BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijrm.ir/article-1-4-en.html
Introduction
Although, several comprehensive studies have emphasized the predictive value of traditional semen analysis for in-vivo fertility and pregnancy outcome (Bostofte et al., 1990; Barrat et al., 1992; Barrat et al., 1995), total or near-total fertilization failure in IVF cycles is not uncommon. This has been led to perform more specific and sensitive tests. These tests must be reliable, cost effective and provide clinically significant information (Barrat et al., 1998).An essential event in the process of fertilization is Acrosome Reaction (AR) .The ability of spermatozoa to undergo AR may reveal its normal function in fertilization and therefore has been proposed as a potential test of sperm function (Bray et al., 1999). In addition, AR is dependent on an increase of intracellular calcium [Ca2+]i and progesterone present, in high concentrations in the follicular fluid and in the cumulus matrix has been shown to be able to enhance AR, sperm zona pellucida penetration as well as sperm-oocyte penetration (Balid, et al, 1995). Krauttz et al. have shown high correlation between sperm responsiveness to progesterone in form of AR or calcium increase [Ca2+]i and fertilization in vitro (Krausz et al.,1995). When spermatozoa are treated with progesterone, a rapid transient elevation of [Ca2+]i occurs (within seconds) (bray et al,1999,Kirkman Brown 2000). The measurement of this calcium increase [Ca2+]i has been proposed as a biological assay of sperm function and may assist in the prediction of sperm fertilizing ability (Krausz et al., 1996).
We have performed this inexpensive and easy test, the [Ca2+]i increase in response to progesterone, in spermatozoa from 86 unselected patients that were devided into three groups according to their semen parameters criteria. The objective was to evaluate the predictive value of semen parameters in sperm fertility progesterone. Also, to find if there is correlation between each individual sperm parameter and [Ca2+]i increase in response to progesterone.

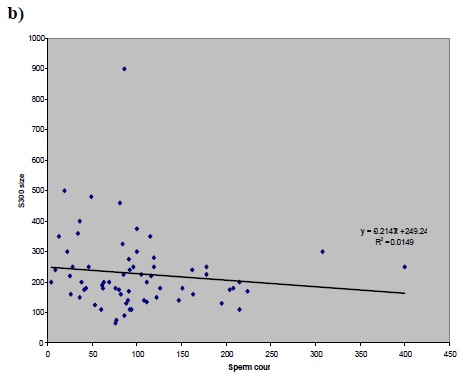
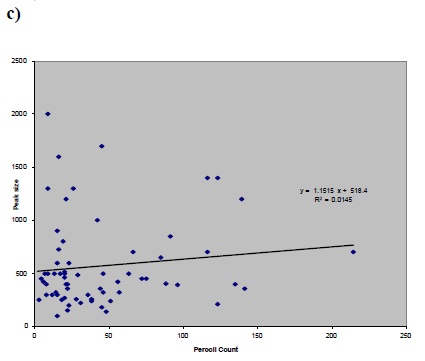
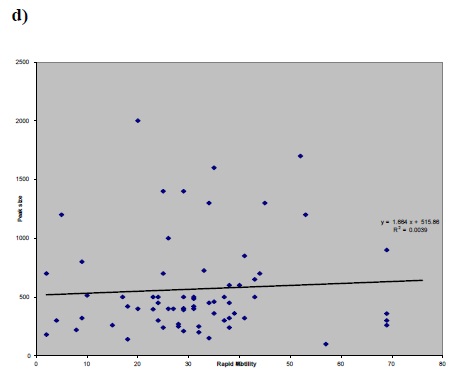
a) Figure 1 (a,b,c and d): Correlation between factor of a) peak size and b) S300 size versus sperm concentration (before preparation) and peak size versus c) CASA rapid motility d) Percoll concentration.a)
Patients
This cross-sectional study took place on 86 patients that referred to andrology lab of ACU in Birmingham women’s hospital. Patients were divided into three groups according to semen analysis criteria as follows:
-
Normal (n=20): >20m/ml sperm count, >50% motility, >25% rapid progressive, >50%
-
viable, >15% normal form, <50% antisperm antibody, before preparing and > 10 m/ml, >75% progressive after preparation.
-
IVF (n=47) 5-20 m/ml sperm concentration, >5% normal forms and >25% rapid with rest of sperm parameters.
-
ICSI (n=21) <5m/ml sperm concentration,<25% rapid and <5% normal forms.
In addition, 6 samples from fertile donor were used as controls.
Chemicals
Fura 2/ AM was purchased from molecular probes (Leiden, Netherlands) at unite size of 50 µg. 0.01 grams pluronic F127 (sigma, pool Dorset, UK) in 50 µl DMSO, dissolved in incubator for 5-10 min. 20 µl pluronic in DMSO was added to one tube of Fura 2/AM. 0.3804 gr. EGTA (sigma, pool Dorset, UK) was dissolved in 4 ml pbs and adjusted with NAOH (PH=7.3). 0.062 gr. Digitonin(sigma, pool Dorset, UK) dissolved in 1 ml DMSO. Progesterone was dissolved in dimethyl sulfoxide (DMSO) at an initial concentration of 2mg/ml.
Preparation of spermatozoa
Semen parameters were assessed according to WHO criteria. After semen analysis, spermatozoa were separated from seminal plasma by Percoll separation solution and then washed in EBSS. Sperm parameters were then assessed and washed spermatozoa was adjusted to 2 ml with final concentration of 6×106 mil/ml with aid of computer-assisted semen analysis (CASA) for flurimetric [Ca2+]i measurements.
Measurement of [Ca2+]i
Spermatozoa at a concentration less than 6×106 mil/ml in the EBSS with final volume of 2 ml were incubated at and co2 5% with 6µl prepared Fura 2/ AM for 12 min. The labelled cells were washed and resuspended in 2ml EBSS, and then incubated for 15 min. [Ca2+]i was measured using a flurimetry method. Spermatozoa were transferred to a quartz cuvette. Fluorescence was measured using a spectrofluorometer (Perkins-Elmer LS1503) set at 340-380nm excitation with emission at 510 nm. The spermatozoa were stimulated with 20 µl (0.5 µg/ml) progesterone directly in the cuvette at 200s of the experiment. For calibration, 20µl of prepared Digitonin (5mM) was added at 500s of the experiment,
for determining of Rmax followed by adding of 200µl EGTA (20mM) at 800s the experiment for determining of Rmax followed by adding of 200µl EGTA (20mM) at 800s the experiment for determining of Rmin. Conversion of fluorescence indicator to values of [Ca2+]i was done by Perkins-Elmer soft ware using dissociation constant (Kd) of Fura2 for calcium of 224 nM. Auto fluorescence of the cells was assessed by measuring the fluorescence of unloaded cells. After Auto fluorescence subtraction,calibration was done by determining of Rmax (ratio maximum) and Rmin (ratio minimum).
Statistical analysis
Microsoft Excel and SPSS software were used for statistical analysis. Results are expressed as mean ± SEM. The distribution of the raw data was examined by scatter gram analysis as well as linear regression.
Results
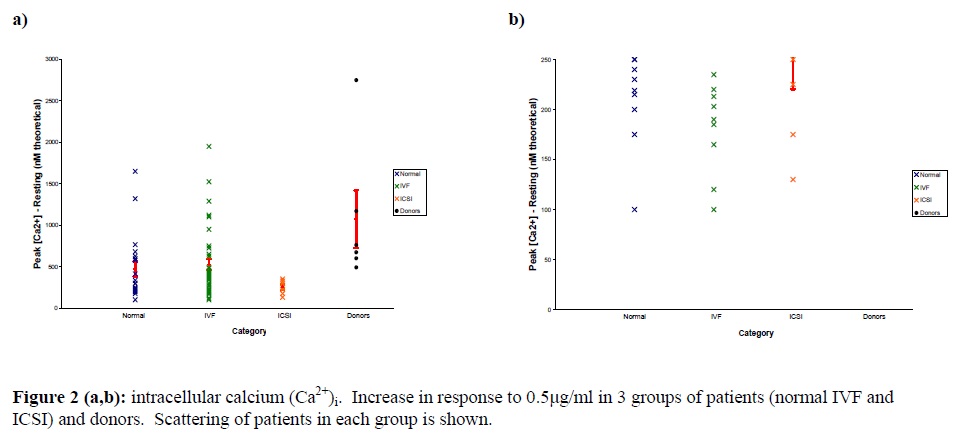
In this cross-sectional study, The average basal [Ca2+]i in group 1 (normal) was 89.9±13.9, in groups 2 (IVF) and 3 (ICSI), it was 88.1±10.7 and 70.7 ±12.6 respectively. However, the average basal [Ca2++] was 162.5±30.6 in control. The average peak size in groups 1, 2, 3 and the donors was 561.35, 615.2, 321.4 and 1236.3, respectively.No significant statistical correlation was found between each individual sperm parameter and [Ca2+]i response to progesterone (Fig.1 : a, b, c, d); between sperm count and peak size P=0.502 , r=-0.085, sperm count and S300 size P=0.607, r=+0.0605, CASA rapid motility and peak size P=0.338, r=-0.124, percoll count and peak size p=0.889, r=0.018.
Intracellular calcium [Ca2+]i increase in response to progesterone in patients of each group; normal, IVF, ICSI In normal category; 8 patients (36.6%) and in IVF group 9 patients (19.36%) had poor responses to progesterone. In addition, in 4 patients, we couldn’t get any response, probably due to lack of cell labelling with Fura 2/AM.
Discussion
Mainly lack of fertilization in cases of male and unexplained infertility is suggested to be due to biochemical alteration in function of spermatozoa in fertilization rather than defects in motility or concentration. For this reason, traditional semen parameters in the prediction of male fertility is probably of little clinical value (Barrat et al., 1998). Acrosome reaction, an essential event in fertilization is dependent on an increase of intracellular calcium [ca2+ ]i and progesterone has been shown to be able to enhance several sperm function including the AR ( Kirkman-Brown et al., 2000).An impaired response of the spermatozoa to progesterone , either in terms of an increase in the intracellular calcium concentration, and/ or induction of the AR is a significant and clinically well recognized defect in male factor infertility ( Krausz et al., 1995). Krausz et al. (1996) showed that the [ca2+ ]i as well as the AR increases in response to progesterone significantly in patients with a fertilization >50%.Furtheremore, they showed that in cases of fertilization failure, no increase of [ca2+ ]i or AR was observed in response to progesterone. These findings indicate that response to progesterone is functionally related to the sperm fertilizing ability. Therefore, measurement of sperm response to progesterone could be used as a diagnostic tool for the evaluation of sperm fertilizing ability. In this study, for evaluation of the value of each individual sperm parameter in predicting of sperm fertilizing ability, no statistically correlation between each parameter and [ca2+ ]i peak size was observed. The finding indicates that each individual sperm parameter has no significant value in prediction of fertility ability of sperm.On the basis of sperm parameters criteria, patients that referred to ACU andrology lab were assigned into in normal, IVF or ICSI groups for future treatments. For evaluation, the magnitude of intracellular calcium increase in response to progesterone in each category and scattering of patients in each group were analyzed. The average basal and average peak size in the fertile donor samples had significant difference with other three patients groups (Table I).All patients in ICSI group had poor responses to progesterone. In addition 36.6% in IVF group and 19.6% in normal group had poor responses to progesterone. These findings show that our categories based on semen parameters criteria as a whole have a value to predict the fertility ability of sperm. However, there were samples in IVF and normal group that had poor response to progesterone. This result probably indicates a functional defect that leads to fertilization failure in IVF cycles. It seems that this test is reliable because an intracellular calcium increase was noticed in response to progesterone in a wide range of sperm parameters. Intracellular calcium [Ca2+]i increase in response to progesterone is related to predicting value of sperm parameters in most cases. However, the response of sperm to progesterone could be different in some cases that are expected in normal or IVF category based on our Semen analysis criteria. We suggest that the [Ca2+]i measurements can be done easily and quickly which may reveal more about IVF outcome.
Acknowledgement
I would like to thank the Prof. CLR Barratt, Dr. S.J Publicover, Dr. J.C. Kirkman-Brown, and students of the Reproductive Biology and Genetics; E. Punt, C.Harper, M. Forman and K.Bedu-Addo at the University of Birmingham.
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




