Fri, Feb 20, 2026
[Archive]
Volume 23, Issue 11 (November 2025)
IJRM 2025, 23(11): 881-896 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Baradaran Bagheri R, Mohammadian S. Unveiling the molecular nexus: Long non-coding RNAs, RNA-binding proteins, and DNA damage in ovarian ischemia-reperfusion injury: A narrative review. IJRM 2025; 23 (11) :881-896
URL: http://ijrm.ir/article-1-3693-en.html
URL: http://ijrm.ir/article-1-3693-en.html
1- Department of Obstetrics and Gynecology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
2- Department of Obstetrics and Gynecology, Kamali Hospital, Alborz University of Medical Sciences, Karaj, Iran. ,shima.mohammadian@abzums.ac.ir
2- Department of Obstetrics and Gynecology, Kamali Hospital, Alborz University of Medical Sciences, Karaj, Iran. ,
Full-Text [PDF 428 kb]
(290 Downloads)
| Abstract (HTML) (168 Views)
Full-Text: (14 Views)
1. Introduction
Ovarian ischemia-reperfusion injury (IRI) represents a significant challenge in gynecological health, frequently associated with conditions such as ovarian torsion, surgery, or transplantation. The disruption of blood flow followed by its restoration can cause substantial damage to ovarian tissues, leading to impaired follicular development, compromised ovarian function, and infertility. Elucidating the molecular mechanisms underlying ovarian IRI is essential for developing targeted therapies and mitigating their detrimental effects (1-4).
In recent years, emerging evidence has shed light on the significant role of non-coding RNAs (ncRNAs) in regulating gene expression and orchestrating cellular processes. Among these, long non-coding RNAs (lncRNAs) have emerged as key players in the intricate network of molecular interactions governing ovarian physiology and pathology. Long considered as transcriptional noise, lncRNAs are now recognized for their diverse functions in chromatin remodeling, transcriptional regulation, and post-transcriptional processing, making them critical regulators of cellular homeostasis and disease pathogenesis (5-7).
RNA-binding proteins (RBPs) are essential mediators of RNA metabolism, modulating the fate and function of cellular RNA molecules. By binding to specific RNA sequences or structures, RBPs regulate messenger RNAs (mRNA) stability, translation, and localization, profoundly influencing gene expression and cellular phenotype. RBP dysregulation has been implicated in various diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases, underscoring their importance as therapeutic targets and diagnostic biomarkers (8-10).
In ovarian IRI, the interplay between lncRNAs, RBPs, and DNA damage represents a promising but insufficiently explored area. Ischemic insult triggers a cascade of molecular events, including oxidative stress, inflammation, and DNA damage, that culminate in cellular dysfunction and death. Within this complex environment, lncRNAs and RBPs are hypothesized to be critical regulators of DNA damage response (DDR) pathways, potentially modulating the cellular response to genotoxic stress and influencing ovarian IRI outcomes (11, 12).
This narrative review aims to explore the potential interplay between lncRNAs, RBPs, and DNA damage in ovarian IRI. We synthesize evidence from related fields to propose a conceptual model for their roles and mechanisms in this specific condition. It is crucial to note that direct experimental evidence for this molecular nexus in ovarian tissue remains limited; therefore, this review serves to outline a framework for future hypothesis-driven research. Through comprehensive examination of current literature, we seek to advance the conceptual understanding of ovarian IRI pathophysiology and identify promising avenues for future investigation.
2. Methods
This narrative review was conducted to synthesize existing knowledge and propose hypotheses on the roles of lncRNAs, RBPs, and DNA damage in ovarian IRI. A literature search was performed using electronic databases, including PubMed, Scopus, and Web of Science. The search strategy employed a combination of keywords and MeSH terms related to: “long non-coding RNA” OR “lncRNA”, “RNA binding protein” OR “RBP”, “DNA damage” OR “DNA damage response”, and “ovarian ischemia reperfusion injury” OR “ovarian torsion” OR “ovarian IRI” OR “ischemia reperfusion injury”. To ensure contemporary relevance, the search was not restricted by date, but priority was given to recent publications (2018-2025). Given the scarcity of studies directly investigating lncRNAs and RBPs in ovarian IRI, the scope was broadened to include foundational and mechanistic studies from other organ systems (e.g., cardiac, cerebral, and renal IRI) and related pathologies (e.g., cancer and neurodegenerative diseases) where these molecules are well-characterized in the context of genotoxic stress. The inclusion criteria focused on original research articles and reviews published in English. The selected literature was analyzed to identify common mechanisms, potential interactions, and conceptual frameworks that could be extrapolated to the context of ovarian IRI.
3. Ovarian IRI: Pathophysiology and mechanisms
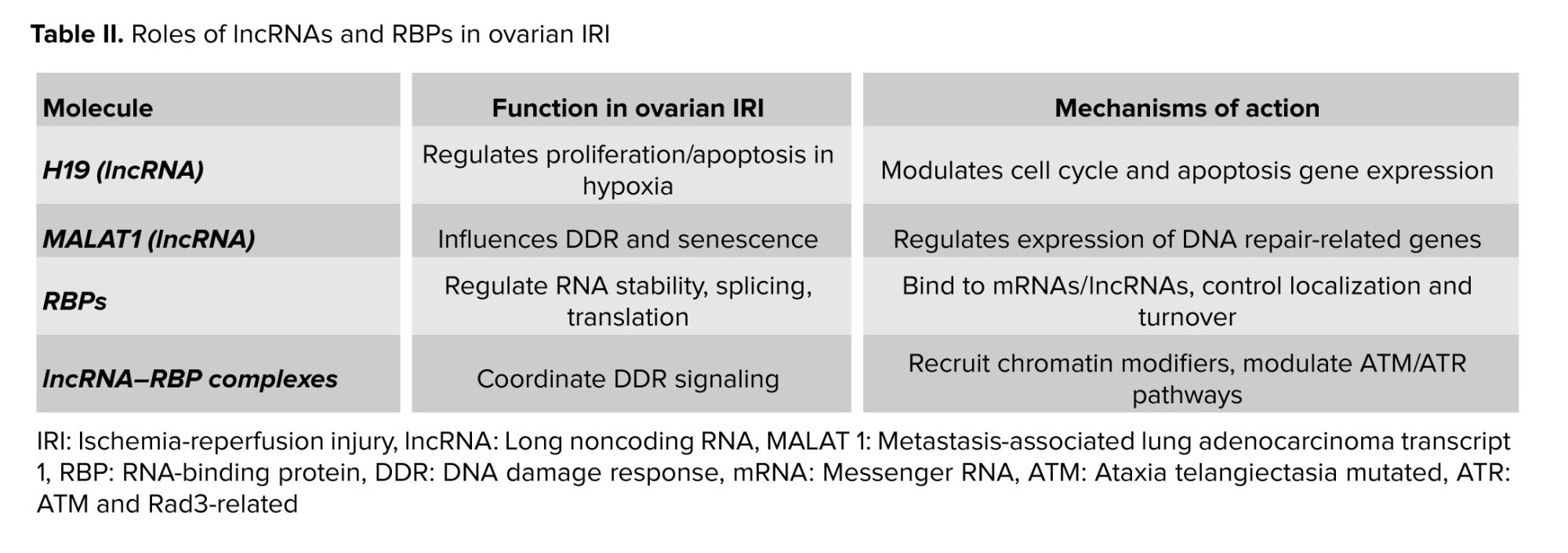
Ovarian IRI represents a complex pathological condition characterized by the temporary disruption of blood flow to the ovaries followed by the subsequent restoration of perfusion. This phenomenon can occur in various clinical scenarios, including ovarian torsion, surgical procedures involving the ovaries, and organ transplantation. Despite being a transient event, IRI can have profound and long-lasting consequences on ovarian function and reproductive health (13-15).
The pathophysiology of ovarian IRI involves a cascade of interconnected molecular and cellular events that contribute to tissue damage and dysfunction (16, 17). The initial phase of ischemia results in oxygen and nutrient deprivation, leading to metabolic alterations, ATP depletion, and accumulation of metabolic byproducts such as lactate and reactive oxygen species (ROS). This hypoxic microenvironment triggers a series of adaptive responses, including activation of hypoxia-inducible factors, inflammatory mediators, and cell death pathways (16, 18, 19).
Upon reperfusion, restoration of blood flow paradoxically exacerbates tissue injury through several mechanisms (20). The abrupt reintroduction of oxygen-rich blood leads to the generation of additional ROS, exacerbating oxidative stress and causing oxidative damage to cellular macromolecules, including lipids, proteins, and DNA. The sudden influx of inflammatory cells and cytokines further amplifies the inflammatory response, triggering endothelial dysfunction, leukocyte infiltration, and tissue inflammation (21).
At the cellular level, IRI disrupts cellular homeostasis and impairs essential cellular functions, including energy metabolism, ion transport, and protein synthesis. Mitochondrial dysfunction, calcium overload, and activation of pro-apoptotic pathways contribute to cellular damage and programmed cell death, leading to tissue injury and loss of ovarian follicles. Moreover, IRI induces DNA damage, including single-strand breaks (SSBs), double-strand breaks (DSBs), and DNA base modifications, further compromising genomic integrity and cellular viability (22, 23).
The interplay between oxidative stress, inflammation, and DNA damage plays a central role in the pathogenesis of ovarian IRI. ROS generated during IRI oxidative damage to cellular components, including DNA, leading to the activation of DDR pathways. DDR mechanisms, including DNA repair, cell cycle arrest, and apoptosis, are orchestrated by a complex network of signaling pathways involving DNA damage sensors, signal transducers, and effector proteins (24, 25).
Furthermore, emerging evidence suggests that lncRNAs and RBPs play critical roles in modulating the cellular response to IRI (26, 27). These ncRNA molecules and RBPs regulate gene expression, RNA stability, and post-transcriptional processing, influencing cellular fate and function in response to genotoxic stress. The dysregulation of lncRNAs and RBPs has been implicated in various pathological conditions, including ischemic injury and tissue damage (27, 28).
In summary, ovarian IRI is a multifaceted pathological condition characterized by oxidative stress, inflammation, and DNA damage. Elucidating the underlying mechanisms of ovarian IRI is essential for developing targeted therapeutic interventions aimed at mitigating tissue damage and preserving ovarian function. Understanding the roles of lncRNAs, RBPs, and DDR pathways in ovarian IRI may offer novel insights into its pathogenesis and potential therapeutic targets for future intervention strategies (Table I).
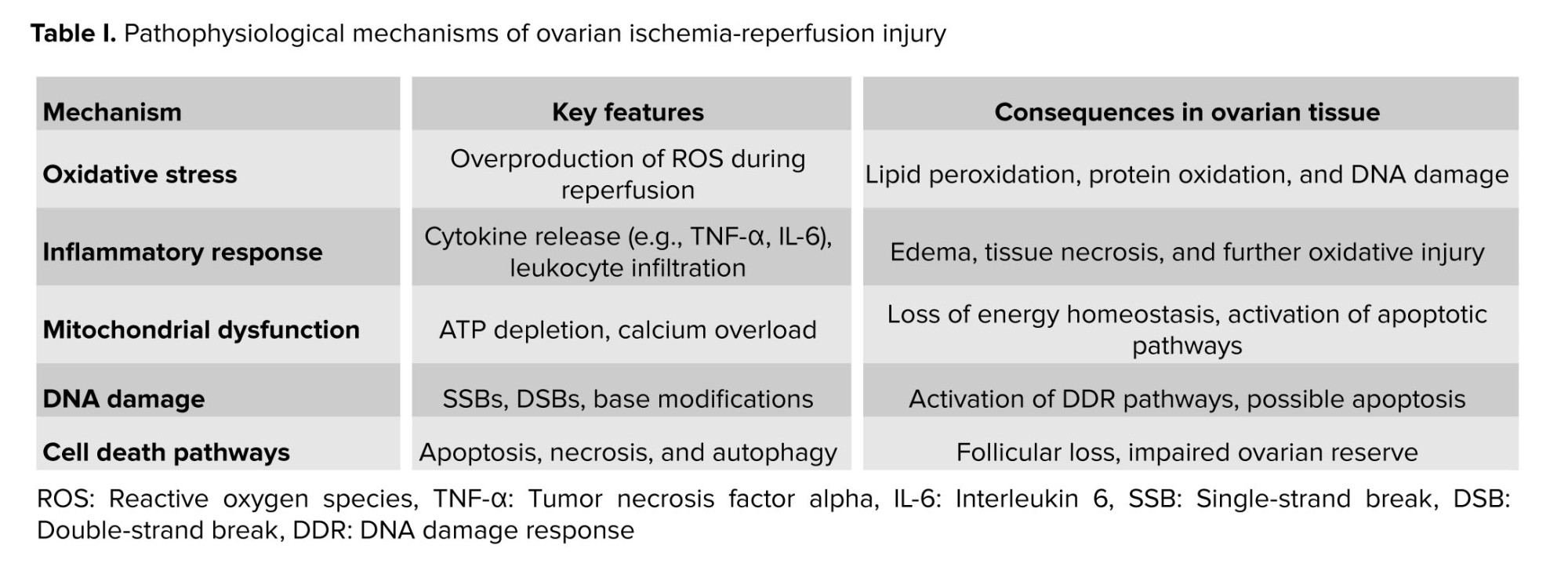
4. lncRNAs: Roles and functions
lncRNAs have emerged as crucial regulators of gene expression and cellular processes, contributing to various physiological and pathological conditions, including ovarian IRI (29). Despite lacking protein-coding capacity, lncRNAs exert diverse functions through intricate molecular mechanisms, making them key players in the complex interplay between genomic regulation and cellular homeostasis (29, 30).
In the context of ovarian IRI, lncRNAs participate in the orchestration of cellular responses to ischemic insult and reperfusion injury. These ncRNA molecules modulate gene expression at multiple levels, including transcriptional, post-transcriptional, and epigenetic regulation, thereby influencing cellular fate and function in response to genotoxic stress (31, 32).
At the transcriptional level, lncRNAs act as molecular scaffolds, decoys, or guides to recruit chromatin-modifying complexes and transcription factors to specific genomic loci, regulating the expression of target genes involved in cellular pathways relevant to ovarian IRI. Additionally, lncRNAs can function as enhancers or suppressors of gene transcription, fine-tuning gene expression patterns in response to environmental cues and cellular signals (33).
Furthermore, lncRNAs participate in post-transcriptional gene regulation by modulating mRNA stability, splicing, and translation. Through interactions with RBPs or microRNAs (miRNAs), lncRNAs influence the processing and turnover of target mRNAs, thereby regulating gene expression programs involved in cellular stress responses, apoptosis, and DNA damage repair pathways (34, 35).
Moreover, lncRNAs contribute to epigenetic regulation by modulating chromatin structure and histone modifications, thereby influencing gene accessibility and transcriptional activity. By recruiting chromatin-modifying enzymes or interacting with regulatory elements such as enhancers or promoters, lncRNAs exert long-range effects on gene expression and cellular phenotype, shaping the cellular response to ischemic insult and reperfusion injury in the ovary (5, 36).
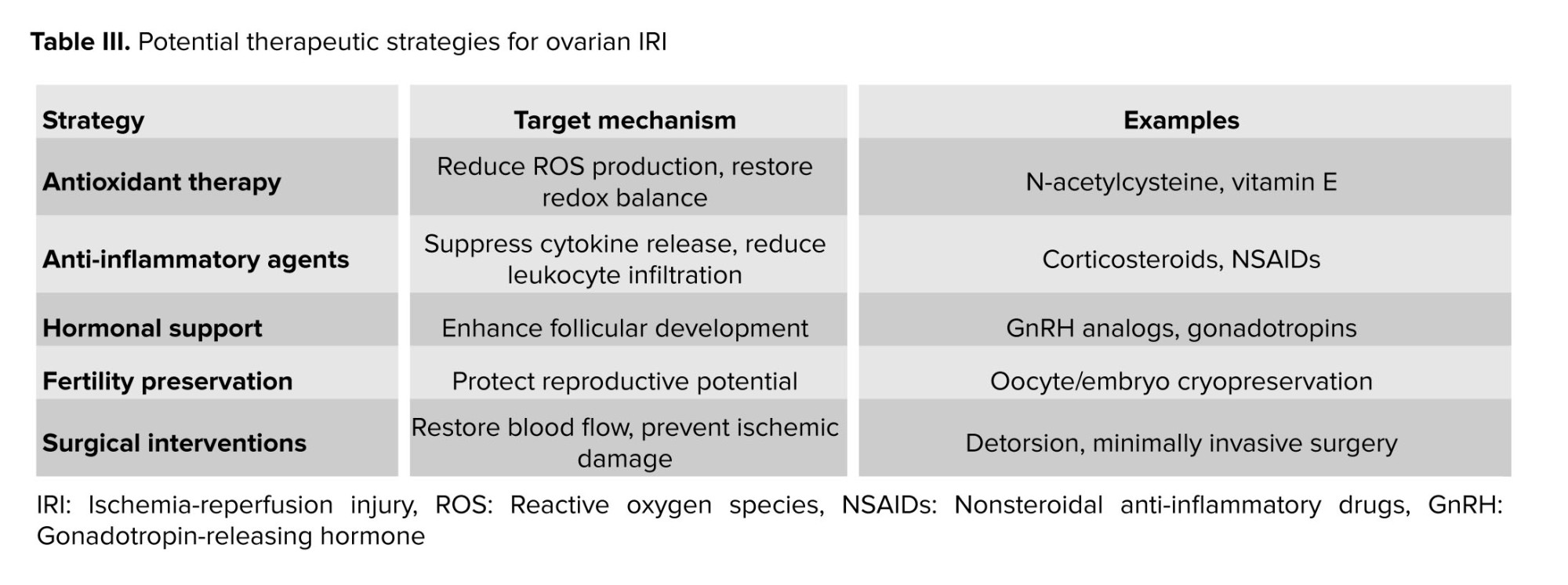
Several lncRNAs have been implicated in the pathogenesis of ovarian IRI, either as mediators of cellular adaptation to hypoxic stress or as regulators of DDR pathways. For example, H19, a maternally expressed lncRNA, has been shown to regulate cell proliferation and apoptosis in response to hypoxia by modulating the expression of genes involved in cell cycle progression and apoptosis. Similarly, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been implicated in the regulation of DDR pathways and cellular senescence in various cell types, suggesting its potential involvement in ovarian IRI pathophysiology (37-39).
Overall, lncRNAs play multifaceted roles in regulating gene expression and cellular processes potentially relevant to ovarian IRI. Understanding their potential functions could provide novel insights and rationale for future experimental validation. Specifically, profiling lncRNA expression in ovarian IRI models and functionally characterizing their roles represent critical research priorities.
5. RBPs: Significance in cellular processes
RBPs play crucial roles in a myriad of cellular processes, including gene expression regulation, RNA metabolism, and post-transcriptional control. These multifunctional proteins interact with various RNA molecules, including mRNAs, lncRNAs, and miRNAs, exerting profound effects on RNA stability, translation, and localization (40, 41). In the context of ovarian IRI, RBPs emerge as key mediators of cellular responses to genotoxic stress and oxidative damage, influencing cellular fate and function in the face of ischemic insult and reperfusion injury (42).
One of the primary functions of RBPs is to modulate mRNA stability and translation, thereby regulating gene expression patterns in response to cellular stressors. RBPs can bind to specific sequences or structural motifs within the mRNA molecules, either stabilizing or destabilizing them, depending on the context and cellular conditions. By regulating mRNA turnover rates and translational efficiency, RBPs control the abundance of target proteins involved in cellular pathways relevant to ovarian IRI, including DNA damage repair, apoptosis, and oxidative stress response (43, 44).
Moreover, RBPs play critical roles in RNA processing and maturation, including pre-mRNA splicing, alternative splicing, and polyadenylation. By interacting with pre-mRNA transcripts and spliceosomal complexes, RBPs modulate the splicing patterns of target genes, generating diverse mRNA isoforms with distinct functional properties. Dysregulation of RBP-mediated splicing events has been implicated in various pathological conditions, including cancer, neurodegenerative diseases, and cardiovascular disorders, highlighting the significance of RBPs in cellular homeostasis and disease pathogenesis (45, 46).
Furthermore, RBPs are involved in the regulation of RNA localization and subcellular trafficking, ensuring proper spatial and temporal control of gene expression. By binding to specific mRNA sequences or structures, RBPs facilitate the transport of target transcripts to subcellular compartments, such as the cytoplasm, nucleus, or dendrites, where they undergo localized translation or storage. This dynamic regulation of RNA localization by RBPs is essential for cellular processes such as synaptic plasticity, cell migration, and embryonic development, underscoring the importance of RBPs in shaping cellular phenotype and function (47, 48). In addition to their roles in RNA metabolism, RBPs have been implicated in the regulation of ncRNA functions, including lncRNAs and miRNAs. RBPs interact with these ncRNA molecules, modulating their stability, activity, and interactions with target transcripts. Through these interactions, RBPs influence diverse cellular processes, including chromatin remodeling, transcriptional regulation, and epigenetic modification, thereby exerting profound effects on gene expression and cellular phenotype (49, 50).
In summary, RBPs likely play essential roles in cellular processes relevant to ovarian IRI. By regulating gene expression, RNA metabolism, and post-transcriptional control, RBPs may contribute to cellular adaptation to genotoxic stress and oxidative damage, potentially influencing ovarian IRI outcomes. Future work should determine whether specific RBPs, such as member of the Hu family of RBPs or quaking, exhibit altered expression or function in ovarian IRI and whether their manipulation affects DNA repair efficiency and follicular survival (51) (Table II).
6. DDR mechanisms in ovarian IRI
DDR mechanisms are crucial for maintaining genomic integrity and cellular homeostasis in response to genotoxic stress, such as that induced by ovarian IRI. Ovarian IRI, characterized by the temporary interruption of blood flow to the ovaries followed by the subsequent restoration of perfusion, triggers a cascade of molecular events that can result in DNA damage and genomic instability. In this context, DDR mechanisms play a central role in sensing, signaling, and repairing DNA lesions, thereby mitigating the detrimental effects of genotoxic stress and preserving ovarian function (52-54).
Upon exposure to genotoxic insults, cells activate a complex network of signaling pathways collectively known as the DDR (55). The DDR involves the coordinated action of sensor proteins, signal transducers, and effector proteins, culminating in cell cycle arrest, DNA repair, or programmed cell death, depending on the extent and severity of DNA damage. In ovarian IRI, ischemic insult and reperfusion injury induce various types of DNA lesions, including SSBs, DSBs, and DNA base modifications, triggering DDR activation to maintain genomic integrity and cellular viability (56, 57).
Central to DDR activation is the recruitment and activation of sensor proteins, such as the ataxia telangiectasia mutated (ATM) kinase and the ataxia telangiectasia and Rad3-related (ATR) kinase, which phosphorylate downstream effector proteins in response to DNA damage (58). ATM is primarily involved in the response to DSBs, whereas ATR responds to replication stress and SSBs, ensuring a coordinated and efficient DDR to different types of DNA lesions. Additionally, DNA-dependent protein kinase, another member of the phosphoinositide 3-kinase-related protein kinase family, is activated in response to DSBs, contributing to DNA repair and cell survival (59, 60).
Upon activation, DDR effector proteins orchestrate downstream cellular responses, including cell cycle checkpoint activation, DNA repair, and apoptosis (59, 61). Cell cycle checkpoints, such as the G1/S and G2/M checkpoints, are activated to temporarily arrest cell cycle progression, allowing time for DNA repair or triggering apoptosis if the damage is irreparable. DNA repair mechanisms, including homologous recombination (HR), non-homologous end joining, and base excision repair (BER), are activated to restore genomic integrity and prevent the accumulation of mutations. In cases of severe or irreparable DNA damage, apoptosis pathways are activated to eliminate damaged cells and prevent the propagation of genomic instability (61).
Furthermore, emerging evidence suggests that lncRNAs and RBPs play critical roles in modulating DDR pathways and cellular responses to genotoxic stress (62). These ncRNA molecules and RBPs regulate gene expression, RNA metabolism, and post-transcriptional control, influencing the cellular fate and function in response to DNA damage. Dysregulation of lncRNAs and RBPs has been implicated in various pathological conditions, including ovarian IRI, highlighting their significance as potential therapeutic targets for intervention strategies aimed at preserving ovarian function and mitigating tissue damage (58-61).
In summary, DDR mechanisms play a central role in the cellular response to ovarian IRI, orchestrating cell cycle arrest, DNA repair, and apoptosis to maintain genomic integrity and cellular viability. Understanding the molecular mechanisms underlying DDR activation and regulation in ovarian IRI may offer novel insights into its pathogenesis and provide potential therapeutic targets for intervention strategies aimed at preserving ovarian function and mitigating tissue damage. Further research is warranted to elucidate the specific roles of DDR pathways, lncRNAs, and RBPs in ovarian IRI and to explore their therapeutic potential in the management of this condition.
7. Interactions between lncRNAs, RBPs, and DNA damage: Molecular insights
Interactions between lncRNAs, RBPs, and DNA damage represent a complex molecular interplay that regulates cellular responses to genotoxic stress, including that induced by ovarian IRI. These interactions contribute to the coordination of DDR pathways, DNA repair mechanisms, and cellular fate decisions, ultimately shaping the outcome of genotoxic insults on cellular function and viability (28, 63).
At the molecular level, lncRNAs act as scaffolds, guides, or decoys for RBPs, facilitating their recruitment to specific genomic loci or RNA molecules and modulating their activity and function. By interacting with RBPs, lncRNAs influence various aspects of RNA metabolism, including RNA splicing, stability, translation, and localization, thereby regulating gene expression programs involved in DDR and DNA repair pathways (28, 64).
RBPs, in turn, recognize specific RNA sequences or structural motifs within lncRNAs, mediating their interactions with target RNAs or protein complexes involved in DDR. RBPs can modulate the stability and activity of lncRNAs, regulate their subcellular localization, or facilitate their association with other cellular components, such as chromatin-modifying complexes or DNA repair machinery (28).
The dynamic interplay between lncRNAs, RBPs, and DDR pathways influences cellular responses to genotoxic stress, including cell cycle arrest, DNA repair, and apoptosis. lncRNAs and RBPs participate in the regulation of DDR signaling cascades, modulating the activation and function of key DDR proteins, such as ATM, ATR, and DNA-dependent protein kinase, in response to DNA damage (28, 65).
Furthermore, lncRNAs and RBPs regulate DNA repair mechanisms, including HR, non-homologous end joining, and BER, by modulating the expression, activity, or localization of DNA repair factors. These interactions ensure the efficient and accurate repair of DNA lesions, preventing the accumulation of mutations and genomic instability (66, 67).
In addition to their roles in DDR and DNA repair, lncRNAs and RBPs influence cellular fate decisions in response to genotoxic stress, including cell cycle arrest, senescence, or apoptosis. By modulating the expression of genes involved in cell cycle regulation, apoptosis, or cellular senescence, lncRNAs and RBPs regulate the balance between cell survival and death in the face of DNA damage (68).
lncRNA, RBP, or DDR pathways dysregulation occurs in various pathologies. In ovarian IRI, we hypothesize that aberrant interactions between these elements may contribute to tissue damage and ovarian dysfunction (28, 58, 61). Future research should specifically investigate whether complexes such as lncRNA H19 with RBP HuR, or MALAT1 with specific splicing factors, form in ovarian cells during IRI and how they impact DDR efficacy.
8. Experimental models and methodologies in studying ovarian IRI
Experimental models and methodologies play a pivotal role in elucidating the pathophysiology of ovarian IRI and exploring potential therapeutic interventions. These models aim to recapitulate key aspects of ovarian IRI observed in clinical settings, including the transient interruption of blood flow to the ovaries followed by reperfusion-induced tissue damage. Additionally, sophisticated methodologies are employed to assess various aspects of ovarian function, tissue damage, and molecular changes associated with IRI (15, 23, 69).
Animal models: particularly rodent models such as rats and mice, are commonly used to study ovarian IRI due to their physiological relevance and feasibility of manipulation. Ovariectomy followed by ischemic insult induction through methods like vascular occlusion or torsion and subsequent reperfusion allows researchers to mimic the IRI observed clinically. These models enable the investigation of histological changes, functional alterations, and molecular mechanisms underlying ovarian IRI (15, 69, 70). For example, the rat ovarian torsion model typically involves 3 hr of torsion followed by detorsion, with tissue collection at various reperfusion timepoints to assess acute and subacute damage.
In vitro models: cell culture models using ovarian cell lines or primary ovarian cells provide a controlled environment to investigate cellular responses to ischemic insult and reperfusion. Ovarian granulosa cells, theca cells, and follicular cells can be subjected to hypoxic conditions followed by reoxygenation to simulate IRI. These models allow for the examination of cellular viability, apoptosis, oxidative stress, and DDR pathways in response to genotoxic stress (71, 72).
Molecular and biochemical analyses: various molecular and biochemical techniques are employed to characterize the molecular mechanisms underlying ovarian IRI. These include quantitative real-time polymerase chain reaction to assess gene expression changes, western blotting and immunohistochemistry to evaluate protein levels and localization, and enzyme-linked immunosorbent assays to quantify inflammatory cytokines, oxidative stress markers, and DNA damage markers (67, 69, 70, 72). Histological and morphological assessments: histological analysis of ovarian tissue sections using hematoxylin and eosin staining allows assessment of tissue morphology, structural integrity, and cellular damage following IRI. Other staining techniques, such as terminal deoxynucleotidyl transferase dUTP nick-end labeling assay for apoptosis detection and immunofluorescence staining for specific protein markers, provide insights into cellular responses and pathological changes induced by IRI (14, 73).
Functional assessments: functional assays, including hormone assays to measure ovarian hormone levels (e.g., estrogen and progesterone), ovarian reserve assessments (e.g., follicle counting), and reproductive function tests (e.g., mating studies and fertility assessments), allow for the evaluation of ovarian function and reproductive outcomes following IRI (14, 69, 70, 73).
Imaging techniques: non-invasive imaging modalities such as ultrasound, magnetic resonance imaging, and computed tomography scanning are utilized to visualize ovarian morphology, blood flow dynamics, and structural changes in response to IRI. These techniques provide valuable insights into the temporal and spatial progression of tissue damage and recovery following IRI (74, 75). Overall, integrating diverse experimental models and methodologies enables comprehensive ovarian IRI pathophysiology investigations and facilitates novel therapeutic strategy development for mitigating tissue damage and preserving ovarian function. Continued experimental technique and model system advancements are essential for advancing ovarian IRI understanding and translating experimental findings into clinical applications for improving patient outcomes (14, 69, 70, 72-74).
9. Clinical implications and therapeutic strategies
Clinical implications and therapeutic strategies for ovarian IRI encompass a broad spectrum of approaches aimed at mitigating tissue damage, preserving ovarian function, and improving patient outcomes. Given the complexity of ovarian IRI and its potential impact on reproductive health, it is essential to consider multifaceted interventions targeting various aspects of the pathophysiological cascade (76). Early detection and diagnosis: timely detection and diagnosis of ovarian IRI are crucial for initiating appropriate interventions and minimizing tissue damage. Clinical evaluation, including history taking, physical examination, and imaging studies such as ultrasound or magnetic resonance imaging, can aid in identifying patients at risk of ovarian IRI, particularly those undergoing surgeries associated with ovarian manipulation or vascular compromise. Surgical techniques and ovarian preservation: in cases where ovarian ischemia is anticipated, such as during ovarian cystectomy or ovarian torsion, surgical techniques aimed at minimizing ischemic insult and preserving ovarian blood supply are paramount. Strategies such as detorsion of twisted ovaries, ovarian auto-transplantation, or minimally invasive surgical approaches can help preserve ovarian function and fertility potential (77).
Pharmacological interventions: pharmacological agents targeting oxidative stress, inflammation, and apoptosis represent promising therapeutic avenues for mitigating tissue damage and improving outcomes in ovarian IRI. Antioxidants such as N-acetylcysteine, which scavenges ROS and restores cellular redox balance, have shown potential in preclinical studies for protecting against IRI in various organs, including the ovary (78, 79).
Hormonal support and ovarian stimulation: hormonal support with exogenous gonadotropins or gonadotropin-releasing hormone analogs may be considered to support ovarian function and follicular development in patients at risk of ovarian IRI. Ovarian stimulation protocols aimed at enhancing follicular recruitment and development may also be utilized to optimize fertility outcomes in women undergoing assisted reproductive technologies following ovarian injury (80, 81).
Fertility preservation techniques: for women facing the risk of ovarian dysfunction or premature ovarian failure due to IRI, fertility preservation techniques such as oocyte or embryo cryopreservation offer the possibility of preserving reproductive potential for future use. These techniques are particularly relevant for young cancer patients undergoing gonadotoxic treatments or individuals undergoing surgeries with potential ovarian damage (77).
Multidisciplinary care and counseling: multidisciplinary collaboration involving gynecologists, reproductive endocrinologists, oncologists, and fertility specialists is essential for providing comprehensive care to patients at risk of ovarian IRI. Counseling regarding the potential impact of ovarian injury on fertility, the available treatment options, and the importance of fertility preservation is critical for empowering patients to make informed decisions about their reproductive health (82).
Long-term follow-up and monitoring: long-term follow-up and monitoring of ovarian function, hormonal status, and reproductive outcomes are essential for assessing the efficacy of therapeutic interventions and addressing potential complications arising from ovarian IRI. Regular evaluation of ovarian reserve markers, menstrual cycles, and fertility outcomes allows for timely intervention and optimization of patient care (82, 83).
Overall, clinical implications and therapeutic strategies for ovarian IRI encompass a multifaceted approach for mitigating tissue damage, preserving ovarian function, and optimizing reproductive outcomes. Through surgical techniques, pharmacological interventions, fertility preservation techniques, and multidisciplinary care, clinicians can effectively manage ovarian IRI and improve affected individuals' quality of life. Continued research efforts and clinical practice advancements are essential for further refining therapeutic strategies and enhancing patient care in this challenging clinical scenario (83) (Table III).
10. Challenges and future directions in research
Challenges and future directions in research on ovarian IRI represent critical areas of focus aimed at addressing current limitations, advancing scientific understanding, and improving clinical management strategies. Despite significant progress in elucidating the pathophysiology of ovarian IRI, several challenges persist, necessitating innovative approaches and interdisciplinary collaborations to overcome them effectively (76).
Heterogeneity of ovarian IRI: one of the primary challenges in ovarian IRI research is the inherent heterogeneity of the condition, encompassing diverse etiologies, patient populations, and clinical presentations. Variability in ischemic insult severity, duration, and reperfusion dynamics complicates the interpretation of experimental findings and hinders the development of standardized diagnostic criteria and treatment protocols (84, 85).
Limited experimental models: current experimental models of ovarian IRI often fail to fully recapitulate the complexity of the clinical condition, posing challenges for translational research and therapeutic development. Improved animal models that better mimic the pathophysiological features of human ovarian IRI, including dynamic blood flow changes, tissue oxygenation levels, and hormonal fluctuations, are needed to enhance the relevance and predictive value of preclinical studies (86).
Mechanistic understanding of tissue damage: despite considerable efforts to elucidate the molecular mechanisms underlying ovarian IRI, gaps remain in our understanding of the specific pathways and signaling cascades driving tissue damage and dysfunction. Future research must delineate oxidative stress, inflammation, apoptosis, autophagy, and DDR pathway roles in mediating ovarian injury and identify novel therapeutic targets. A key priority is to move from associative observations to mechanistic studies, for example, by using knockout models to define the functional importance of specific lncRNAs (e.g., MALAT1) in the ovarian response to IRI.
Therapeutic intervention strategies: developing effective therapeutic interventions for ovarian IRI represents a significant research challenge, given the limited pharmacological agents and treatment modalities currently available. Innovative approaches targeting key pathophysiological mechanisms, such as oxidative stress scavengers, anti-inflammatory agents, and pro-survival signaling pathways, hold promise for mitigating tissue damage and preserving ovarian function (14, 69, 70, 72-74).
Fertility preservation and reproductive outcomes: optimizing fertility preservation strategies and reproductive outcomes in women at risk of ovarian IRI remains a critical research priority. Advances in assisted reproductive technologies, ovarian tissue cryopreservation, and in vitro follicle maturation techniques offer new avenues for preserving fertility potential and improving pregnancy rates following ovarian injury.
Long-term follow-up and outcome assessment: longitudinal studies with extended follow-up periods are essential for evaluating the long-term impact of ovarian IRI on ovarian function, reproductive outcomes, and overall quality of life. Longitudinal cohort studies and registry databases can provide valuable insights into the natural history of ovarian IRI and inform the development of evidence-based guidelines for patient management (82, 83).
Translational and clinical trials: translating preclinical findings into clinical practice represents a significant challenge in ovarian IRI research, requiring well-designed clinical trials and multicenter collaborations to validate novel therapeutic interventions and treatment algorithms. Rigorous clinical trials incorporating standardized outcome measures, patient-reported outcomes, and long-term follow-up are essential for establishing the efficacy and safety of emerging therapeutic strategies (80, 82, 83, 86).
Addressing the challenges and embracing the future directions outlined above will be instrumental in advancing research on ovarian IRI, improving our understanding of its pathophysiology, and enhancing clinical management strategies for affected individuals. By fostering interdisciplinary collaborations, leveraging innovative technologies, and prioritizing patient-centered research approaches, we can work toward alleviating the burden of ovarian IRI and improving outcomes for women facing this challenging clinical condition.
11. Conclusion
In conclusion, ovarian IRI is a complex condition with major reproductive health implications. The interplay between ischemic insult and reperfusion triggers molecular events, such as oxidative stress, inflammation, and DNA damage, leading to tissue damage and functional impairment. This review has proposed a conceptual framework where lncRNAs, RBPs, and DNA damage interact dynamically, potentially influencing ovarian IRI outcomes. We emphasize that this nexus remains hypothetical and is presented to stimulate targeted research.
Addressing the identified challenges, particularly the need for ovarian tissue-specific mechanistic data, is essential for advancing the field. Future efforts should prioritize developing refined experimental models, functionally validating candidate molecules, and exploring the biomarker potential of lncRNAs and RBPs. Through interdisciplinary collaboration and focused investigation on the proposed hypotheses, we can work toward mitigating ovarian IRI burden and improving outcomes for affected women.
Data Availability
Data supporting the findings of this study are derived from the literature available in the public domain. All data sources are cited within the article and listed in the reference section.
Acknowledgments
We sincerely acknowledge the “Clinical Research Development Unit of Tabriz Valiasr hospital” at Tabriz University of Medical Sciences, Tabriz, Iran, for their valuable assistance in this research. Additionally, we extend our appreciation to the creators of the DeepSeek AI tool, as we used it for translation and to enhance the language of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ovarian ischemia-reperfusion injury (IRI) represents a significant challenge in gynecological health, frequently associated with conditions such as ovarian torsion, surgery, or transplantation. The disruption of blood flow followed by its restoration can cause substantial damage to ovarian tissues, leading to impaired follicular development, compromised ovarian function, and infertility. Elucidating the molecular mechanisms underlying ovarian IRI is essential for developing targeted therapies and mitigating their detrimental effects (1-4).
In recent years, emerging evidence has shed light on the significant role of non-coding RNAs (ncRNAs) in regulating gene expression and orchestrating cellular processes. Among these, long non-coding RNAs (lncRNAs) have emerged as key players in the intricate network of molecular interactions governing ovarian physiology and pathology. Long considered as transcriptional noise, lncRNAs are now recognized for their diverse functions in chromatin remodeling, transcriptional regulation, and post-transcriptional processing, making them critical regulators of cellular homeostasis and disease pathogenesis (5-7).
RNA-binding proteins (RBPs) are essential mediators of RNA metabolism, modulating the fate and function of cellular RNA molecules. By binding to specific RNA sequences or structures, RBPs regulate messenger RNAs (mRNA) stability, translation, and localization, profoundly influencing gene expression and cellular phenotype. RBP dysregulation has been implicated in various diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases, underscoring their importance as therapeutic targets and diagnostic biomarkers (8-10).
In ovarian IRI, the interplay between lncRNAs, RBPs, and DNA damage represents a promising but insufficiently explored area. Ischemic insult triggers a cascade of molecular events, including oxidative stress, inflammation, and DNA damage, that culminate in cellular dysfunction and death. Within this complex environment, lncRNAs and RBPs are hypothesized to be critical regulators of DNA damage response (DDR) pathways, potentially modulating the cellular response to genotoxic stress and influencing ovarian IRI outcomes (11, 12).
This narrative review aims to explore the potential interplay between lncRNAs, RBPs, and DNA damage in ovarian IRI. We synthesize evidence from related fields to propose a conceptual model for their roles and mechanisms in this specific condition. It is crucial to note that direct experimental evidence for this molecular nexus in ovarian tissue remains limited; therefore, this review serves to outline a framework for future hypothesis-driven research. Through comprehensive examination of current literature, we seek to advance the conceptual understanding of ovarian IRI pathophysiology and identify promising avenues for future investigation.
2. Methods
This narrative review was conducted to synthesize existing knowledge and propose hypotheses on the roles of lncRNAs, RBPs, and DNA damage in ovarian IRI. A literature search was performed using electronic databases, including PubMed, Scopus, and Web of Science. The search strategy employed a combination of keywords and MeSH terms related to: “long non-coding RNA” OR “lncRNA”, “RNA binding protein” OR “RBP”, “DNA damage” OR “DNA damage response”, and “ovarian ischemia reperfusion injury” OR “ovarian torsion” OR “ovarian IRI” OR “ischemia reperfusion injury”. To ensure contemporary relevance, the search was not restricted by date, but priority was given to recent publications (2018-2025). Given the scarcity of studies directly investigating lncRNAs and RBPs in ovarian IRI, the scope was broadened to include foundational and mechanistic studies from other organ systems (e.g., cardiac, cerebral, and renal IRI) and related pathologies (e.g., cancer and neurodegenerative diseases) where these molecules are well-characterized in the context of genotoxic stress. The inclusion criteria focused on original research articles and reviews published in English. The selected literature was analyzed to identify common mechanisms, potential interactions, and conceptual frameworks that could be extrapolated to the context of ovarian IRI.
3. Ovarian IRI: Pathophysiology and mechanisms
Ovarian IRI represents a complex pathological condition characterized by the temporary disruption of blood flow to the ovaries followed by the subsequent restoration of perfusion. This phenomenon can occur in various clinical scenarios, including ovarian torsion, surgical procedures involving the ovaries, and organ transplantation. Despite being a transient event, IRI can have profound and long-lasting consequences on ovarian function and reproductive health (13-15).
The pathophysiology of ovarian IRI involves a cascade of interconnected molecular and cellular events that contribute to tissue damage and dysfunction (16, 17). The initial phase of ischemia results in oxygen and nutrient deprivation, leading to metabolic alterations, ATP depletion, and accumulation of metabolic byproducts such as lactate and reactive oxygen species (ROS). This hypoxic microenvironment triggers a series of adaptive responses, including activation of hypoxia-inducible factors, inflammatory mediators, and cell death pathways (16, 18, 19).
Upon reperfusion, restoration of blood flow paradoxically exacerbates tissue injury through several mechanisms (20). The abrupt reintroduction of oxygen-rich blood leads to the generation of additional ROS, exacerbating oxidative stress and causing oxidative damage to cellular macromolecules, including lipids, proteins, and DNA. The sudden influx of inflammatory cells and cytokines further amplifies the inflammatory response, triggering endothelial dysfunction, leukocyte infiltration, and tissue inflammation (21).
At the cellular level, IRI disrupts cellular homeostasis and impairs essential cellular functions, including energy metabolism, ion transport, and protein synthesis. Mitochondrial dysfunction, calcium overload, and activation of pro-apoptotic pathways contribute to cellular damage and programmed cell death, leading to tissue injury and loss of ovarian follicles. Moreover, IRI induces DNA damage, including single-strand breaks (SSBs), double-strand breaks (DSBs), and DNA base modifications, further compromising genomic integrity and cellular viability (22, 23).
The interplay between oxidative stress, inflammation, and DNA damage plays a central role in the pathogenesis of ovarian IRI. ROS generated during IRI oxidative damage to cellular components, including DNA, leading to the activation of DDR pathways. DDR mechanisms, including DNA repair, cell cycle arrest, and apoptosis, are orchestrated by a complex network of signaling pathways involving DNA damage sensors, signal transducers, and effector proteins (24, 25).
Furthermore, emerging evidence suggests that lncRNAs and RBPs play critical roles in modulating the cellular response to IRI (26, 27). These ncRNA molecules and RBPs regulate gene expression, RNA stability, and post-transcriptional processing, influencing cellular fate and function in response to genotoxic stress. The dysregulation of lncRNAs and RBPs has been implicated in various pathological conditions, including ischemic injury and tissue damage (27, 28).
In summary, ovarian IRI is a multifaceted pathological condition characterized by oxidative stress, inflammation, and DNA damage. Elucidating the underlying mechanisms of ovarian IRI is essential for developing targeted therapeutic interventions aimed at mitigating tissue damage and preserving ovarian function. Understanding the roles of lncRNAs, RBPs, and DDR pathways in ovarian IRI may offer novel insights into its pathogenesis and potential therapeutic targets for future intervention strategies (Table I).

4. lncRNAs: Roles and functions
lncRNAs have emerged as crucial regulators of gene expression and cellular processes, contributing to various physiological and pathological conditions, including ovarian IRI (29). Despite lacking protein-coding capacity, lncRNAs exert diverse functions through intricate molecular mechanisms, making them key players in the complex interplay between genomic regulation and cellular homeostasis (29, 30).
In the context of ovarian IRI, lncRNAs participate in the orchestration of cellular responses to ischemic insult and reperfusion injury. These ncRNA molecules modulate gene expression at multiple levels, including transcriptional, post-transcriptional, and epigenetic regulation, thereby influencing cellular fate and function in response to genotoxic stress (31, 32).
At the transcriptional level, lncRNAs act as molecular scaffolds, decoys, or guides to recruit chromatin-modifying complexes and transcription factors to specific genomic loci, regulating the expression of target genes involved in cellular pathways relevant to ovarian IRI. Additionally, lncRNAs can function as enhancers or suppressors of gene transcription, fine-tuning gene expression patterns in response to environmental cues and cellular signals (33).
Furthermore, lncRNAs participate in post-transcriptional gene regulation by modulating mRNA stability, splicing, and translation. Through interactions with RBPs or microRNAs (miRNAs), lncRNAs influence the processing and turnover of target mRNAs, thereby regulating gene expression programs involved in cellular stress responses, apoptosis, and DNA damage repair pathways (34, 35).
Moreover, lncRNAs contribute to epigenetic regulation by modulating chromatin structure and histone modifications, thereby influencing gene accessibility and transcriptional activity. By recruiting chromatin-modifying enzymes or interacting with regulatory elements such as enhancers or promoters, lncRNAs exert long-range effects on gene expression and cellular phenotype, shaping the cellular response to ischemic insult and reperfusion injury in the ovary (5, 36).
Several lncRNAs have been implicated in the pathogenesis of ovarian IRI, either as mediators of cellular adaptation to hypoxic stress or as regulators of DDR pathways. For example, H19, a maternally expressed lncRNA, has been shown to regulate cell proliferation and apoptosis in response to hypoxia by modulating the expression of genes involved in cell cycle progression and apoptosis. Similarly, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been implicated in the regulation of DDR pathways and cellular senescence in various cell types, suggesting its potential involvement in ovarian IRI pathophysiology (37-39).
Overall, lncRNAs play multifaceted roles in regulating gene expression and cellular processes potentially relevant to ovarian IRI. Understanding their potential functions could provide novel insights and rationale for future experimental validation. Specifically, profiling lncRNA expression in ovarian IRI models and functionally characterizing their roles represent critical research priorities.
5. RBPs: Significance in cellular processes
RBPs play crucial roles in a myriad of cellular processes, including gene expression regulation, RNA metabolism, and post-transcriptional control. These multifunctional proteins interact with various RNA molecules, including mRNAs, lncRNAs, and miRNAs, exerting profound effects on RNA stability, translation, and localization (40, 41). In the context of ovarian IRI, RBPs emerge as key mediators of cellular responses to genotoxic stress and oxidative damage, influencing cellular fate and function in the face of ischemic insult and reperfusion injury (42).
One of the primary functions of RBPs is to modulate mRNA stability and translation, thereby regulating gene expression patterns in response to cellular stressors. RBPs can bind to specific sequences or structural motifs within the mRNA molecules, either stabilizing or destabilizing them, depending on the context and cellular conditions. By regulating mRNA turnover rates and translational efficiency, RBPs control the abundance of target proteins involved in cellular pathways relevant to ovarian IRI, including DNA damage repair, apoptosis, and oxidative stress response (43, 44).
Moreover, RBPs play critical roles in RNA processing and maturation, including pre-mRNA splicing, alternative splicing, and polyadenylation. By interacting with pre-mRNA transcripts and spliceosomal complexes, RBPs modulate the splicing patterns of target genes, generating diverse mRNA isoforms with distinct functional properties. Dysregulation of RBP-mediated splicing events has been implicated in various pathological conditions, including cancer, neurodegenerative diseases, and cardiovascular disorders, highlighting the significance of RBPs in cellular homeostasis and disease pathogenesis (45, 46).
Furthermore, RBPs are involved in the regulation of RNA localization and subcellular trafficking, ensuring proper spatial and temporal control of gene expression. By binding to specific mRNA sequences or structures, RBPs facilitate the transport of target transcripts to subcellular compartments, such as the cytoplasm, nucleus, or dendrites, where they undergo localized translation or storage. This dynamic regulation of RNA localization by RBPs is essential for cellular processes such as synaptic plasticity, cell migration, and embryonic development, underscoring the importance of RBPs in shaping cellular phenotype and function (47, 48). In addition to their roles in RNA metabolism, RBPs have been implicated in the regulation of ncRNA functions, including lncRNAs and miRNAs. RBPs interact with these ncRNA molecules, modulating their stability, activity, and interactions with target transcripts. Through these interactions, RBPs influence diverse cellular processes, including chromatin remodeling, transcriptional regulation, and epigenetic modification, thereby exerting profound effects on gene expression and cellular phenotype (49, 50).
In summary, RBPs likely play essential roles in cellular processes relevant to ovarian IRI. By regulating gene expression, RNA metabolism, and post-transcriptional control, RBPs may contribute to cellular adaptation to genotoxic stress and oxidative damage, potentially influencing ovarian IRI outcomes. Future work should determine whether specific RBPs, such as member of the Hu family of RBPs or quaking, exhibit altered expression or function in ovarian IRI and whether their manipulation affects DNA repair efficiency and follicular survival (51) (Table II).

6. DDR mechanisms in ovarian IRI
DDR mechanisms are crucial for maintaining genomic integrity and cellular homeostasis in response to genotoxic stress, such as that induced by ovarian IRI. Ovarian IRI, characterized by the temporary interruption of blood flow to the ovaries followed by the subsequent restoration of perfusion, triggers a cascade of molecular events that can result in DNA damage and genomic instability. In this context, DDR mechanisms play a central role in sensing, signaling, and repairing DNA lesions, thereby mitigating the detrimental effects of genotoxic stress and preserving ovarian function (52-54).
Upon exposure to genotoxic insults, cells activate a complex network of signaling pathways collectively known as the DDR (55). The DDR involves the coordinated action of sensor proteins, signal transducers, and effector proteins, culminating in cell cycle arrest, DNA repair, or programmed cell death, depending on the extent and severity of DNA damage. In ovarian IRI, ischemic insult and reperfusion injury induce various types of DNA lesions, including SSBs, DSBs, and DNA base modifications, triggering DDR activation to maintain genomic integrity and cellular viability (56, 57).
Central to DDR activation is the recruitment and activation of sensor proteins, such as the ataxia telangiectasia mutated (ATM) kinase and the ataxia telangiectasia and Rad3-related (ATR) kinase, which phosphorylate downstream effector proteins in response to DNA damage (58). ATM is primarily involved in the response to DSBs, whereas ATR responds to replication stress and SSBs, ensuring a coordinated and efficient DDR to different types of DNA lesions. Additionally, DNA-dependent protein kinase, another member of the phosphoinositide 3-kinase-related protein kinase family, is activated in response to DSBs, contributing to DNA repair and cell survival (59, 60).
Upon activation, DDR effector proteins orchestrate downstream cellular responses, including cell cycle checkpoint activation, DNA repair, and apoptosis (59, 61). Cell cycle checkpoints, such as the G1/S and G2/M checkpoints, are activated to temporarily arrest cell cycle progression, allowing time for DNA repair or triggering apoptosis if the damage is irreparable. DNA repair mechanisms, including homologous recombination (HR), non-homologous end joining, and base excision repair (BER), are activated to restore genomic integrity and prevent the accumulation of mutations. In cases of severe or irreparable DNA damage, apoptosis pathways are activated to eliminate damaged cells and prevent the propagation of genomic instability (61).
Furthermore, emerging evidence suggests that lncRNAs and RBPs play critical roles in modulating DDR pathways and cellular responses to genotoxic stress (62). These ncRNA molecules and RBPs regulate gene expression, RNA metabolism, and post-transcriptional control, influencing the cellular fate and function in response to DNA damage. Dysregulation of lncRNAs and RBPs has been implicated in various pathological conditions, including ovarian IRI, highlighting their significance as potential therapeutic targets for intervention strategies aimed at preserving ovarian function and mitigating tissue damage (58-61).
In summary, DDR mechanisms play a central role in the cellular response to ovarian IRI, orchestrating cell cycle arrest, DNA repair, and apoptosis to maintain genomic integrity and cellular viability. Understanding the molecular mechanisms underlying DDR activation and regulation in ovarian IRI may offer novel insights into its pathogenesis and provide potential therapeutic targets for intervention strategies aimed at preserving ovarian function and mitigating tissue damage. Further research is warranted to elucidate the specific roles of DDR pathways, lncRNAs, and RBPs in ovarian IRI and to explore their therapeutic potential in the management of this condition.
7. Interactions between lncRNAs, RBPs, and DNA damage: Molecular insights
Interactions between lncRNAs, RBPs, and DNA damage represent a complex molecular interplay that regulates cellular responses to genotoxic stress, including that induced by ovarian IRI. These interactions contribute to the coordination of DDR pathways, DNA repair mechanisms, and cellular fate decisions, ultimately shaping the outcome of genotoxic insults on cellular function and viability (28, 63).
At the molecular level, lncRNAs act as scaffolds, guides, or decoys for RBPs, facilitating their recruitment to specific genomic loci or RNA molecules and modulating their activity and function. By interacting with RBPs, lncRNAs influence various aspects of RNA metabolism, including RNA splicing, stability, translation, and localization, thereby regulating gene expression programs involved in DDR and DNA repair pathways (28, 64).
RBPs, in turn, recognize specific RNA sequences or structural motifs within lncRNAs, mediating their interactions with target RNAs or protein complexes involved in DDR. RBPs can modulate the stability and activity of lncRNAs, regulate their subcellular localization, or facilitate their association with other cellular components, such as chromatin-modifying complexes or DNA repair machinery (28).
The dynamic interplay between lncRNAs, RBPs, and DDR pathways influences cellular responses to genotoxic stress, including cell cycle arrest, DNA repair, and apoptosis. lncRNAs and RBPs participate in the regulation of DDR signaling cascades, modulating the activation and function of key DDR proteins, such as ATM, ATR, and DNA-dependent protein kinase, in response to DNA damage (28, 65).
Furthermore, lncRNAs and RBPs regulate DNA repair mechanisms, including HR, non-homologous end joining, and BER, by modulating the expression, activity, or localization of DNA repair factors. These interactions ensure the efficient and accurate repair of DNA lesions, preventing the accumulation of mutations and genomic instability (66, 67).
In addition to their roles in DDR and DNA repair, lncRNAs and RBPs influence cellular fate decisions in response to genotoxic stress, including cell cycle arrest, senescence, or apoptosis. By modulating the expression of genes involved in cell cycle regulation, apoptosis, or cellular senescence, lncRNAs and RBPs regulate the balance between cell survival and death in the face of DNA damage (68).
lncRNA, RBP, or DDR pathways dysregulation occurs in various pathologies. In ovarian IRI, we hypothesize that aberrant interactions between these elements may contribute to tissue damage and ovarian dysfunction (28, 58, 61). Future research should specifically investigate whether complexes such as lncRNA H19 with RBP HuR, or MALAT1 with specific splicing factors, form in ovarian cells during IRI and how they impact DDR efficacy.
8. Experimental models and methodologies in studying ovarian IRI
Experimental models and methodologies play a pivotal role in elucidating the pathophysiology of ovarian IRI and exploring potential therapeutic interventions. These models aim to recapitulate key aspects of ovarian IRI observed in clinical settings, including the transient interruption of blood flow to the ovaries followed by reperfusion-induced tissue damage. Additionally, sophisticated methodologies are employed to assess various aspects of ovarian function, tissue damage, and molecular changes associated with IRI (15, 23, 69).
Animal models: particularly rodent models such as rats and mice, are commonly used to study ovarian IRI due to their physiological relevance and feasibility of manipulation. Ovariectomy followed by ischemic insult induction through methods like vascular occlusion or torsion and subsequent reperfusion allows researchers to mimic the IRI observed clinically. These models enable the investigation of histological changes, functional alterations, and molecular mechanisms underlying ovarian IRI (15, 69, 70). For example, the rat ovarian torsion model typically involves 3 hr of torsion followed by detorsion, with tissue collection at various reperfusion timepoints to assess acute and subacute damage.
In vitro models: cell culture models using ovarian cell lines or primary ovarian cells provide a controlled environment to investigate cellular responses to ischemic insult and reperfusion. Ovarian granulosa cells, theca cells, and follicular cells can be subjected to hypoxic conditions followed by reoxygenation to simulate IRI. These models allow for the examination of cellular viability, apoptosis, oxidative stress, and DDR pathways in response to genotoxic stress (71, 72).
Molecular and biochemical analyses: various molecular and biochemical techniques are employed to characterize the molecular mechanisms underlying ovarian IRI. These include quantitative real-time polymerase chain reaction to assess gene expression changes, western blotting and immunohistochemistry to evaluate protein levels and localization, and enzyme-linked immunosorbent assays to quantify inflammatory cytokines, oxidative stress markers, and DNA damage markers (67, 69, 70, 72). Histological and morphological assessments: histological analysis of ovarian tissue sections using hematoxylin and eosin staining allows assessment of tissue morphology, structural integrity, and cellular damage following IRI. Other staining techniques, such as terminal deoxynucleotidyl transferase dUTP nick-end labeling assay for apoptosis detection and immunofluorescence staining for specific protein markers, provide insights into cellular responses and pathological changes induced by IRI (14, 73).
Functional assessments: functional assays, including hormone assays to measure ovarian hormone levels (e.g., estrogen and progesterone), ovarian reserve assessments (e.g., follicle counting), and reproductive function tests (e.g., mating studies and fertility assessments), allow for the evaluation of ovarian function and reproductive outcomes following IRI (14, 69, 70, 73).
Imaging techniques: non-invasive imaging modalities such as ultrasound, magnetic resonance imaging, and computed tomography scanning are utilized to visualize ovarian morphology, blood flow dynamics, and structural changes in response to IRI. These techniques provide valuable insights into the temporal and spatial progression of tissue damage and recovery following IRI (74, 75). Overall, integrating diverse experimental models and methodologies enables comprehensive ovarian IRI pathophysiology investigations and facilitates novel therapeutic strategy development for mitigating tissue damage and preserving ovarian function. Continued experimental technique and model system advancements are essential for advancing ovarian IRI understanding and translating experimental findings into clinical applications for improving patient outcomes (14, 69, 70, 72-74).
9. Clinical implications and therapeutic strategies
Clinical implications and therapeutic strategies for ovarian IRI encompass a broad spectrum of approaches aimed at mitigating tissue damage, preserving ovarian function, and improving patient outcomes. Given the complexity of ovarian IRI and its potential impact on reproductive health, it is essential to consider multifaceted interventions targeting various aspects of the pathophysiological cascade (76). Early detection and diagnosis: timely detection and diagnosis of ovarian IRI are crucial for initiating appropriate interventions and minimizing tissue damage. Clinical evaluation, including history taking, physical examination, and imaging studies such as ultrasound or magnetic resonance imaging, can aid in identifying patients at risk of ovarian IRI, particularly those undergoing surgeries associated with ovarian manipulation or vascular compromise. Surgical techniques and ovarian preservation: in cases where ovarian ischemia is anticipated, such as during ovarian cystectomy or ovarian torsion, surgical techniques aimed at minimizing ischemic insult and preserving ovarian blood supply are paramount. Strategies such as detorsion of twisted ovaries, ovarian auto-transplantation, or minimally invasive surgical approaches can help preserve ovarian function and fertility potential (77).
Pharmacological interventions: pharmacological agents targeting oxidative stress, inflammation, and apoptosis represent promising therapeutic avenues for mitigating tissue damage and improving outcomes in ovarian IRI. Antioxidants such as N-acetylcysteine, which scavenges ROS and restores cellular redox balance, have shown potential in preclinical studies for protecting against IRI in various organs, including the ovary (78, 79).
Hormonal support and ovarian stimulation: hormonal support with exogenous gonadotropins or gonadotropin-releasing hormone analogs may be considered to support ovarian function and follicular development in patients at risk of ovarian IRI. Ovarian stimulation protocols aimed at enhancing follicular recruitment and development may also be utilized to optimize fertility outcomes in women undergoing assisted reproductive technologies following ovarian injury (80, 81).
Fertility preservation techniques: for women facing the risk of ovarian dysfunction or premature ovarian failure due to IRI, fertility preservation techniques such as oocyte or embryo cryopreservation offer the possibility of preserving reproductive potential for future use. These techniques are particularly relevant for young cancer patients undergoing gonadotoxic treatments or individuals undergoing surgeries with potential ovarian damage (77).
Multidisciplinary care and counseling: multidisciplinary collaboration involving gynecologists, reproductive endocrinologists, oncologists, and fertility specialists is essential for providing comprehensive care to patients at risk of ovarian IRI. Counseling regarding the potential impact of ovarian injury on fertility, the available treatment options, and the importance of fertility preservation is critical for empowering patients to make informed decisions about their reproductive health (82).
Long-term follow-up and monitoring: long-term follow-up and monitoring of ovarian function, hormonal status, and reproductive outcomes are essential for assessing the efficacy of therapeutic interventions and addressing potential complications arising from ovarian IRI. Regular evaluation of ovarian reserve markers, menstrual cycles, and fertility outcomes allows for timely intervention and optimization of patient care (82, 83).
Overall, clinical implications and therapeutic strategies for ovarian IRI encompass a multifaceted approach for mitigating tissue damage, preserving ovarian function, and optimizing reproductive outcomes. Through surgical techniques, pharmacological interventions, fertility preservation techniques, and multidisciplinary care, clinicians can effectively manage ovarian IRI and improve affected individuals' quality of life. Continued research efforts and clinical practice advancements are essential for further refining therapeutic strategies and enhancing patient care in this challenging clinical scenario (83) (Table III).

10. Challenges and future directions in research
Challenges and future directions in research on ovarian IRI represent critical areas of focus aimed at addressing current limitations, advancing scientific understanding, and improving clinical management strategies. Despite significant progress in elucidating the pathophysiology of ovarian IRI, several challenges persist, necessitating innovative approaches and interdisciplinary collaborations to overcome them effectively (76).
Heterogeneity of ovarian IRI: one of the primary challenges in ovarian IRI research is the inherent heterogeneity of the condition, encompassing diverse etiologies, patient populations, and clinical presentations. Variability in ischemic insult severity, duration, and reperfusion dynamics complicates the interpretation of experimental findings and hinders the development of standardized diagnostic criteria and treatment protocols (84, 85).
Limited experimental models: current experimental models of ovarian IRI often fail to fully recapitulate the complexity of the clinical condition, posing challenges for translational research and therapeutic development. Improved animal models that better mimic the pathophysiological features of human ovarian IRI, including dynamic blood flow changes, tissue oxygenation levels, and hormonal fluctuations, are needed to enhance the relevance and predictive value of preclinical studies (86).
Mechanistic understanding of tissue damage: despite considerable efforts to elucidate the molecular mechanisms underlying ovarian IRI, gaps remain in our understanding of the specific pathways and signaling cascades driving tissue damage and dysfunction. Future research must delineate oxidative stress, inflammation, apoptosis, autophagy, and DDR pathway roles in mediating ovarian injury and identify novel therapeutic targets. A key priority is to move from associative observations to mechanistic studies, for example, by using knockout models to define the functional importance of specific lncRNAs (e.g., MALAT1) in the ovarian response to IRI.
Therapeutic intervention strategies: developing effective therapeutic interventions for ovarian IRI represents a significant research challenge, given the limited pharmacological agents and treatment modalities currently available. Innovative approaches targeting key pathophysiological mechanisms, such as oxidative stress scavengers, anti-inflammatory agents, and pro-survival signaling pathways, hold promise for mitigating tissue damage and preserving ovarian function (14, 69, 70, 72-74).
Fertility preservation and reproductive outcomes: optimizing fertility preservation strategies and reproductive outcomes in women at risk of ovarian IRI remains a critical research priority. Advances in assisted reproductive technologies, ovarian tissue cryopreservation, and in vitro follicle maturation techniques offer new avenues for preserving fertility potential and improving pregnancy rates following ovarian injury.
Long-term follow-up and outcome assessment: longitudinal studies with extended follow-up periods are essential for evaluating the long-term impact of ovarian IRI on ovarian function, reproductive outcomes, and overall quality of life. Longitudinal cohort studies and registry databases can provide valuable insights into the natural history of ovarian IRI and inform the development of evidence-based guidelines for patient management (82, 83).
Translational and clinical trials: translating preclinical findings into clinical practice represents a significant challenge in ovarian IRI research, requiring well-designed clinical trials and multicenter collaborations to validate novel therapeutic interventions and treatment algorithms. Rigorous clinical trials incorporating standardized outcome measures, patient-reported outcomes, and long-term follow-up are essential for establishing the efficacy and safety of emerging therapeutic strategies (80, 82, 83, 86).
Addressing the challenges and embracing the future directions outlined above will be instrumental in advancing research on ovarian IRI, improving our understanding of its pathophysiology, and enhancing clinical management strategies for affected individuals. By fostering interdisciplinary collaborations, leveraging innovative technologies, and prioritizing patient-centered research approaches, we can work toward alleviating the burden of ovarian IRI and improving outcomes for women facing this challenging clinical condition.
11. Conclusion
In conclusion, ovarian IRI is a complex condition with major reproductive health implications. The interplay between ischemic insult and reperfusion triggers molecular events, such as oxidative stress, inflammation, and DNA damage, leading to tissue damage and functional impairment. This review has proposed a conceptual framework where lncRNAs, RBPs, and DNA damage interact dynamically, potentially influencing ovarian IRI outcomes. We emphasize that this nexus remains hypothetical and is presented to stimulate targeted research.
Addressing the identified challenges, particularly the need for ovarian tissue-specific mechanistic data, is essential for advancing the field. Future efforts should prioritize developing refined experimental models, functionally validating candidate molecules, and exploring the biomarker potential of lncRNAs and RBPs. Through interdisciplinary collaboration and focused investigation on the proposed hypotheses, we can work toward mitigating ovarian IRI burden and improving outcomes for affected women.
Data Availability
Data supporting the findings of this study are derived from the literature available in the public domain. All data sources are cited within the article and listed in the reference section.
Acknowledgments
We sincerely acknowledge the “Clinical Research Development Unit of Tabriz Valiasr hospital” at Tabriz University of Medical Sciences, Tabriz, Iran, for their valuable assistance in this research. Additionally, we extend our appreciation to the creators of the DeepSeek AI tool, as we used it for translation and to enhance the language of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Pattology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |