Mon, Feb 2, 2026
[Archive]
Volume 4, Issue 1 (7-2006)
IJRM 2006, 4(1): 35-39 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dehghan M H, Dehghanan R. Plasma level of vitamin C in women with pre-eclampsia in Ardabil, Iran. IJRM 2006; 4 (1) :35-39
URL: http://ijrm.ir/article-1-43-en.html
URL: http://ijrm.ir/article-1-43-en.html
1- Department of Biochemistry, Ardabil University of Medical Sciences, Ardabil, Iran , mh.dehghan@arums.ac.ir
2- Ardabil Educational Organization, Ardabil, Iran
2- Ardabil Educational Organization, Ardabil, Iran
Full-Text [PDF 115 kb]
(630 Downloads)
| Abstract (HTML) (3380 Views)
Full-Text: (425 Views)
Introduction
Pre-eclampsia affects between 0.4% to 2.8% of all pregnancies in developed countries and even more in developing countries, leading to as many as 8,370,000 cases worldwide per year (1). Maternal mortality and morbidity from hypertensive disorders of pregnancy, including pre-eclampsia, remain high worldwide and represent a troublesome issue of modern perinatology (2). The risk of pre-eclampsia significantly increases in women with previous history of pre-eclampsia and in those with either preexisting vascular diseases or conditions associated with increased cardiovascular risk, such as renal diseases, hypertension, diabetes, thrombophilia, and obesity (body mass index >29) (3). However, aetiology and pathogenesis of the pre-eclampsia is still unknown.
Pathophysiological characteristics of pre-eclampsia contain altered vascular reactivity, loss of vascular integrity, activation of coagulation cascade and inadequate trophoblastic invasion of spiral arteries, which suggests dysfunction of the vascular endothelium (4-7). In addition, It has been documented that pre-eclampsia is accompanied by oxidative stress that contributes to vascular dysfunction (6-8). A recent study in which oxidative stress was investigated in maternal blood by a wide variety of markers showed evidence of oxidative stress in pre-eclmapsia by some (notably reduced plasma vitamin C concentrations), but not all markers used, leading to the conclusion of a mild state of oxidative stress in blood of pre-eclamptic women (9).
The inadequate trophoblastic invasion results in a high resistance and low flow uteroplacental circulation that develop placental ischaemia and hypoxia (7). These could trigger lipid peroxidation and induce endothelial damage in pre-eclampsia (6,8).
Oxidative stress plays an important role in the pathophysiology of pre-eclampsia. It has been postulated that the placental ischaemia initiates excessive free radical production and triggers multiple chain reactions, which ultimately results in widespread endothelial damage. Under hypoxic condition, the enzyme xanthine dehydrogenase is shifted to the oxidase generating free oxygen radicals (7) that attach unsaturated lipids and thiol containing proteins in cell membranes (10). This oxidative attack causes damage to enzymes, cell membranes, proteins and nuclear materials. The superoxide anion, an important free radical, can inactivate endothelium-derived-relaxing factor (11) and inhibit prostacyclin synthesis (12,13) resulting in the platelet aggregation and vasospasm, which are the two important characteristics of pre-eclampsia. Thus, oxidative stress arises from increased production of reactive oxygen species or deficiency in antioxidant nutrients. It has been suggested that deficiency in antioxidant vitamins would developed pre-eclampsia (6,14). Antioxidant vitamins, with the ability to stabilise highly reactive free radicals, act as the first line of defense against free radical attack and lipid peroxidation (15). Vitamin E, vitamin C and beta-carotene (pro-vitamin A) are known to be powerful antioxidants (15-17).
Vitamin E (α-tocopherol) is the major lipid-soluble antioxidant that protects cells against lipid peroxidation.Vitamin C is a quencher of free radicals as well as singlet oxygen. It also regenerates vitamin E. Beta-carotene, by quenching singlet oxygen, also acts as a potential antioxidant. Antioxidant vitamins have been reported to have an important function in regulating blood pressure (18). Nitrous oxide (NO) is the most important endothelium dependent vasodilator and is highly susceptible to oxidative damage. The antioxidants vitamins, C and E, are able to inhibit formation of free radicals thereby inhibiting the oxidation of NO, and thus maintaining the vasodilator status of blood vessels. Vitamin C has been reported to have a direct acute effect on the inhibition of the constrictor response of resistance arteries to a variety of stimulies. It is reported that there is synergism between the actions of vitamin E and vitamin C (19-20). However, there is little information regarding the functions of antioxidant vitamins particularly plasma concentrations of vitamin C in pre-eclampsia especially in Iranian popultations. Therefore, we tried to evaluate the plasma concentrations of vitamin C in pre-eclampsia and normo-tensive pregnant women in Ardabil province, Iran.
Materials and Methods
Study population
This study was designed as a cross sectional case control study and was conducted in the department of Obstetrics and Gynecology, Ardabil Educational Hospital “Allavi”, Ardabil, Iran. The biochemical analysis was carried out in the department of biochemistry.
The study included 40 pre-eclampsia and 80 normotensive pregnant women of singleton gestation in third trimester. The subjects were selected under defined criteria. Pre-eclamptic patients were at 28 to 42 weeks of singleton gestation, with one measurement of diastolic pressure of 110 mmHg or more, or two measurements of 90 mmHg or more on two consecutive occasions 6 hours or more apart, and urinary protein of 2+ or more (100mg/dl; dipstick reagent strip, Boehringer Mannheim, Germany) (21). The exclusion criteria included; previous history of hypertension and proteinuria before conception or before 20 weeks of gestation, any associated disorders, a history of antioxidant vitamin therapy during last one year, and smoking. The patients who developed convulsions were considered to be suffering from eclampsia and were excluded. As cohort control, age and socio-economically matched healthy normo-tensives women at 28 to 42 weeks of singleton gestation with no urinary protein were recruited by convenience.
Body Mass Index (BMI)
BMI is a widely used method of calculating percentage of body fat as a continuum rather than simple conventional classification units. This study tries to determine the mechanisms by which body fat increases pre-eclampsia risk.
BMI was calculated as weight before pregnancy (kg) divided by squared height (m2).
Plasma analysis
Peripheral blood samples (5ml) were collected from antecubital vein of cases and controls in heparin tubes. The samples were kept undisturbed on wet ice for at least 10 minutes, protected from ultraviolet light, and were processed within 30 minutes. The samples were centrifuged at 3000 rpm for 10 minutes in a cooling centrifuge. Plasma was decanted into cryovials, preserved with metaphosphoric acid/ dithiothreitol solution, and stored at -24oC for analysis. The concentration of ascorbic acid in the plasma was determined by spectrophotometric within 48 hours as described by Kyaw (22). Absorbance was measured against a blank reagent at 700 nm by a spectrophotometer (UV-VIS, Ependorf Co., Germany). Plasma vitamin C was categorized as deficiency (less than 0.2 mg/dl), marginal deficiency (0.2-0.4 mg/dl) and normal (more than 0.4 mg/dl).
Statistical analysis
SPSS software package (version 10.0, SPSS Inc. Chicago, USA) was used to analyse the data. Descriptive statistics were calculated for all variables. The associations of plasma vitamin levels and BMI were assessed using multiple regression analysis. In the regression model, plasma vitamin levels, maternal age and body mass index (BMI) were adjusted. The results were statistically analyzed using student’s t-test and regression analysis. Values were expressed as percentage and mean ± SE.
Results
Clinical parameters of the patients and normotensive controls are shown in Table I. The mean maternal and gestational ages of the patients and controls were similar. Patients had different gravida distribution and had nearly equal proteinurea. The systolic and diastolic pressures of pre-eclamptic patients were significantly (p<0.001) higher than controls (Table I).

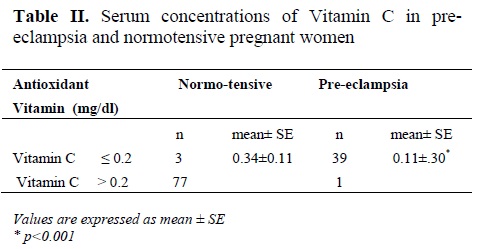
Table II shows plasma vitamin C concentration in the cases and controls. It was observed that vitamin C concentration in pre-eclamptic patients was significantly lower than control (p< 0.001).
Vitamin C level in pre-eclamptic patirnts was found to be influenced by maternal age (p<0.01). In total, the correlation between the age and vitamin C level was determined and it was found to be positively related to each other (p<0.01). In controls, gestational age showed positive influence on vitamin C level (p<0.01).
Discussion
The etiology of pre-eclampsia is elusive. There is no definite way to prevent pre-eclampsia. Oxidative stress is thought to be associated with pre-eclampsia (5-7). From early pregnancy, the human placenta influences maternal homeostasis. It is rich in mitochondria and when fully developed consumes about 1% of the basal metabolic rate of the pregnant woman (23). Placenta is also highly vascular and is exposed to high maternal oxygen partial pressure. These characteristics explain, in part, the generation of superoxide, because about 5% of all electrons in the mitochondrial respiratory chain leak out of the mitochondrion (24,25). Initially the placenta, has a hypoxic environment. As it matures and its vascularization develops, it changes to an oxygen-rich environment and its abundant mitochondrial mass favors the production of reactive oxygen species (ROS) (26).
Vitamin C deficiency affects placental structure, ROS production and facilitates placental infection, which result in increased risk of premature rupture of placental membranes and premature births (27,28). The placenta is avid to absorb vitamin C so when maternal plasma ascorbic acid concentration is low it is absorbed by active mechanisms. At higher plasma ascorbic acid concentrations, it enters the placenta by passive diffusion. Curiously, ascorbate is preferentially taken up in the oxidized form (dehydroascorbic acid), which more easily passes the lipid layer. However, it is transformed to its reduced form at the expense of other reducing agents, and donate electrons before transferring to the fetus by active transport (29).
Plasma antioxidant activity has been reported to increase progressively throughout pregnancy (4,10,18). This study investigated plasma vitamin C concentration in pre-eclampsia and attempted to evaluate its correlation with the etiology of this disease.
Some reports documented an increase in plasma vitamin E levels in pre-eclampsia (10,27), but others have found a decreased concentration (5,7).
Vitamins E (alpha-tocopherol) and C, have differences in the contribution they make to antioxidant potential, as vitamin E is the major lipid-soluble chain-breaking antioxidant in cell-membranes while vitamin C is an important aqueous phase antioxidant.
Antioxidants may act synergistically, for instance when vitamin C regenerates alpha-tocopherol from the tocopherol radical (30), this ‘sacrificial’ antioxidant acts more by sparing vitamin E than by recycling (31). Thus, it might be important to evaluate the effectiveness of potential antioxidant defense systems in limiting scale.
This study provides evidence for the relationship between plasma vitamin C levels during the third trimester and pre-eclampsia. Our results indicated the plasma vitamin C concentration to be significantly lower in pre-eclampsia, as compared to control group which is similar to the other studies (32-35).
Ascorbic acid has been described as the major ‘front line’ water-soluble antioxidant. Vitamin C can regenerate vitamin E from the alpha-tocopheroxyl radical. In addition, it has also been documented that vitamin C, as an effective antioxidant in human plasma, provides major defense against the diseases caused by oxidative stress (18,19).
It is likely that severe oxidative stress in eclampsia utilises a higher amount of vitamin C to fight the oxidative stress leading to a depletion of the vitamin. The higher vitamin C levels in pre-eclampsia may serve to prevent oxidation of NO (endothelium dependent vasodilator) to maintain the vasodilatation of blood vessels (18).
The vitamin C levels in pre-eclampsia showed positive correlation with maternal age which is in agreement to some extent with the report of Kharb (35).
Deleterious effects of free radicals include initiation of lipid peroxidation, oxidative damage of biomolecules, and cellular dysfunction, which may initiate maternal vascular endothelial dysfunction and leukocyte activation (20). Free radical chain oxidation and the interaction of various antioxidants are now attracting the attention of nutritionists (32). The important role of vitamin C in pre-eclampsia, suggests that changes in its concentration may influence susceptibility of vascular endothelium to oxygen toxicity (36). Thus, analyzing vitamin C concentration may provide a means of assessing the total capacity of the chain-breaking antioxidants to prevent lipid peroxidation in plasma.
Conclusion
The results of our study showed that plasma vitamin C concentration of the pre-eclamptic women was significantly lower than that of controls. Vitamin C level in pre-eclampsia was also found to be influenced by maternal age, gestational age and BMI. More studies should be done before pre-eclampsia is clinically evident. Vitamin C assessment should be done at different stages of pregnancy with tools validated for each stage of pregnancy.
In addition, in view of low plasma vitamin C concentrations in pre-eclampsia, a combination of vitamins C and E is a promising prophylactic strategy for prevention of pre-eclampsia.
Acknowledgement
This research was supported in part by an award from the Deputy for Research and Technology, Ministry of Health, Iran, which is greatly appreciated.
The authors are indebted to the participants of the study for their cooperation. They are also grateful for the technical expertise contributed by Dr. Ehdaivand and the staff of Gynecology Departement, Allavi Hospital, Ardabil, Iran.
Our sincere thanks go to Dr. Firooz Amani for his excellent cooperation in statistical analysis.
Pre-eclampsia affects between 0.4% to 2.8% of all pregnancies in developed countries and even more in developing countries, leading to as many as 8,370,000 cases worldwide per year (1). Maternal mortality and morbidity from hypertensive disorders of pregnancy, including pre-eclampsia, remain high worldwide and represent a troublesome issue of modern perinatology (2). The risk of pre-eclampsia significantly increases in women with previous history of pre-eclampsia and in those with either preexisting vascular diseases or conditions associated with increased cardiovascular risk, such as renal diseases, hypertension, diabetes, thrombophilia, and obesity (body mass index >29) (3). However, aetiology and pathogenesis of the pre-eclampsia is still unknown.
Pathophysiological characteristics of pre-eclampsia contain altered vascular reactivity, loss of vascular integrity, activation of coagulation cascade and inadequate trophoblastic invasion of spiral arteries, which suggests dysfunction of the vascular endothelium (4-7). In addition, It has been documented that pre-eclampsia is accompanied by oxidative stress that contributes to vascular dysfunction (6-8). A recent study in which oxidative stress was investigated in maternal blood by a wide variety of markers showed evidence of oxidative stress in pre-eclmapsia by some (notably reduced plasma vitamin C concentrations), but not all markers used, leading to the conclusion of a mild state of oxidative stress in blood of pre-eclamptic women (9).
The inadequate trophoblastic invasion results in a high resistance and low flow uteroplacental circulation that develop placental ischaemia and hypoxia (7). These could trigger lipid peroxidation and induce endothelial damage in pre-eclampsia (6,8).
Oxidative stress plays an important role in the pathophysiology of pre-eclampsia. It has been postulated that the placental ischaemia initiates excessive free radical production and triggers multiple chain reactions, which ultimately results in widespread endothelial damage. Under hypoxic condition, the enzyme xanthine dehydrogenase is shifted to the oxidase generating free oxygen radicals (7) that attach unsaturated lipids and thiol containing proteins in cell membranes (10). This oxidative attack causes damage to enzymes, cell membranes, proteins and nuclear materials. The superoxide anion, an important free radical, can inactivate endothelium-derived-relaxing factor (11) and inhibit prostacyclin synthesis (12,13) resulting in the platelet aggregation and vasospasm, which are the two important characteristics of pre-eclampsia. Thus, oxidative stress arises from increased production of reactive oxygen species or deficiency in antioxidant nutrients. It has been suggested that deficiency in antioxidant vitamins would developed pre-eclampsia (6,14). Antioxidant vitamins, with the ability to stabilise highly reactive free radicals, act as the first line of defense against free radical attack and lipid peroxidation (15). Vitamin E, vitamin C and beta-carotene (pro-vitamin A) are known to be powerful antioxidants (15-17).
Vitamin E (α-tocopherol) is the major lipid-soluble antioxidant that protects cells against lipid peroxidation.Vitamin C is a quencher of free radicals as well as singlet oxygen. It also regenerates vitamin E. Beta-carotene, by quenching singlet oxygen, also acts as a potential antioxidant. Antioxidant vitamins have been reported to have an important function in regulating blood pressure (18). Nitrous oxide (NO) is the most important endothelium dependent vasodilator and is highly susceptible to oxidative damage. The antioxidants vitamins, C and E, are able to inhibit formation of free radicals thereby inhibiting the oxidation of NO, and thus maintaining the vasodilator status of blood vessels. Vitamin C has been reported to have a direct acute effect on the inhibition of the constrictor response of resistance arteries to a variety of stimulies. It is reported that there is synergism between the actions of vitamin E and vitamin C (19-20). However, there is little information regarding the functions of antioxidant vitamins particularly plasma concentrations of vitamin C in pre-eclampsia especially in Iranian popultations. Therefore, we tried to evaluate the plasma concentrations of vitamin C in pre-eclampsia and normo-tensive pregnant women in Ardabil province, Iran.
Materials and Methods
Study population
This study was designed as a cross sectional case control study and was conducted in the department of Obstetrics and Gynecology, Ardabil Educational Hospital “Allavi”, Ardabil, Iran. The biochemical analysis was carried out in the department of biochemistry.
The study included 40 pre-eclampsia and 80 normotensive pregnant women of singleton gestation in third trimester. The subjects were selected under defined criteria. Pre-eclamptic patients were at 28 to 42 weeks of singleton gestation, with one measurement of diastolic pressure of 110 mmHg or more, or two measurements of 90 mmHg or more on two consecutive occasions 6 hours or more apart, and urinary protein of 2+ or more (100mg/dl; dipstick reagent strip, Boehringer Mannheim, Germany) (21). The exclusion criteria included; previous history of hypertension and proteinuria before conception or before 20 weeks of gestation, any associated disorders, a history of antioxidant vitamin therapy during last one year, and smoking. The patients who developed convulsions were considered to be suffering from eclampsia and were excluded. As cohort control, age and socio-economically matched healthy normo-tensives women at 28 to 42 weeks of singleton gestation with no urinary protein were recruited by convenience.
Body Mass Index (BMI)
BMI is a widely used method of calculating percentage of body fat as a continuum rather than simple conventional classification units. This study tries to determine the mechanisms by which body fat increases pre-eclampsia risk.
BMI was calculated as weight before pregnancy (kg) divided by squared height (m2).
Plasma analysis
Peripheral blood samples (5ml) were collected from antecubital vein of cases and controls in heparin tubes. The samples were kept undisturbed on wet ice for at least 10 minutes, protected from ultraviolet light, and were processed within 30 minutes. The samples were centrifuged at 3000 rpm for 10 minutes in a cooling centrifuge. Plasma was decanted into cryovials, preserved with metaphosphoric acid/ dithiothreitol solution, and stored at -24oC for analysis. The concentration of ascorbic acid in the plasma was determined by spectrophotometric within 48 hours as described by Kyaw (22). Absorbance was measured against a blank reagent at 700 nm by a spectrophotometer (UV-VIS, Ependorf Co., Germany). Plasma vitamin C was categorized as deficiency (less than 0.2 mg/dl), marginal deficiency (0.2-0.4 mg/dl) and normal (more than 0.4 mg/dl).
Statistical analysis
SPSS software package (version 10.0, SPSS Inc. Chicago, USA) was used to analyse the data. Descriptive statistics were calculated for all variables. The associations of plasma vitamin levels and BMI were assessed using multiple regression analysis. In the regression model, plasma vitamin levels, maternal age and body mass index (BMI) were adjusted. The results were statistically analyzed using student’s t-test and regression analysis. Values were expressed as percentage and mean ± SE.
Results
Clinical parameters of the patients and normotensive controls are shown in Table I. The mean maternal and gestational ages of the patients and controls were similar. Patients had different gravida distribution and had nearly equal proteinurea. The systolic and diastolic pressures of pre-eclamptic patients were significantly (p<0.001) higher than controls (Table I).

Table II shows plasma vitamin C concentration in the cases and controls. It was observed that vitamin C concentration in pre-eclamptic patients was significantly lower than control (p< 0.001).
Vitamin C level in pre-eclamptic patirnts was found to be influenced by maternal age (p<0.01). In total, the correlation between the age and vitamin C level was determined and it was found to be positively related to each other (p<0.01). In controls, gestational age showed positive influence on vitamin C level (p<0.01).

Discussion
The etiology of pre-eclampsia is elusive. There is no definite way to prevent pre-eclampsia. Oxidative stress is thought to be associated with pre-eclampsia (5-7). From early pregnancy, the human placenta influences maternal homeostasis. It is rich in mitochondria and when fully developed consumes about 1% of the basal metabolic rate of the pregnant woman (23). Placenta is also highly vascular and is exposed to high maternal oxygen partial pressure. These characteristics explain, in part, the generation of superoxide, because about 5% of all electrons in the mitochondrial respiratory chain leak out of the mitochondrion (24,25). Initially the placenta, has a hypoxic environment. As it matures and its vascularization develops, it changes to an oxygen-rich environment and its abundant mitochondrial mass favors the production of reactive oxygen species (ROS) (26).
Vitamin C deficiency affects placental structure, ROS production and facilitates placental infection, which result in increased risk of premature rupture of placental membranes and premature births (27,28). The placenta is avid to absorb vitamin C so when maternal plasma ascorbic acid concentration is low it is absorbed by active mechanisms. At higher plasma ascorbic acid concentrations, it enters the placenta by passive diffusion. Curiously, ascorbate is preferentially taken up in the oxidized form (dehydroascorbic acid), which more easily passes the lipid layer. However, it is transformed to its reduced form at the expense of other reducing agents, and donate electrons before transferring to the fetus by active transport (29).
Plasma antioxidant activity has been reported to increase progressively throughout pregnancy (4,10,18). This study investigated plasma vitamin C concentration in pre-eclampsia and attempted to evaluate its correlation with the etiology of this disease.
Some reports documented an increase in plasma vitamin E levels in pre-eclampsia (10,27), but others have found a decreased concentration (5,7).
Vitamins E (alpha-tocopherol) and C, have differences in the contribution they make to antioxidant potential, as vitamin E is the major lipid-soluble chain-breaking antioxidant in cell-membranes while vitamin C is an important aqueous phase antioxidant.
Antioxidants may act synergistically, for instance when vitamin C regenerates alpha-tocopherol from the tocopherol radical (30), this ‘sacrificial’ antioxidant acts more by sparing vitamin E than by recycling (31). Thus, it might be important to evaluate the effectiveness of potential antioxidant defense systems in limiting scale.
This study provides evidence for the relationship between plasma vitamin C levels during the third trimester and pre-eclampsia. Our results indicated the plasma vitamin C concentration to be significantly lower in pre-eclampsia, as compared to control group which is similar to the other studies (32-35).
Ascorbic acid has been described as the major ‘front line’ water-soluble antioxidant. Vitamin C can regenerate vitamin E from the alpha-tocopheroxyl radical. In addition, it has also been documented that vitamin C, as an effective antioxidant in human plasma, provides major defense against the diseases caused by oxidative stress (18,19).
It is likely that severe oxidative stress in eclampsia utilises a higher amount of vitamin C to fight the oxidative stress leading to a depletion of the vitamin. The higher vitamin C levels in pre-eclampsia may serve to prevent oxidation of NO (endothelium dependent vasodilator) to maintain the vasodilatation of blood vessels (18).
The vitamin C levels in pre-eclampsia showed positive correlation with maternal age which is in agreement to some extent with the report of Kharb (35).
Deleterious effects of free radicals include initiation of lipid peroxidation, oxidative damage of biomolecules, and cellular dysfunction, which may initiate maternal vascular endothelial dysfunction and leukocyte activation (20). Free radical chain oxidation and the interaction of various antioxidants are now attracting the attention of nutritionists (32). The important role of vitamin C in pre-eclampsia, suggests that changes in its concentration may influence susceptibility of vascular endothelium to oxygen toxicity (36). Thus, analyzing vitamin C concentration may provide a means of assessing the total capacity of the chain-breaking antioxidants to prevent lipid peroxidation in plasma.
Conclusion
The results of our study showed that plasma vitamin C concentration of the pre-eclamptic women was significantly lower than that of controls. Vitamin C level in pre-eclampsia was also found to be influenced by maternal age, gestational age and BMI. More studies should be done before pre-eclampsia is clinically evident. Vitamin C assessment should be done at different stages of pregnancy with tools validated for each stage of pregnancy.
In addition, in view of low plasma vitamin C concentrations in pre-eclampsia, a combination of vitamins C and E is a promising prophylactic strategy for prevention of pre-eclampsia.
Acknowledgement
This research was supported in part by an award from the Deputy for Research and Technology, Ministry of Health, Iran, which is greatly appreciated.
The authors are indebted to the participants of the study for their cooperation. They are also grateful for the technical expertise contributed by Dr. Ehdaivand and the staff of Gynecology Departement, Allavi Hospital, Ardabil, Iran.
Our sincere thanks go to Dr. Firooz Amani for his excellent cooperation in statistical analysis.
Type of Study: Original Article |
References
1. Villar K, Say L, Gülmezoglu AM, Merialdi M, Lindheimer MD, Betran AP, Piaggio G. Eclampsia and pre-eclampsia: a health problem for 2000 years. In: Critchley H, MacLean AB, Poston L, Walker JJ, eds. Pre-eclampsia. London: RCOG Press 2003; 189-207.
2. Walker JJ: Pre-eclampsia. Lancet 2000; 356:1260-1265. [DOI:10.1016/S0140-6736(00)02800-2]
3. Nelson-Piercy C. Pre-eclampsia: the women at risk. In: Critchley H, MacLean AB, Poston L, Walker JJ, eds. Pre-eclampsia. London: RCOG Press 2003; 342-353.
4. Staff AC, Ranheim T, Khoury J, Henriksen T. Increased contents of phopholipids, cholesterol, and lipid peroxides in decidua basalis in women with pre-eclampsia. Am J Obstet Gynecol 1999; 180: 587-592. [DOI:10.1016/S0002-9378(99)70259-0]
5. Madazli R, Benian A, Gumustas K, Uzun H, Ocak V, Aksu F. Lipid peroxidation and antioxidants in pre-eclampsia. Eur J Obstet Gynecol Reproduct Biol 1999; 85: 205-208. [DOI:10.1016/S0301-2115(99)00023-8]
6. Hubel CA, Kozlov AV, Kagan VE, Evans RW, Davidge ST, McLaughlin MK, Roberts JM. Decreased transferring and increased transferrin saturation in sera of women with pre-eclamsia. Implications for oxidative stress. Am J Obstet Gynecol 1997;175: 692-700. [DOI:10.1053/ob.1996.v175.a74252]
7. Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MMJ. Antioxidants in the treatment of severe pre-eclampsia. an explanatory randomised trial. Br J Obstet Gynecol 1997; 104: 689-696. [DOI:10.1111/j.1471-0528.1997.tb11979.x]
8. Shaarawy M, Aref A, Salem ME, Sheiba M. Radical-scavenging antioxidants in pre-eclampsia and eclampsia. Int J Gynecol Obstet 1998; 60: 123-128. [DOI:10.1016/S0020-7292(97)00256-7]
9. Llurba E, Gratacos E, Martin-Gallan P, Cabero L, Dominguez C. A comprehensive study of oxidative stress and antioxidant status in pre-eclampsia and normal pregnancy. Free Radic Biol Med 2004; 37: 557-570. [DOI:10.1016/j.freeradbiomed.2004.04.035]
10. Freeman C. Free radicals and tissue injury. Lab Invest 1982; 47: 412-426.
11. Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986; 320: 454-456. [DOI:10.1038/320454a0]
12. Wang Y, Walsh SW, Guo J, Zhang J. Maternal levels of prostacyclin, thromboxane, vitamin E, and lipid peroxides throughout normal pregnancy. Am J Obstet Gynecol 1991; 165: 1690-1694. [DOI:10.1016/0002-9378(91)90016-K]
13. Weiss SJ, Turk J, Needleman P. A mechanism for hydroperoxide mediated inactivation of prostacyclin synthetase. Blood 1979; 208: 1191-1196.
14. Ehrenkrarnz RA. Vitamin E and the neonate. Arch Pediatr Adoles Med 1980; 134:1157-1166. [DOI:10.1001/archpedi.1980.02130240041013]
15. Mayes PA . Structure and function of lipid soluble vitamins. In: Herper's Biochemistry. 24th edition. Murray RK, Granner DK, Mayes PA, Rodwell VW (eds). Appleton and Lange, Connecticut.1996; 614-624.
16. Czerinichow S, Hercberg S .International studies concerning the role of antioxidant vitamins in cardiovascular diseases: a review. J Nutr Health Aging 2001;5(3): 188-195.
17. Diplock AT. Antioxidant nutrients and disease prevention: an overview. Am J Clin Nutr 1991; 53: 1893-1935.
18. Dakshinamuti K, Dakshinamuti S. Blood pressure regulation and micronutrients. Nutr Res Review 2001; 14: 3-43. [DOI:10.1079/095442201108729123]
19. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 1989; 86: 6377-6381. [DOI:10.1073/pnas.86.16.6377]
20. Raijmakers MT, Dechend R, Poston L. Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension 2004; 44:374-380. [DOI:10.1161/01.HYP.0000141085.98320.01]
21. Gifford RW, August PA, Cunningham G, Green LA, Lindheimer MD, McNellis D, et al. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183: S1-S22. [DOI:10.1067/mob.2000.107928]
22. Kyaw A. A simple colorimetric method for ascorbic acid determination in blood, plasma. Clin Chim Acta 1978; 86:153-157. [DOI:10.1016/0009-8981(78)90128-6]
23. Sies H. Oxidative stress II. Oxidants and antioxidants. Academic Press, London 1991.
24. Page KR. The physiology of human placenta. UCL Press Limited, London 1993;164.
25. Fridovich I. Hypoxia and oxygen toxicity. Adv Neurol 1979; 26: 255-259.
26. Liochev SI, Friedovich I. How does superoxide dismutase protect against tumor necrosis factor: a hypothesis informed by effect of superoxide on ''free'' iron. Free Radic Biol Med 1997; 23: 668-671. [DOI:10.1016/S0891-5849(97)00060-9]
27. Romero R. Intrauterine infection, premature birth and the Fetal Inflammatory Response Syndrome. J Nutr 2003; 133: 1668S-1673S. [DOI:10.1093/jn/133.5.1668S]
28. Casanueva E, Magan AL, Pfeffer F, Baez A. Incidence of premature rupture of membranes in pregnant women with low leukocyte levels of vitamin C. Eur J Clin Nutr 1991; 45: 401-405.
29. Choi JL, Rose RC. Transport and metabolism of ascorbic acid in human placenta. Am J Physiol 1989; 257: c110-c113. [DOI:10.1152/ajpcell.1989.257.1.C110]
30. Schiff E, Friedman SA, Stampfer M, Kao L,Barrett PH, Sibai BM. Dietary consumption and plasma concentrations of vitamin E in pregnancies Complicated by pre-eclampsia. Am J Obstet Gynecol 1996; 175: 1024-1028. [DOI:10.1016/S0002-9378(96)80046-9]
31. Niki E, Saito T, Kawakami A, Kamiya Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J Biol Chem 1984; 259:4177-4182
32. Lindeman JHN, Grobben DVZ, Schrijver J, Speek AJ, Poorthuis BJHM, Berger HM. The total free radical trapping ability of cord blood plasma in preterm and term babies. Pediatr Res 1989; 26:20-24. [DOI:10.1203/00006450-198907000-00008]
33. Uotila JT, Tuimala RJ, Aarnio TM. Findings on lipid peroxidation and antioxidant function in hypertensive complications of pregnancy. Br J Obstet Gynaecol 1993; 100:270-276. [DOI:10.1111/j.1471-0528.1993.tb15242.x]
34. Kharb S. Total free radical trapping antioxidant potential in pre-eclampsia. Int J Gynecol Obstet 2000; 39:23-26 [DOI:10.1016/S0020-7292(99)00198-8]
35. Kharb S. Vitamins E & C in pre-eclampsia. Eur J Obstet Gynecol Reproduct Biol 2001; 1: 195-197
36. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biolo Endocrinol 2005; 3:28. [DOI:10.1186/1477-7827-3-28]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




