and seminal leucocyte concentration. In fact, Fe concentrations in the seminal plasma of the 79 sub-fertile individuals were significantly higher than in the 29 healthy participants. As such, the authors further concluded that trace element (including Fe) excess is implicated in low sperm quality (37).
Moreover, tin was also believed to affect the quality of human sperm by reducing the creatine kinase activity, which in turn allows for the male reproductive health to regress. Among the 75 Pakistani males, tin counts were the highest among azoospermia individuals as compared to oligozoospermia and normozoospermia ones (45).
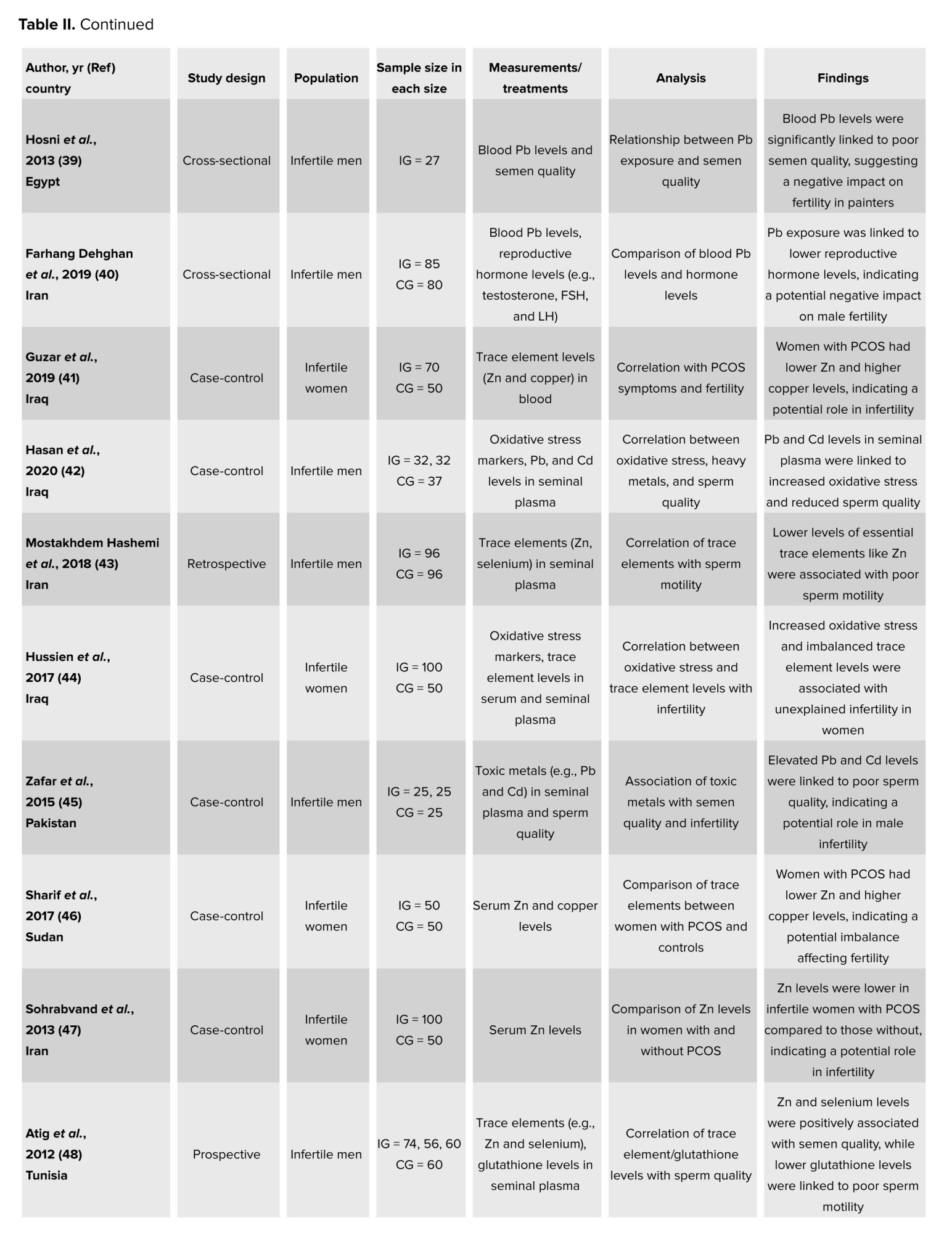
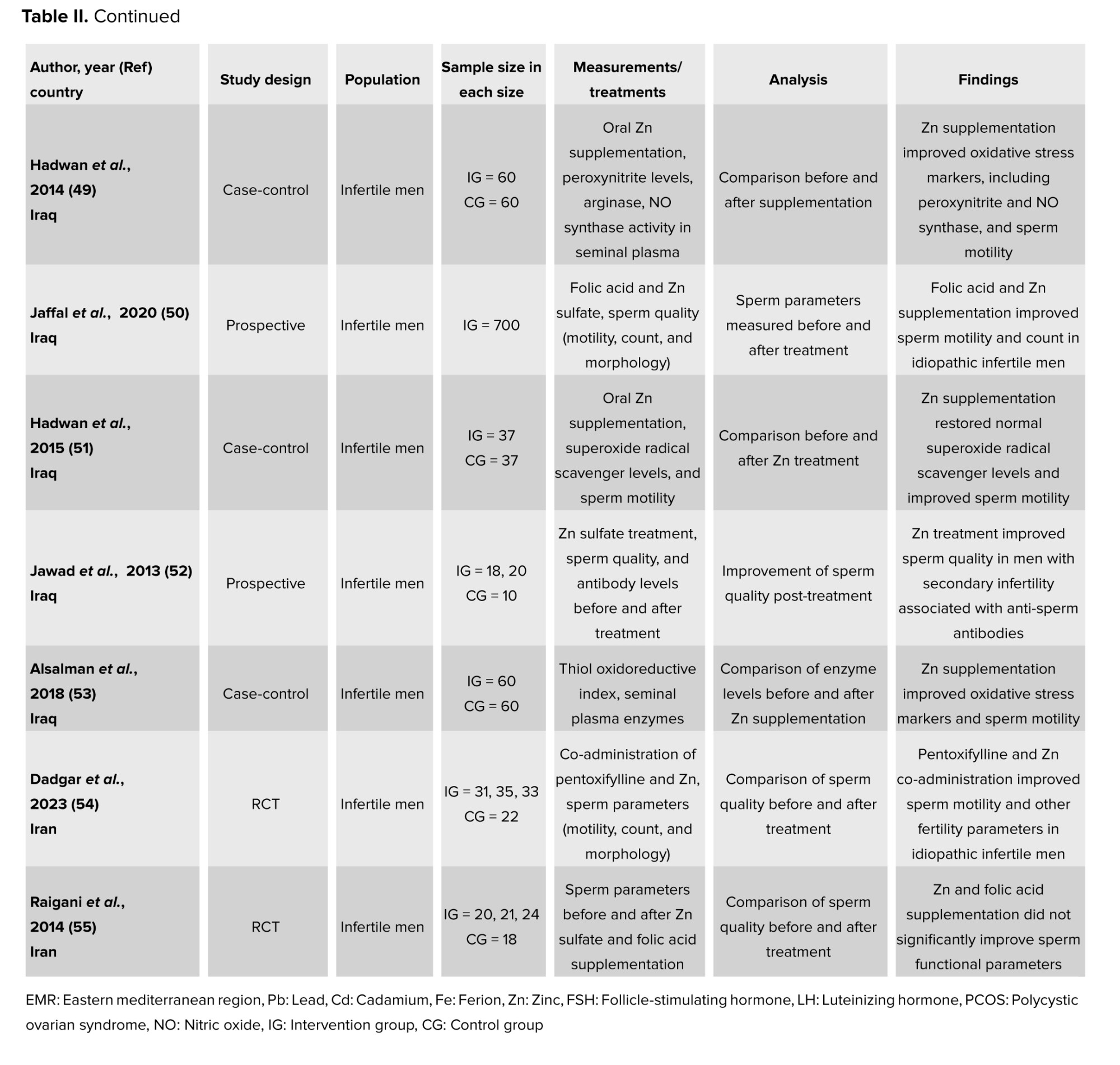
On a more positive note, other metals like Zn were proven to have a positive impact on people’s reproductive health. For instance, unlike the presence of other trace elements, Zn did not have any negative influence on women with PCOS (46, 47). In fact, in their case-control study, no difference was observed in the serum Zn levels among the 50 PCOS women and the 50 control women (46). Furthermore, a standard amount of Zn is crucial for several reproductive processes like the steroidogenesis, testis development, synthesis and secretion of sexual hormones, differentiation of gonads, spermatozoa maturation, acrosome function, and its fertilization capacity (48, 49). This can be reflected in a case-control study, which showed the lower seminal Zn levels in the 190 infertile men as compared to the 60 normospermic men (48). Moreover, Zn coupled with sulfate was also shown to play a huge role in sperm physiology functions, like shaping the sperm membrane, increasing sperm motility, and enhancing seminal fluid parameters (49-51). In a prospective study, 700 men who completed the Zn sulfate and folic acid treatment after 3 months recorded an increase in sperm concentrations and sperm motility as compared to when they first began (50). It was even explained that Zn sulfate treatment can be used to help infertile males suffering from anti-sperm antibodies, as the results of his study reported a decrease in the level of serum and seminal plasma anti-sperm antibodies, in the 38 infertile men who were administered the Zn sulfate treatment over 45 days as compared to the 10 control individuals whose seminal levels remained intact (52). In addition, oral Zn supplementation can augment the normal morphology and reproductive hormones and protect sperm chromatin (53, 54). This was shown in a randomized clinical trial, where the 68 participants who were given either Zn or pentoxifylline + Zn as a treatment for 3 months reported a significant reduction in DNA fragmentation as compared to the 31 individuals who took pentoxifylline alone and the 22 controls. This is because Zn connects protamines with Zn bridges; imidazole groups of histidine and thiol groups of cysteine, to be exact (54). In contrast, while Zn sulfate and folic acid do enhance conditions for sperms, the male reproductive health cannot be improved only by resorting to this kind of treatment, as the impairment is the result of many factors. Hence, in order to ameliorate it effectively, all aspects must be tackled (55). In conclusion, the summarized articles presented a well-developed insight into the different effects, be they positive or negative, that trace elements have on the reproductive health of humans.
4. Air pollution
There remain other factors that also affect male infertility. Air dust pollution has a negative influence on male sperm quality. The findings in their retrospective analytical study, conducted among 850 infertile males in Iran over 4 yr, suggested a significantly negative correlation between air dust pollution and sperm morphology and concentrations (56). Moreover, air pollutants also impact women’s fertility and its exposure to them can lead to stillbirth, miscarriage and fetal loss, preterm delivery, reduced fertility rate, and impaired fetal growth. In their cross-sectional study on 67 women living in a polluted area, the authors showed that long-term exposure to particulate matter (PM) PM1 and PM2.5 was linked to a decrease in the levels of the serum anti-Mullerian hormone, which reflects an individual’s egg count. Not only that but exposure to PM2.5 was also shown to lead to systemic inflammation, portrayed through a variance in the levels of Interleukin-6 and tumor necrosis factor -alpha, in addition to morphological changes in ovarian tissue. This, in turn, can result in ovarian injuries (57).
5. Smoking
Controversial results regarding the effect of smoking frequency on sperm parameters were found. Many studies compared the outcomes of smokers and non-smokers. Smoking water pipes or hookah slightly decreased the semen volume, morphology, and progressive motility (58). Women hookah users did report considerably higher period discomfort (59). In men, hookah enhanced ROS production while decreasing water-soluble antioxidants, resulting in oxidative stress in sperm plasma membranes (60).
A large group of smokers confirmed the negative impact of smoking on motile and normally shaped sperm (61-64). A case-control study on 960 adult males focused on the significant difference seen among heavy smokers (> 20 cigarettes/day), especially in the values of sperm concentration and the percent of shaking and immotile spermatozoa, which was lower in comparison to non-smokers (61). Heavy smokers also exhibited higher leukocyte inflation than other subgroups. In another case-control study, Cd and Zn levels were evaluated among smokers for their potential effect on sperm parameters. This is because cigarettes are known to be a major cause of Cd toxicity and are related to Zn deficiency. As such, the results demonstrated lower levels of Zn in the 35 smokers as compared to the 35 non-smokers. Furthermore, the changes in sperm motility, viability, and morphology, opposite to Cd, were positively linked to higher serum and seminal levels of Zn (65).
In addition, non-smokers had lower concentrations of Pb and Arsenic in seminal fluid, and better sperm count and viability, respectively (66). 2 case-control studies conducted in KSA and Pakistan, respectively, and one cross-sectional study conducted in Pakistan, addressed the variation of reproductive hormones of men who smoke cigarettes, dip tobacco, or cannabis (62-64). All types of drugs were correlated to lower levels of testosterone, luteinizing hormone, cortisol, and sex hormone-binding globulins in smoker infertile men vs. non-smoker infertile men as compared to higher levels of prolactin and follicle stimulating hormone in smoker participants, especially those who smoke cannabis (62-64). An Egyptian study found that sperm motility has been affected negatively by tobacco, and it decreased drastically. In contrast, it has been affected positively by tramadol and cannabis as measured by both traditional and computer-assisted semen analysis techniques (67). The spermatozoa movement of 64 smokers was compared to that of 84 non-smokers. The weakened motility found in the first group was attributed to lower sperm creatine kinase activity and impaired energy production (68). Many studies investigated DNA methylation changes in sperm (69-71). Investigating the DNA methylation and transcription of several gene expressions in 15 infertile smoker men, significant changes were noticed in the methylation of all 9, 18, 7, 17, and 3 cytosine-phosphate guanine sequences within PRICKLE2, ALS2CR12, ALDH3B2, PTGIR, GAA, MAPK8IP3, and ANXA2 genes amplicon, respectively (69, 70). Similarly, evidence on other altered gene expressions, such as PGAM5, PTPRN2, TYRO3, was found, but no correlation was observed between smoking and H2BFWT, PRM1, and 2 gene variants (72, 73). Smoking at least 25 cigarettes per day for at least 10 yr also leads to poor DNA integrity by altering sperm chromatin condensation (69, 70, 74). Finally, it was shown that cigarette smoking also increases nitric oxide and malondialdehyde levels in normospermic smokers, which in turn were found to reduce sperm motility (75). As for the embryological results, 3 groups of 50 couples were assessed: overweight infertile men, overweight men who smoke, and controls. In the second group, higher levels of abnormal sperm shape were associated with lower numbers of fertilization, pregnancy, and embryos of good quality (76). In contrast, 2 studies gathering infertile women and men did not find a significant correlation between smoking and infertility (77, 78).
Broadly, this review found that environmental exposures and other lifestyle-related pollutants have negative impacts on the reproduction of EMR couples. Studies showed how increased temperatures lead to impairments in sperm production and function, as well as elevated rates of abortion. These findings align with research conducted in Europe; for example, after analyzing data from 32 European countries, for over 244 million live births between 1969 and 2021, high temperature was suggested to increase the risk of miscarriage and leave detrimental impacts on live births (79). Most of the available study designs recruiting patients from the EMR are cross-sectional in nature, which limits the ability to infer causality due to potential confounding factors. Prospective cohort and longitudinal studies should be prioritized in future research.
This is concerning as the EMR, similarly to the rest of the globe, is experiencing climate change and rising temperature patterns, which paired with limited access to reproductive health services, can negatively affect fertility rates in the region. As for chemicals, BPA has a positive relation with DNA damage and a negative one with semen quality and antioxidant levels. The literature also showed the negative impact that BPA has on fertility (80, 81). Surprisingly, BPA levels found in the urine samples in Egypt were notably higher than levels found in samples in the US and China (82-84). This discrepancy can be attributed to lifestyle differences with increased use of plastics in Egypt, which is a common pattern in many other low-income countries in the EMR.
Moreover, the review outlines phthalates ability to alter testis development in males and induce failed clinical pregnancy and live birth in females. Similar findings are outlined in studies conducted in the US, Korea, and Canada, but with lower levels of exposure (85-88). These findings can be attributed to several factors, which include lifestyle changes as well as cultural practices, dietary habits, and personal care product use, as well as governmental legislation regulating phthalate use. A key limitation of several EMR studies is the lack of confounding factors adjustment, which might exaggerate or obscure the observed associations between chemical exposures and infertility. The control for body mass index, smoking status, maternal age, alcohol consumption, and physical activity are important covariates identified in the literature. The impact of trace element deficiency or the outcome of their high exposures is still poorly understood. In men, similar findings of both positive and negative effects of micronutrients on semen characteristics were summarized (89). According to 3 publications, pesticides, as well as air pollution, are also found to have negative effects on fertility, as they lead to reproductive disorders, including pregnancy issues in females, and decrease seminal parameters and increase genital abnormalities in males. Similar findings were identified in Barcelona, Spain, which showed that exposure to air pollution, resulting from different sources, can lead to a significant reduction in fertility rates (90). In an overview on pesticides, organochlorines such as dieldrin, DDT, and aldrin showed a wide-ranging impact on ecosystems and pose threats to human health, including fertility, circulatory, neurological, hormonal, and endocrine functions (91). There remains a dearth of literature on air pollution’s impact on perinatal health in the EMR, and even fewer studies have been conducted on its impact on fertility, which is problematic since air pollution continues to be a critical environmental and public health problem in the region (92). For instance, the annual average of PM10 and PM2.5 concentrations in both Beirut and Tabriz cities surpassed the standard values set by the World Health Organization (93, 94). Although EMR studies investigating pesticide contamination and fertility impairment represent a limited sample size, they provide important preliminary insights and reflect the challenges of conducting environmental health research in this region. Future research with larger sample sizes is necessary to validate existing findings regarding air pollution and pesticide exposure.
This review also highlighted the negative reproductive health outcomes associated with smoking, which aligns with international literature (95). For example, within the recruitment of 1222 Russian men, heavy smokers (> 10 cigarettes/day) had decreased total sperm counts and sperm concentrations (96). Moreover, smoking, which is a major cause of Cd toxicity, is also of a retrogressive effect on male reproductivity, as it can lower levels of testosterone and weaken sperm movement (65). Smoking is an exposure of particular importance in the EMR as the number of smokers continues to grow, with the prevalence of tobacco use ranging between 8.1% in Oman and 35% in Jordan and Lebanon. The high prevalence of tobacco use in the EMR can be partially attributed to the popularity of waterpipe or hookah smoking, which has a reduced harm perception and inadequate policies and regulations (97).
Based on this review, it is evident that the data on the effects of environmental exposures on male reproductive health were more prevalent. The ease of semen sample collection and the fewer ethical restrictions related to male reproductive health investigations explain this imbalance in the research distribution. Only a few studies focused on occupational exposures such as PBA, phthalates, and pesticides, and they indicated a reduced performance of the human reproductive system and lower numbers of successful artificially induced pregnancies. The effects of trace elements included more information about women with PCOS but did not tackle assisted reproductive technology or IVF methods.
As for smoking, some publications on men found a small impact on semen parameters, while others mentioned that heavy smokers might witness fertility issues. Although no specific definition was observed for heavy smoking, the overall trend consistently indicated that frequent smoking habits are associated with reduced fertility. These publications delivered the results of small groups of participants, which is why a bigger sample size and dose-response assessment protocols might be more helpful in proving the actual impact of smoking frequency. Also, only one paper correlated the effect of smoking with clinical outcomes of IVF cycles. As such, further studies on the ART results are much needed, along with more observational studies among healthy or infertile smoking women. In general, case-control and cross-sectional studies were most used in tackling those factors. While each serves a different purpose, together, they can give a comprehensive overview of the matter. This aligns with the limitations observed by a previous scoping review on perinatal health in the EMR (92).
6. Conclusion
Despite some environmental aspects being extensively studied across different populations, there are still significant limitations in terms of the number of studies and the data itself derived from the EMR. As such, more research is needed to set instructions for daily routines of people and set guidelines on the use of chemicals for all people working in agriculture, manufacturing, and other industries with high risks of exposure. Also, the EMR is witnessing rapid population growth and requires better public health management.
Addressing the vast health, cultural, and social discrepancies within the region can be instrumental in enhancing public awareness, education, policy, and regulation. This, in turn, can inform decision-makers, helping them adopt new guidelines, setting standards for air and water quality, waste management, and industrial emission regulations, and decreasing the adverse impacts on human fertility. Overall, by conducting further research on this topic, researchers can contribute to enhance the quality of life and have more effective and sustainable strategies to tackle the region’s problems.
Acknowledgements
The authors affirm that no artificial intelligence tools were used in the writing, editing, data analysis, or preparation of this manuscript. All work was conducted and prepared by the authors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Reports indicate that infertility, which affects more than 70 million couples around the globe, is a growing medical and social challenge (1). Moreover, this issue has been found to worsen over the years in the Eastern Mediterranean Region (EMR), comprising 22 countries with over 597 million people by 2013 (2, 3). For instance, in Lebanon, the fertility rate dropped from 3 in 1993 to 2.1 in 2020 (4). Similarly, a sample from Babol, Iran, showed that more than 120,000 new infertile couples are registered every year (5). In 2015, a meta-analysis of 13 papers involving 55,658 Iranian couples found a lifetime infertility frequency of 13.2%, which is markedly higher than the global rate of 10.5% (6). In the Kingdom of Saudi Arabia (KSA), 18.93% of couples in the reproductive age failed to achieve at least one live birth (7).
Consequently, centers that offer assisted reproduction technologies (ART), especially intracytoplasmic sperm injection have become widely available in the region (8). A Lebanese survey demonstrated that most infertile couples (87.5%) opt for ART, which are socially and religiously accepted (82.2% and 66.2%, respectively) (9).
Several environmental and occupational exposures have been suggested to interfere with reproduction. Few reviews have published data concerning these factors among infertile couples living in the EMR. For men, environmental, industrial, and air pollution can decrease sperm counts, while for women, ovarian dysfunction is a leading factor (10). A review of evidence among Qatari couples highlighted the impact of smoking, coffee and alcohol consumption, stress, body weight, and environmental exposures (11). Another review focused on the effect of multiple trace elements necessary for normal spermatogenesis, adequate levels of sex hormones, and satisfactory sperm characteristics (12). The available reviews describe the results of some EMR countries, or are limited to specific environmental exposures, or study either infertile men or infertile women separately. Since previous research published contains little data on their effect on the embryological and clinical outcomes of ART, a broader review, in scope, is crucial to identify literature gaps and guide research in this area. For that purpose, we focused on existing information concerning these risk factors in the EMR. Findings were divided into 2 reviews: the first one focused on the dietary habits and nutritional supplementation and their impact on fertility (13). The second discussed the influence of chemical, climatic, and other environmental exposures on the reproductive functions.
The literature search was conducted using PubMed and Scopus databases. Publications from January 2012-June 2024 were considered. A total of 63 studies were found eligible for the synthesis. Around 59% (n = 37) of the publications evaluated male factor infertility, while almost 36% (n = 23) examined the outcomes of infertile women. The search was constrained in scope and depended on specific databases and keywords, without the application of formal inclusion/exclusion criteria or quality assessment of studies. Consequently, certain references may have been involuntarily excluded. The findings should be regarded as a summary and synthesis of specific evidence rather than a systematic review.
2. Temperature
Climatic factors, including temperature, have a detrimental impact on fertility rates (14-17). A cross-sectional study conducted on 3670 women showed that seasonality affects reproduction without any particular pattern, mainly due to its temperature and photoperiod parameters (15).
For instance, in summer, sperm concentration and motility were lower than usual compared to the number of metaphase II oocytes, which was relatively higher (15, 18). A correlational study conducted on 51,065 women found that a global warming-induced temperature increase has negative effects on human fertility. On one hand, cool environmental conditions were found to have a positive impact on the quality and quantity of the spermatogenesis process in men; whereas, a lively lifestyle with jobs depending on agricultural productions led to efficient economic conditions, which in turn promoted better access to calorie sources, resulting in higher productivity potential for women (16). Cross-sectional studies conducted in Pakistan and KSA on 360 and 137 males, respectively, explain that the successful spermatogenesis process depends on scrotal temperature stability; any increase in scrotal temperature as compared to the normal core body temperature can lead to an impairment in sperm production and function (17, 19). Moreover, males exposed to high temperatures in their jobs, like bakers, textile industry workers, and ceramic oven operators, were more likely to have poor sperm qualities, a loss of libido, and high levels of stress, which in turn led to a disturbance in the nervous system and the hormonal equilibrium (19). Similarly, 2 cross-sectional studies found that in such jobs, exposure to harsh temperatures in addition to stressful work conditions, including financial, psychological, chemical, and physical ones, also lead to increased abortion rates, decreased sperm motility, and abnormal sperm morphology, with defects in the tail and head (17, 20). While other studies shed light on how occupational exposures, coupled with varicocele for infertile males, resulted in decreased semen parameters and ubiquitination median, which is used as a marker to measure the ubiquitin-proteasome system’s function. It was shown that a group of 90 infertile men exposed to high temperatures and a group of 51 infertile men with different grades of varicocele, had a significantly lower mean of sperm parameters when compared to a control group of 60 normospermic men who did not present varicocele and were not exposed to any chemicals (21).
3. Chemical exposures
3.1. Bisphenol A (BPA)
Exposure to chemicals, like BPA, a substance used in the plastic industry, which may enter the body through contaminated food or drinks, domestic or medical equipment, and occupational sources, also has adverse effects on fertility (Table I). For instance, BPA was positively linked to the deoxyribonucleic acid (DNA) damage and negatively correlated to semen quality and antioxidant levels. In fact, 2 case-control studies discussed the BPA’s severe effects among Egyptian groups. They explained that BPA tends to bind to estrogen receptors, thus inducing non-genomic steroid action. Consequently, because of the exposure to BPA, testicular testosterone levels decrease, and the risk for prostatic hyperplasia and predisposition to carcinoma increases. Moreover, it was shown that when taken in, BPA leads to errors in the hypothalamo-pituitary-gonadal axis and sperm functions through increased sperm DNA damage, oxidative stress, and epigenetic methylation, in addition to a potential dysfunction of steroidogenesis and spermatogenesis (22, 23). Also, all of the 50 infertile men had increased levels of urinary BPA as compared to the 50 fertile controls, and in turn exhibited notable distinctions in terms of sperm morphology, motility, count, and oxidative stress (22). High urine BPA also raised levels of sex hormone binding globulin and estradiol, while lowering levels of active androgens (24).

3.2. Phthalates
Phthalates are industrial chemicals, also known as plasticizers, used in several consumer products, including food, toys, and cosmetics (Table I). They are known to have reproductive and developmental health risks. In fact, in a cross-sectional study done in KSA, after exposure to phthalates during fetal and early development, alterations in testis development and function can happen, thus affecting a male’s fertility (25). Their findings showed that exposure to phthalates suppresses the level of Leydig cells, which play a crucial role in the production of growth factor 3 and testosterone, leading to certain aspects of testicular dysgenesis syndrome, like impaired spermatogenesis.
Mono(2-ethyl-5-oxohexyl) phthalate, one of the Di-(2-ethylhexyl) phthalate metabolites, has been measured in Jordanian females urine. Its exposure, stemming from microwave heat and cosmetic products, shows an association with infertility (26). Results of over 500 Saudi individuals, both males and females, pinpointed that being exposed to phthalates could also be associated with DNA methylation in genes related to embryogenesis, with lower egg count, egg quality, implantation, clinical pregnancy, and live birth rates, and with polycystic ovarian syndrome (PCOS) in humans (27-29).
Moreover, it was found that increased levels of certain phthalates (like the percentage of mono-2-ethylhexylphthalate being less than 29%) in women led to increased risks of biochemical pregnancy, in addition to failed clinical pregnancy and live birth. For instance, mono-2-ethylhexylphthalate exposure was positively associated with failed clinical pregnancies in 599 couples undergoing in vitro fertilization (IVF) (30).
3.3. Pesticides
Pesticides, substances used to control pests in agriculture, are also known to have adverse effects on a person’s reproductive health (Table I). In 2020, a study on Iranian women working in greenhouses found that reproductive disorders, including pregnancy complications, menstrual disturbances, reduced fertility, stillbirth, spontaneous abortion, preterm or post-term birth, congenital abnormalities, ovarian dysfunction, and hormonal problems, are all more common among women in agricultural-related industries. For example, the authors even showed that ovary or fallopian tube problems were 4 to 16 times higher among women who worked in agriculture than in those who worked in different fields. It was also shown that exposure to pesticides could decrease the number and motility of Y-bearing sperm, thus affecting the fetus’s gender, increasing the odds of getting a girl (31).
In Tunisia, one study on 2122 males showed how pesticides can impact the post-testicular sperm maturation, which involves the epididymis, prostate, and seminal vesicles, and eventually lead to an increase in pH, and a decrease in seminal volume, motility, percentage of normally shaped sperm, and seminal zinc (Zn) concentration (20). Furthermore, individuals who were exposed showed higher levels of DNA breakage and chromatin decondensation (32). They can also disrupt the sex hormonal activities, thus resulting in the secretory dysfunction of male accessory glands (20). In particular, organochlorine pollutants, including dichlorodiphenyltrichloroethanes (DDTs), polychlorinated biphenyls, and hexachlorobenzene essentially used for pest control in agriculture, are known to affect a male’s reproductive system negatively. For instance, DDTs and their respective metabolites have high androgenic and estrogenic activities, acting as hormonal receptor antagonists or agonists, thus hindering the work of reproductive hormones. Increased serum levels of polychlorinated biphenyls are shown to decrease seminal parameters, and hexachlorobenzene was seen to be associated with genital abnormalities such as hypospadias and with defects in sperm motility (33).
3.4. Trace elements
Trace elements, also known as trace metals, are minerals found in living organisms in minor quantities. If ingested in significant amounts over extended periods, any trace element can become harmful (34). Many publications focused on their impact on reproductive health in the EMR (Table II). In fact, these metals can affect human reproductive health in 2 ways: either directly, by targeting set reproductive organs, or indirectly, by working on the neuroendocrine system (35). Nonetheless, in general, these metals have been associated with a decline in fertility rates and were believed to negatively affect fine-tuning of biochemical reactions responsible for the regulation of the function of the male reproductive system (36, 37). In their case-control study, trace elements were found in higher levels in the 64 infertile males as compared to the 32 fertile men (35).
Lead (Pb), for instance, was also found to have adverse effects on different systems of the human body, especially the reproductive one (38). Constantly being subjected to Pb, such as painters, has the potential to impact the quality of semen, particularly affecting sperm movement (motility) and causing an elevation in testosterone levels. This also damages Leydig and Sertoli cells, thus impairing testosterone formation and the process of spermatogenesis, respectively, and limits the motility of sperm (35, 36, 39). Similarly, in a cross-sectional study, 85 welders (exposed to Pb fumes) had much higher blood Pb levels than the 80 administrative staff (not exposed), as well as a relatively lower sperm count and motility than the control group (40).
Pb was also shown to result in the absence of libido in males and issues, and spontaneous abortions in females (38). Moreover, Pb coupled with nickel was found to be in significantly higher amounts in the 70 women with PCOS than in the 50 control participants. This can be explained by the fact that these trace elements increase the reproductive hormone levels that relate to oxidative stress and hinder the physiological balance, allowing for deregulation of glucose via reactive oxygen species (ROS) pathways (41).
Cadmium (Cd) was also found to have negative effects on male fertility rates. The presence of Cd is linked to an increased amount of ROS that surpasses the antioxidant defense, in addition to a decreased number of sperm. In their case-control study, the authors observed augmented levels of Cd in the 64 infertile participants as compared to the 37 control individuals. This was linked to an increase and an imbalance in the levels of ROS, which led to oxidative stress, resulting in lipid peroxidation, alterations in DNA and protein structures, and sperm death. This result can be explained through Cd’s ability to compete with essential metals over protein-binding sites, leading to the release of Fe2+ and Cu2+ ions, which induce the production of ROS, and in turn, increase oxidative stress (42).
Iron (Fe) was thought to decrease the motility and the quality of sperm, by inducing lipid peroxidation and forming hydroxyl radicals on one hand, and to affect women’s infertility on the other (37, 43, 44). To better understand this mechanism, a prospective control study found a positive and significant association between Fe and the multiple anomalies index and seminal leucocyte concentration. In fact, Fe concentrations in the seminal plasma of the 79 sub-fertile individuals were significantly higher than in the 29 healthy participants. As such, the authors further concluded that trace element (including Fe) excess is implicated in low sperm quality (37).
Moreover, tin was also believed to affect the quality of human sperm by reducing the creatine kinase activity, which in turn allows for the male reproductive health to regress. Among the 75 Pakistani males, tin counts were the highest among azoospermia individuals as compared to oligozoospermia and normozoospermia ones (45).
On a more positive note, other metals like Zn were proven to have a positive impact on people’s reproductive health. For instance, unlike the presence of other trace elements, Zn did not have any negative influence on women with PCOS (46, 47). In fact, in their case-control study, no difference was observed in the serum Zn levels among the 50 PCOS women and the 50 control women (46). Furthermore, a standard amount of Zn is crucial for several reproductive processes like the steroidogenesis, testis development, synthesis and secretion of sexual hormones, differentiation of gonads, spermatozoa maturation, acrosome function, and its fertilization capacity (48, 49). This can be reflected in a case-control study, which showed the lower seminal Zn levels in the 190 infertile men as compared to the 60 normospermic men (48). Moreover, Zn coupled with sulfate was also shown to play a huge role in sperm physiology functions, like shaping the sperm membrane, increasing sperm motility, and enhancing seminal fluid parameters (49-51). In a prospective study, 700 men who completed the Zn sulfate and folic acid treatment after 3 months recorded an increase in sperm concentrations and sperm motility as compared to when they first began (50). It was even explained that Zn sulfate treatment can be used to help infertile males suffering from anti-sperm antibodies, as the results of his study reported a decrease in the level of serum and seminal plasma anti-sperm antibodies, in the 38 infertile men who were administered the Zn sulfate treatment over 45 days as compared to the 10 control individuals whose seminal levels remained intact (52). In addition, oral Zn supplementation can augment the normal morphology and reproductive hormones and protect sperm chromatin (53, 54). This was shown in a randomized clinical trial, where the 68 participants who were given either Zn or pentoxifylline + Zn as a treatment for 3 months reported a significant reduction in DNA fragmentation as compared to the 31 individuals who took pentoxifylline alone and the 22 controls. This is because Zn connects protamines with Zn bridges; imidazole groups of histidine and thiol groups of cysteine, to be exact (54). In contrast, while Zn sulfate and folic acid do enhance conditions for sperms, the male reproductive health cannot be improved only by resorting to this kind of treatment, as the impairment is the result of many factors. Hence, in order to ameliorate it effectively, all aspects must be tackled (55). In conclusion, the summarized articles presented a well-developed insight into the different effects, be they positive or negative, that trace elements have on the reproductive health of humans.



4. Air pollution
There remain other factors that also affect male infertility. Air dust pollution has a negative influence on male sperm quality. The findings in their retrospective analytical study, conducted among 850 infertile males in Iran over 4 yr, suggested a significantly negative correlation between air dust pollution and sperm morphology and concentrations (56). Moreover, air pollutants also impact women’s fertility and its exposure to them can lead to stillbirth, miscarriage and fetal loss, preterm delivery, reduced fertility rate, and impaired fetal growth. In their cross-sectional study on 67 women living in a polluted area, the authors showed that long-term exposure to particulate matter (PM) PM1 and PM2.5 was linked to a decrease in the levels of the serum anti-Mullerian hormone, which reflects an individual’s egg count. Not only that but exposure to PM2.5 was also shown to lead to systemic inflammation, portrayed through a variance in the levels of Interleukin-6 and tumor necrosis factor -alpha, in addition to morphological changes in ovarian tissue. This, in turn, can result in ovarian injuries (57).
5. Smoking
Controversial results regarding the effect of smoking frequency on sperm parameters were found. Many studies compared the outcomes of smokers and non-smokers. Smoking water pipes or hookah slightly decreased the semen volume, morphology, and progressive motility (58). Women hookah users did report considerably higher period discomfort (59). In men, hookah enhanced ROS production while decreasing water-soluble antioxidants, resulting in oxidative stress in sperm plasma membranes (60).
A large group of smokers confirmed the negative impact of smoking on motile and normally shaped sperm (61-64). A case-control study on 960 adult males focused on the significant difference seen among heavy smokers (> 20 cigarettes/day), especially in the values of sperm concentration and the percent of shaking and immotile spermatozoa, which was lower in comparison to non-smokers (61). Heavy smokers also exhibited higher leukocyte inflation than other subgroups. In another case-control study, Cd and Zn levels were evaluated among smokers for their potential effect on sperm parameters. This is because cigarettes are known to be a major cause of Cd toxicity and are related to Zn deficiency. As such, the results demonstrated lower levels of Zn in the 35 smokers as compared to the 35 non-smokers. Furthermore, the changes in sperm motility, viability, and morphology, opposite to Cd, were positively linked to higher serum and seminal levels of Zn (65).
In addition, non-smokers had lower concentrations of Pb and Arsenic in seminal fluid, and better sperm count and viability, respectively (66). 2 case-control studies conducted in KSA and Pakistan, respectively, and one cross-sectional study conducted in Pakistan, addressed the variation of reproductive hormones of men who smoke cigarettes, dip tobacco, or cannabis (62-64). All types of drugs were correlated to lower levels of testosterone, luteinizing hormone, cortisol, and sex hormone-binding globulins in smoker infertile men vs. non-smoker infertile men as compared to higher levels of prolactin and follicle stimulating hormone in smoker participants, especially those who smoke cannabis (62-64). An Egyptian study found that sperm motility has been affected negatively by tobacco, and it decreased drastically. In contrast, it has been affected positively by tramadol and cannabis as measured by both traditional and computer-assisted semen analysis techniques (67). The spermatozoa movement of 64 smokers was compared to that of 84 non-smokers. The weakened motility found in the first group was attributed to lower sperm creatine kinase activity and impaired energy production (68). Many studies investigated DNA methylation changes in sperm (69-71). Investigating the DNA methylation and transcription of several gene expressions in 15 infertile smoker men, significant changes were noticed in the methylation of all 9, 18, 7, 17, and 3 cytosine-phosphate guanine sequences within PRICKLE2, ALS2CR12, ALDH3B2, PTGIR, GAA, MAPK8IP3, and ANXA2 genes amplicon, respectively (69, 70). Similarly, evidence on other altered gene expressions, such as PGAM5, PTPRN2, TYRO3, was found, but no correlation was observed between smoking and H2BFWT, PRM1, and 2 gene variants (72, 73). Smoking at least 25 cigarettes per day for at least 10 yr also leads to poor DNA integrity by altering sperm chromatin condensation (69, 70, 74). Finally, it was shown that cigarette smoking also increases nitric oxide and malondialdehyde levels in normospermic smokers, which in turn were found to reduce sperm motility (75). As for the embryological results, 3 groups of 50 couples were assessed: overweight infertile men, overweight men who smoke, and controls. In the second group, higher levels of abnormal sperm shape were associated with lower numbers of fertilization, pregnancy, and embryos of good quality (76). In contrast, 2 studies gathering infertile women and men did not find a significant correlation between smoking and infertility (77, 78).
Broadly, this review found that environmental exposures and other lifestyle-related pollutants have negative impacts on the reproduction of EMR couples. Studies showed how increased temperatures lead to impairments in sperm production and function, as well as elevated rates of abortion. These findings align with research conducted in Europe; for example, after analyzing data from 32 European countries, for over 244 million live births between 1969 and 2021, high temperature was suggested to increase the risk of miscarriage and leave detrimental impacts on live births (79). Most of the available study designs recruiting patients from the EMR are cross-sectional in nature, which limits the ability to infer causality due to potential confounding factors. Prospective cohort and longitudinal studies should be prioritized in future research.
This is concerning as the EMR, similarly to the rest of the globe, is experiencing climate change and rising temperature patterns, which paired with limited access to reproductive health services, can negatively affect fertility rates in the region. As for chemicals, BPA has a positive relation with DNA damage and a negative one with semen quality and antioxidant levels. The literature also showed the negative impact that BPA has on fertility (80, 81). Surprisingly, BPA levels found in the urine samples in Egypt were notably higher than levels found in samples in the US and China (82-84). This discrepancy can be attributed to lifestyle differences with increased use of plastics in Egypt, which is a common pattern in many other low-income countries in the EMR.
Moreover, the review outlines phthalates ability to alter testis development in males and induce failed clinical pregnancy and live birth in females. Similar findings are outlined in studies conducted in the US, Korea, and Canada, but with lower levels of exposure (85-88). These findings can be attributed to several factors, which include lifestyle changes as well as cultural practices, dietary habits, and personal care product use, as well as governmental legislation regulating phthalate use. A key limitation of several EMR studies is the lack of confounding factors adjustment, which might exaggerate or obscure the observed associations between chemical exposures and infertility. The control for body mass index, smoking status, maternal age, alcohol consumption, and physical activity are important covariates identified in the literature. The impact of trace element deficiency or the outcome of their high exposures is still poorly understood. In men, similar findings of both positive and negative effects of micronutrients on semen characteristics were summarized (89). According to 3 publications, pesticides, as well as air pollution, are also found to have negative effects on fertility, as they lead to reproductive disorders, including pregnancy issues in females, and decrease seminal parameters and increase genital abnormalities in males. Similar findings were identified in Barcelona, Spain, which showed that exposure to air pollution, resulting from different sources, can lead to a significant reduction in fertility rates (90). In an overview on pesticides, organochlorines such as dieldrin, DDT, and aldrin showed a wide-ranging impact on ecosystems and pose threats to human health, including fertility, circulatory, neurological, hormonal, and endocrine functions (91). There remains a dearth of literature on air pollution’s impact on perinatal health in the EMR, and even fewer studies have been conducted on its impact on fertility, which is problematic since air pollution continues to be a critical environmental and public health problem in the region (92). For instance, the annual average of PM10 and PM2.5 concentrations in both Beirut and Tabriz cities surpassed the standard values set by the World Health Organization (93, 94). Although EMR studies investigating pesticide contamination and fertility impairment represent a limited sample size, they provide important preliminary insights and reflect the challenges of conducting environmental health research in this region. Future research with larger sample sizes is necessary to validate existing findings regarding air pollution and pesticide exposure.
This review also highlighted the negative reproductive health outcomes associated with smoking, which aligns with international literature (95). For example, within the recruitment of 1222 Russian men, heavy smokers (> 10 cigarettes/day) had decreased total sperm counts and sperm concentrations (96). Moreover, smoking, which is a major cause of Cd toxicity, is also of a retrogressive effect on male reproductivity, as it can lower levels of testosterone and weaken sperm movement (65). Smoking is an exposure of particular importance in the EMR as the number of smokers continues to grow, with the prevalence of tobacco use ranging between 8.1% in Oman and 35% in Jordan and Lebanon. The high prevalence of tobacco use in the EMR can be partially attributed to the popularity of waterpipe or hookah smoking, which has a reduced harm perception and inadequate policies and regulations (97).
Based on this review, it is evident that the data on the effects of environmental exposures on male reproductive health were more prevalent. The ease of semen sample collection and the fewer ethical restrictions related to male reproductive health investigations explain this imbalance in the research distribution. Only a few studies focused on occupational exposures such as PBA, phthalates, and pesticides, and they indicated a reduced performance of the human reproductive system and lower numbers of successful artificially induced pregnancies. The effects of trace elements included more information about women with PCOS but did not tackle assisted reproductive technology or IVF methods.
As for smoking, some publications on men found a small impact on semen parameters, while others mentioned that heavy smokers might witness fertility issues. Although no specific definition was observed for heavy smoking, the overall trend consistently indicated that frequent smoking habits are associated with reduced fertility. These publications delivered the results of small groups of participants, which is why a bigger sample size and dose-response assessment protocols might be more helpful in proving the actual impact of smoking frequency. Also, only one paper correlated the effect of smoking with clinical outcomes of IVF cycles. As such, further studies on the ART results are much needed, along with more observational studies among healthy or infertile smoking women. In general, case-control and cross-sectional studies were most used in tackling those factors. While each serves a different purpose, together, they can give a comprehensive overview of the matter. This aligns with the limitations observed by a previous scoping review on perinatal health in the EMR (92).
6. Conclusion
Despite some environmental aspects being extensively studied across different populations, there are still significant limitations in terms of the number of studies and the data itself derived from the EMR. As such, more research is needed to set instructions for daily routines of people and set guidelines on the use of chemicals for all people working in agriculture, manufacturing, and other industries with high risks of exposure. Also, the EMR is witnessing rapid population growth and requires better public health management.
Addressing the vast health, cultural, and social discrepancies within the region can be instrumental in enhancing public awareness, education, policy, and regulation. This, in turn, can inform decision-makers, helping them adopt new guidelines, setting standards for air and water quality, waste management, and industrial emission regulations, and decreasing the adverse impacts on human fertility. Overall, by conducting further research on this topic, researchers can contribute to enhance the quality of life and have more effective and sustainable strategies to tackle the region’s problems.
Acknowledgements
The authors affirm that no artificial intelligence tools were used in the writing, editing, data analysis, or preparation of this manuscript. All work was conducted and prepared by the authors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Fertility & Infertility
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





