Sat, Feb 21, 2026
[Archive]
Volume 4, Issue 1 (7-2006)
IJRM 2006, 4(1): 1-5 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Farimani M, Amiri I, Hoseini S. Day 3 serum inhibin-B level is not predictive of ovarian assisted reproductive technologies outcome. IJRM 2006; 4 (1) :1-5
URL: http://ijrm.ir/article-1-49-en.html
URL: http://ijrm.ir/article-1-49-en.html
1- Department of Gynecology & Obstetrics, Facculty of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
2- Depatrment of Anatomy, Facculty of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
2- Depatrment of Anatomy, Facculty of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
Full-Text [PDF 145 kb]
(707 Downloads)
| Abstract (HTML) (3855 Views)
Full-Text: (593 Views)
Introduction
Assisted reproduction and development of in-vitro fertilization (IVF) techniques have revolutionized the treatment of infertility. Because IVF technique is expensive and not completly successful, many researches attempted to determine predictive factors for more successful outcome. Prognostic assessment of ovarian reserve has relied upon indirect markers of ovarian function, such as age (1-3), Follicle Stimulating Hormone (FSH) at baseline (4-7), and estradiol concentrations (8,9). The reduction of ovarian function or “reserve” is apparantly due to reduced number of ovarin premordial follicles, from over 250.000 at menarche to very few at the end of reproductive age. Age and regularity of menses alone are unreliable predictors of ovarian reserve. Neither folliculaor phase FSH nor estradiol concontrations, could finally indicate that ovarian function is normal and unimpaired (1-3). Ovarian volume has also been proposed as a predictor of ovarian response but there were still a substantial number of pregnancies amoung the women with very small ovarian volumes (10,11). In theory, the direct products of granulosa cells might better reflect ovarian secretory capacity and follicle number. Inhibin-B is one of these products which regulates FSH secretion by negative feedback (11-17). The aging of ovary is accompanied by a decrease in inhibin-B secretion (18,19). Early follicular phase serum inhibin-B may be a suitable marker of ovarian follicle reserve and fertility potential (20-24). However, several studies found no or limited clinical value in measuring basal early follicular inhibin-B regarding to ART outcome (25-33).
According to these contraversial data and in order to test the hypothesis that baseline inhibin-B concentration would serve as improved marker of IVF outcome, we examined baseline inhibin-B on day 3, prior to ovarian stimulation and compared it with standard markers of ART outcome.
Materials and Methods
From April 2004 until June 2005, seventy one women undergoing IVF/ICSI treatment were included in this study in the Fatemieh Infertility Research Center. Our inclusion criteria were 1) age under 40 years old, 2) basal FSH level under 15 mIU/mL 3) presence of both ovaries, 4) no evidence of endocrine disorders (normal levels of thyroid-stimulating hormone, testostrone, androstendione, and prolactine), 5) no evidence of ovarian cyst bigger than 2cm in diameter, and 6) written informed consent. All patients received oral contraceptive pills (LD) from the 5th day of their previous menstrual cycle then administration of GnRH agonist (Suprefact, Hoechst, Germany), 500 g/day, was begun on the 19th day. After menstruation, ovarian volumes were calculated as the volume of an elliposoid (length× width× depth×
g/day, was begun on the 19th day. After menstruation, ovarian volumes were calculated as the volume of an elliposoid (length× width× depth×  /6) by transvaginal ultrasound (6.5 MHZ, Dynamic immaging) on day 3. If no follicular cysts larger than 12mm in diameter was detected, Busereline was reduced to 200
/6) by transvaginal ultrasound (6.5 MHZ, Dynamic immaging) on day 3. If no follicular cysts larger than 12mm in diameter was detected, Busereline was reduced to 200  g/day and gonadotropin (Gonal F, Serono, Swiss) was started i.m. daily, depending on age (150 IU in >35 years old and 225 IU in <35 years old). Whenever follicular cysts bigger than 12mm were seen, estradiol was checked and if it was
g/day and gonadotropin (Gonal F, Serono, Swiss) was started i.m. daily, depending on age (150 IU in >35 years old and 225 IU in <35 years old). Whenever follicular cysts bigger than 12mm were seen, estradiol was checked and if it was 50 pg/ml, then the administration of gonadotropin was started. The dose of the gonadotropin was changed according to the folliclular growth. When more than 2 follicles bigger than or equal to 18mm were seen, HCG (Pregnyle, Organon, Germany) 10000 IU were injected to induce final oocyte maturation and 36 hours later, ovum was picked up. After 3 days if fertilization occured, embryo was transfered. Poor response was defined when fewer than four follicules at retrival were collected. Pregnancy was diagnosed by increasing concentrations of ß-HCG 2 weeks after embryo transfer and the subsequent demonstration of an intrauterine gestational sac by transvaginal scan 2 weeks later.
50 pg/ml, then the administration of gonadotropin was started. The dose of the gonadotropin was changed according to the folliclular growth. When more than 2 follicles bigger than or equal to 18mm were seen, HCG (Pregnyle, Organon, Germany) 10000 IU were injected to induce final oocyte maturation and 36 hours later, ovum was picked up. After 3 days if fertilization occured, embryo was transfered. Poor response was defined when fewer than four follicules at retrival were collected. Pregnancy was diagnosed by increasing concentrations of ß-HCG 2 weeks after embryo transfer and the subsequent demonstration of an intrauterine gestational sac by transvaginal scan 2 weeks later.
Hormone analyses
FSH and stradiol concentrations were measured at diagnostic laboratory of the Fatemieh Hospital using routine procedures. To detect Inhibin-B, all of collected sera immediately were freozen and stored at –80°C until assay. Inhibin-B concentration in serum was measured by Inhibin-B assay kit purchased from Serotec (Oxford, UK), using specific two-site enzyme immunoassay. Briefly, standards and samples were diluted as appropriate and mixed with a half volume of distilled water containing 10% sodium dodecyl sulphate (SDS). After 3 min at 100°C, tubes were cooled before adding freshly prepared hydrogen peroxide solution. After additional incubation at room temperature, duplicate aliquots of denatured and oxidized samples and standards were transferred to antibody-coated microtitre plates, that were incubated at room temperature, overnight. After washing with enzyme immunoassay (EIA) wash buffer [0.1 mol/l Tris–HCl, 0.15 mol/l NaCl, 10% (w/v) bovine serum albumin, 5% (v/v) Triton X-100, and 0.1% (w/v) sodium azide, pH 7.5], 50 µl alkaline phosphatase-conjugated mouse anti-human inhibin-α subunit antibody was used. The plates were then incubated for 3 hours. Plates were washed and bound alkaline phosphatase was quantified using a commercially available enzyme immunoassay amplification system (ELISA), which was used according to the supplier's instructions. The inhibin-B plates were read at 490 nm on an automated EIA plate reader (BRIO: Basic Radium Immunoassay Operator, Radim spa, Pomezia, Italy). The assay detection limit for inhibin-B was less than 10 pg/ml. Within and between plate coefficients of variation were <5.0 and 9.0% respectively. Cross-reactions for each assay with the various inhibin-related proteins were <0.5%.
Statistical analysis
Statistical analysis was performed using the commericially available software package SPSS version 11. Student’s t-test and 2 were used for analysis and results were reported as mean SD. p<0.05 was considered as significant level.
2 were used for analysis and results were reported as mean SD. p<0.05 was considered as significant level.
Results
Amoung 71 patients, 11 cases were excluded from the study because of discontinuation of the cycle. The mean age of the women was 29 years (range 20-39). The casuses of infertility were: male factor infertility (40%), tubal infertility (13.3%), ovulatory infertility (18.3%), compound infertility (25%) and unexplained infertility (3.3%).
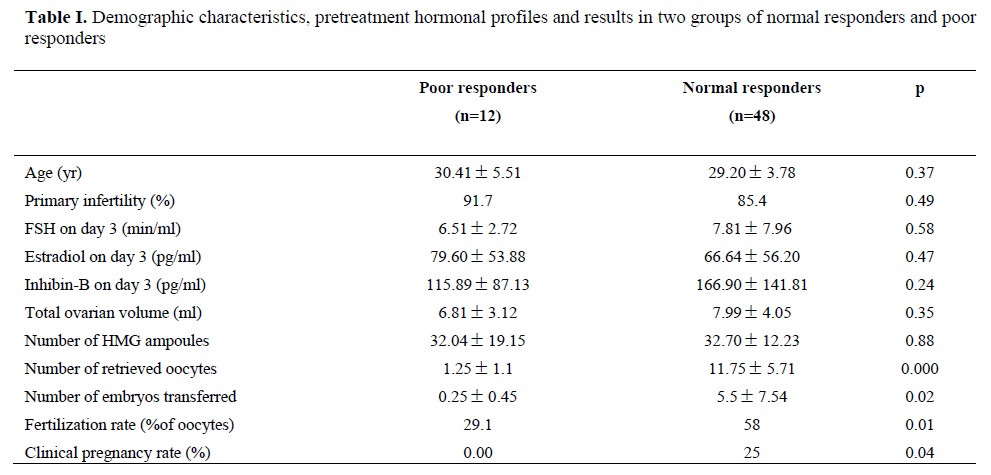
According to the number of retrieved oocytes, the patients were divided into two groups; 12 poor responders (less than 4 oocyte), and 48 normal responders (≥4 oocytes).
Demographic characteristitcs, pretreatment hormonal profiles and results of two groups are shown on table I. Total pregnancy rate was 20%. There were no statistically significant differences in mean of age, mean of duration of infertility, type of infertility, mean of day 3 FSH, E2 and inhibin-B levels, total ovarian volume, number of ampoulses and duration of the ovarian stimulation in two groups. As expected, fertilization rate and clinical pregnancy rate are significantly reduced in poor responders. There was no significant differences in the mean of inhibin-B levels between two groups.
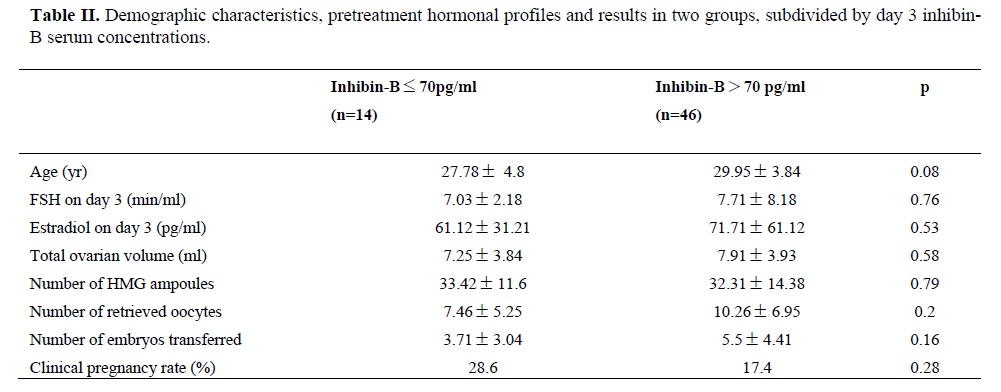
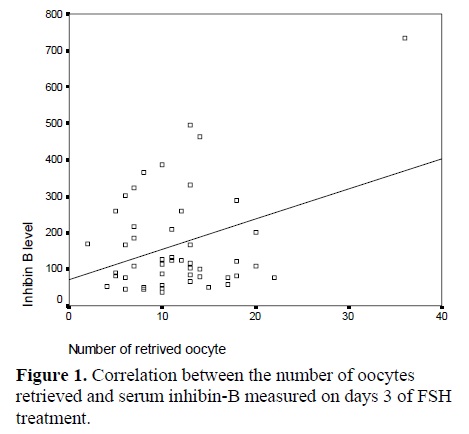
According to inhibin-B levels, the patients were divided into two groups; inhibin-B concentrations <70 pg/ml (14 patients) and inhibin-B concentrations >70 pg/ml (46 patients) (11). Demographic characteristitcs, pretreatment hormonal profiles and results of the two groups are shown on table II. There were no statistically significant differences in FSH and estradiol on day 3 between two groups. However, the number of retrieved oocytes in the patients with inhibin-B <70 pg/ml was lower than this number in the other group but this difference was not statistically significant. Correlation coefficients were determined between the number of oocytes retrieved and serum inhibin-B levels on day 3 administration, and significant correlations (r=0.358) was found between them (fig.1).
Disscusion
The most useful predictive information for an infertile couple should be obtained before beginning assisted reproductive techniques. Basal inhibin concentrations have been evaluated previously as predictive markers for pregnancy in IVF cycles. In two (12,24) of three (13) previous studies, higher inhibin-B on day 2–3 was associated with a greater number of oocytes retrieved in IVF cycles (24) and with subsequent pregnancy (12) which was better predictor of the response to exogenous gonadotrophins than age (24) and was equivalent to day 3 FSH.
Seifer et al (20) demonstrated that, pregnancy rates were higher in patients with day 3 inhibin-B concentrations of over 45 pg/ml than this rate in patients with lower values. In 1999, they compared 109 normal responders with 47 poor responders and they did not find any significant difference in day 3 FSH leveles between the two groups, but poor responders had significantly lower day 3 inhibin-B leveles (21). The study of Hall et al (27) did not support the use of day 3 inhibin-B as a predictive marker of IVF outcome. In addition, base line FSH, E2 and inhibin-B were not significantly different between pregnant and non pregnant patients. In the present study the mean inhibin-B in normal responders was 166.9 141 pg/ml versus 115.8
141 pg/ml versus 115.8 87 pg/ml in poor responders, which the difference was not statistically significant (p= 0.24). In addition, there were no statistically significant differences in day 3 serum FSH, estradiol and ovarian volume between the two groups. A combination of FSH higher than the median value (6.51 mIU/ml) and inhibin-B lower than the median value (115.8 pg/ml) was not seen in poor responders. A cut off of serum inhibin-B conceantrations between normal and poor responders was calculated (cut- offs 45-70-100-120-150 pg/ml), but these cut-offs were not statistically significant.
87 pg/ml in poor responders, which the difference was not statistically significant (p= 0.24). In addition, there were no statistically significant differences in day 3 serum FSH, estradiol and ovarian volume between the two groups. A combination of FSH higher than the median value (6.51 mIU/ml) and inhibin-B lower than the median value (115.8 pg/ml) was not seen in poor responders. A cut off of serum inhibin-B conceantrations between normal and poor responders was calculated (cut- offs 45-70-100-120-150 pg/ml), but these cut-offs were not statistically significant.
There was a positive correlation between inhibin-B level on day 3 and retrived oocyte number but there was not any correlation between inhibin-B level on day 3 and ART outcome. There was tremendous overlap in baseline inhibin-B concentrations between pregnant and non-pregnant subjects, and inhibin-B alone failed to predict pregnancy.The results of this study confirm Hall et al findings (27) and are contraversal with Tsuchia et al (12) and Seifer et al (20) results.
Conclusion
The present study shows that inhibin-B concentration on day 3 positevly correlate with the number of oocytes retrieved during ART. Our results do not support the use of day 3 inhibin-B as a predictive marker of IVF outcome.
Acknowledgment
The authors wish to express thank to Mr. Mani Kashani, and other staff and personel of Fatemieh Infertility Research Center, specially Mrs. Ramazani for their kind assistance in this study.
Assisted reproduction and development of in-vitro fertilization (IVF) techniques have revolutionized the treatment of infertility. Because IVF technique is expensive and not completly successful, many researches attempted to determine predictive factors for more successful outcome. Prognostic assessment of ovarian reserve has relied upon indirect markers of ovarian function, such as age (1-3), Follicle Stimulating Hormone (FSH) at baseline (4-7), and estradiol concentrations (8,9). The reduction of ovarian function or “reserve” is apparantly due to reduced number of ovarin premordial follicles, from over 250.000 at menarche to very few at the end of reproductive age. Age and regularity of menses alone are unreliable predictors of ovarian reserve. Neither folliculaor phase FSH nor estradiol concontrations, could finally indicate that ovarian function is normal and unimpaired (1-3). Ovarian volume has also been proposed as a predictor of ovarian response but there were still a substantial number of pregnancies amoung the women with very small ovarian volumes (10,11). In theory, the direct products of granulosa cells might better reflect ovarian secretory capacity and follicle number. Inhibin-B is one of these products which regulates FSH secretion by negative feedback (11-17). The aging of ovary is accompanied by a decrease in inhibin-B secretion (18,19). Early follicular phase serum inhibin-B may be a suitable marker of ovarian follicle reserve and fertility potential (20-24). However, several studies found no or limited clinical value in measuring basal early follicular inhibin-B regarding to ART outcome (25-33).
According to these contraversial data and in order to test the hypothesis that baseline inhibin-B concentration would serve as improved marker of IVF outcome, we examined baseline inhibin-B on day 3, prior to ovarian stimulation and compared it with standard markers of ART outcome.
Materials and Methods
From April 2004 until June 2005, seventy one women undergoing IVF/ICSI treatment were included in this study in the Fatemieh Infertility Research Center. Our inclusion criteria were 1) age under 40 years old, 2) basal FSH level under 15 mIU/mL 3) presence of both ovaries, 4) no evidence of endocrine disorders (normal levels of thyroid-stimulating hormone, testostrone, androstendione, and prolactine), 5) no evidence of ovarian cyst bigger than 2cm in diameter, and 6) written informed consent. All patients received oral contraceptive pills (LD) from the 5th day of their previous menstrual cycle then administration of GnRH agonist (Suprefact, Hoechst, Germany), 500
Hormone analyses
FSH and stradiol concentrations were measured at diagnostic laboratory of the Fatemieh Hospital using routine procedures. To detect Inhibin-B, all of collected sera immediately were freozen and stored at –80°C until assay. Inhibin-B concentration in serum was measured by Inhibin-B assay kit purchased from Serotec (Oxford, UK), using specific two-site enzyme immunoassay. Briefly, standards and samples were diluted as appropriate and mixed with a half volume of distilled water containing 10% sodium dodecyl sulphate (SDS). After 3 min at 100°C, tubes were cooled before adding freshly prepared hydrogen peroxide solution. After additional incubation at room temperature, duplicate aliquots of denatured and oxidized samples and standards were transferred to antibody-coated microtitre plates, that were incubated at room temperature, overnight. After washing with enzyme immunoassay (EIA) wash buffer [0.1 mol/l Tris–HCl, 0.15 mol/l NaCl, 10% (w/v) bovine serum albumin, 5% (v/v) Triton X-100, and 0.1% (w/v) sodium azide, pH 7.5], 50 µl alkaline phosphatase-conjugated mouse anti-human inhibin-α subunit antibody was used. The plates were then incubated for 3 hours. Plates were washed and bound alkaline phosphatase was quantified using a commercially available enzyme immunoassay amplification system (ELISA), which was used according to the supplier's instructions. The inhibin-B plates were read at 490 nm on an automated EIA plate reader (BRIO: Basic Radium Immunoassay Operator, Radim spa, Pomezia, Italy). The assay detection limit for inhibin-B was less than 10 pg/ml. Within and between plate coefficients of variation were <5.0 and 9.0% respectively. Cross-reactions for each assay with the various inhibin-related proteins were <0.5%.
Statistical analysis
Statistical analysis was performed using the commericially available software package SPSS version 11. Student’s t-test and
Results
Amoung 71 patients, 11 cases were excluded from the study because of discontinuation of the cycle. The mean age of the women was 29 years (range 20-39). The casuses of infertility were: male factor infertility (40%), tubal infertility (13.3%), ovulatory infertility (18.3%), compound infertility (25%) and unexplained infertility (3.3%).
According to the number of retrieved oocytes, the patients were divided into two groups; 12 poor responders (less than 4 oocyte), and 48 normal responders (≥4 oocytes).
Demographic characteristitcs, pretreatment hormonal profiles and results of two groups are shown on table I. Total pregnancy rate was 20%. There were no statistically significant differences in mean of age, mean of duration of infertility, type of infertility, mean of day 3 FSH, E2 and inhibin-B levels, total ovarian volume, number of ampoulses and duration of the ovarian stimulation in two groups. As expected, fertilization rate and clinical pregnancy rate are significantly reduced in poor responders. There was no significant differences in the mean of inhibin-B levels between two groups.
According to inhibin-B levels, the patients were divided into two groups; inhibin-B concentrations <70 pg/ml (14 patients) and inhibin-B concentrations >70 pg/ml (46 patients) (11). Demographic characteristitcs, pretreatment hormonal profiles and results of the two groups are shown on table II. There were no statistically significant differences in FSH and estradiol on day 3 between two groups. However, the number of retrieved oocytes in the patients with inhibin-B <70 pg/ml was lower than this number in the other group but this difference was not statistically significant. Correlation coefficients were determined between the number of oocytes retrieved and serum inhibin-B levels on day 3 administration, and significant correlations (r=0.358) was found between them (fig.1).



Disscusion
The most useful predictive information for an infertile couple should be obtained before beginning assisted reproductive techniques. Basal inhibin concentrations have been evaluated previously as predictive markers for pregnancy in IVF cycles. In two (12,24) of three (13) previous studies, higher inhibin-B on day 2–3 was associated with a greater number of oocytes retrieved in IVF cycles (24) and with subsequent pregnancy (12) which was better predictor of the response to exogenous gonadotrophins than age (24) and was equivalent to day 3 FSH.
Seifer et al (20) demonstrated that, pregnancy rates were higher in patients with day 3 inhibin-B concentrations of over 45 pg/ml than this rate in patients with lower values. In 1999, they compared 109 normal responders with 47 poor responders and they did not find any significant difference in day 3 FSH leveles between the two groups, but poor responders had significantly lower day 3 inhibin-B leveles (21). The study of Hall et al (27) did not support the use of day 3 inhibin-B as a predictive marker of IVF outcome. In addition, base line FSH, E2 and inhibin-B were not significantly different between pregnant and non pregnant patients. In the present study the mean inhibin-B in normal responders was 166.9
There was a positive correlation between inhibin-B level on day 3 and retrived oocyte number but there was not any correlation between inhibin-B level on day 3 and ART outcome. There was tremendous overlap in baseline inhibin-B concentrations between pregnant and non-pregnant subjects, and inhibin-B alone failed to predict pregnancy.The results of this study confirm Hall et al findings (27) and are contraversal with Tsuchia et al (12) and Seifer et al (20) results.
Conclusion
The present study shows that inhibin-B concentration on day 3 positevly correlate with the number of oocytes retrieved during ART. Our results do not support the use of day 3 inhibin-B as a predictive marker of IVF outcome.
Acknowledgment
The authors wish to express thank to Mr. Mani Kashani, and other staff and personel of Fatemieh Infertility Research Center, specially Mrs. Ramazani for their kind assistance in this study.
Type of Study: Original Article |
References
1. Scott RT, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril 1995; 63; 1-11. [DOI:10.1016/S0015-0282(16)57287-2]
2. Sharara F, Scott RT. Assessmant of ovarian reserve and treatment of low responders. Infertil Reprod Med Clin North Amer 1997;8:501-522.
3. Navot D, Rosenwaks Z, Margalioth EJ. Prognostic assessment of female fecundity. Lancet 1997; 2: 645-647. [DOI:10.1016/S0140-6736(87)92439-1]
4. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Resenwaks Z. Follicle- stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril 1989;51: 651-654. [DOI:10.1016/S0015-0282(16)60615-5]
5. Muasher SJ, Oehninger S, Simonetti S. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril 1988; 50: 298-307. [DOI:10.1016/S0015-0282(16)60077-8]
6. Toner JP, Philput CB, Jones GS, Muasher SJ. Basal follicle - stimulating hrmone level is a better predictor of in vitro fertilization performance than age. Fertil Steril 1991; 55: 784-791. [DOI:10.1016/S0015-0282(16)54249-6]
7. Yong Y, Baird D,Thong K, Neily A, Anderson R. Prospective analysis of the relationship between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of normal menstrual cycle and after pituitary down-regulation. Hum Reprod 2003;18(1):35-44. [DOI:10.1093/humrep/deg019] [PMID]
8. Evers JL, Slaats P, Land JA, Dumoulin JC, Dunselman GA. Elevated levels of basal estradiol- 17B predict poor response in patients with normal basal levels of follicle-stimulating hormone undergoing in vitro fertilization. Fertil Steril 1998; 69: 1010-1014. [DOI:10.1016/S0015-0282(98)00080-6]
9. Smotrich DB, Widra EA, Gindoff PR, Levy MJ, Hall JL, Stillman RJ. Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil steril 1995; 64: 1136- 1140. [DOI:10.1016/S0015-0282(16)57974-6]
10. Syrop CHW, lhoite A, Van Voorhis BJ. Ovarian volume a novel outcome predictor assisted reproduction. Fertil Streil 1995; 64: 1167- 1171. [DOI:10.1016/S0015-0282(16)57979-5]
11. Bancsi M, Broekmans FJ. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril 2002; 77: 328-337. [DOI:10.1016/S0015-0282(01)02983-1]
12. Tsuchiya K, Seki M, Itoh M, Hasegawa Y, Miyamoto K, Igarashi M. Correlation of serum inhibin concentrations with results in an ovarian hyperstimulation program. Fertil Steril 1989;52, 88-94. [DOI:10.1016/S0015-0282(16)60795-1]
13. Hughes EG, Robertson DM, Handelsman DJ, Hayward S, Healy DL, Kretser DM de. Inhibin and estradiol responses to ovarian hyperstimulation: effects of age and predictive value for in vitro fertilization outcome. J Clin Endocrinol Metab 1990; 70: 358-364. [DOI:10.1210/jcem-70-2-358] [PMID]
14. Ohshima K, Kishi H, Itoh M, Arai KY, Watanabe G, Arai K, et al. Secretory pattern of inhibin A, inhibin B and inhibin pro-α C during induced follicular atresia and subsequent follicular development in the golden hamster (Mesocricetus auratus). J Endocrinol 2002;171:575-581. [DOI:10.1677/joe.0.1720575] [PMID]
15. Nancy A, Brenda S, Karl R, Teresa K, Patrick M, William J, Michael R. Age-Related Analysis of Inhibin A, Inhibin B, and Activin A Relative to the Intercycle Monotropic Follicle-Stimulating Hormone Rise in Normal Ovulatory Women. Clin Endocrinol Metab 2004; 89(6): 2977-2981. [DOI:10.1210/jc.2003-031515] [PMID]
16. Corrine K, Welt Zachary A, Smith Donna K, Hall JE. Differential Regulation of Inhibin A and Inhibin B by Luteinizing Hormone, Follicle-Stimulating Hormone, and Stage of Follicle Development. Clin Endocrinol Metab 2001; 86(6): 2531-2537. [DOI:10.1210/jc.86.6.2531]
17. Casper F, Scufert R, Pollow K. Inversa correlation between basline inhibin B and FSH after stimulation with GnRH: a study of serum levels of inhibin A and B, pro alpha-C and activin A in women with ovulatory disturbances before and after stimulation with GnRH. E J Endocrinol 2000;143:77-84. [DOI:10.1530/eje.0.1430077] [PMID]
18. Klein NA, IIIingworth PJ, Groome NP, McNeilly A, Battaglia D, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise on older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab 1996;81: 2742- 2745. [DOI:10.1210/jcem.81.7.8675606] [PMID]
19. Danforth D, Arbogast L , Mroueh J, Kim H, Kennard A, Seifer B, Friedman I. Dimeric inhibin: a direct marker of ovarian aging. Fertil Steril 1998 ; 70: 119- 123. [DOI:10.1016/S0015-0282(98)00127-7]
20. Seifer DB, Lambert-Messerlian G, Gardiner AC, Blazer AS, Berk CA. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril 1997; 67: 110-114 [DOI:10.1016/S0015-0282(97)81865-1]
21. Seifer B, Scott, RT, Bergh PA, Abrogast LK, Friedman CI, Mack CK, Danforth DR. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle stimulating hormone. Fertil Steril 1999; 72: 63-65. [DOI:10.1016/S0015-0282(99)00193-4]
22. Matson PL, Morris D, Sun JG, Ibrahim ZH, Lieberman BA. Serum inhibin as an index of ovarian function in women undergoing pituitary suppression and ovarian stimulation in an in vitro fertilization program. Horm Res 1991: 35, 173-177. [DOI:10.1159/000181897] [PMID]
23. Balasch J, Creus M, Fabregues F, Carmona F, Casamitjana R, Ascaso C, Vanrell JA. Inhibin, follicle-stimulating hormone and age as predictors of ovarian response in in vitro fertilization cycles stimulated with gonadotropin-releasing hormone agonist-gonadotropin treatment. Am J Obstet Gynecol 1996; 127: 1226-1230. [DOI:10.1016/S0002-9378(96)70032-7]
24. Lockwood GM, Muttukrishna S, Groome NP, Ledger WL. Circulating inhibins and activin A during GnRH-analogue down-regulation and ovarian hyperstimulation with recombinant FSH for in-vitro fertilization-embryo transfer. Clin Endocrinol 1996; 45: 741-748. [DOI:10.1046/j.1365-2265.1996.8510861.x] [PMID]
25. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin B as a test of ovarian reserve for infertile women. Hum Reprod 1999; 14: 2818-2821. [DOI:10.1093/humrep/14.11.2818] [PMID]
26. Elder-Geva T, Margalioth EJ, Ben-Chetrit A, Robertson D, Healy DL, Diamanat Y. Serum inhibin B levels measured early during FSH administration for IVF may be of value in predicting the number of oocytes to be retrived in normal and low responders. Hum Reprod 2002;17(9): 2331-2337. [DOI:10.1093/humrep/17.9.2331] [PMID]
27. Hall E, Welt CK, Gramer DW. Inhibin A and inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Hum Reprod 1999; 14: 409-415. [DOI:10.1093/humrep/14.2.409] [PMID]
28. Tinkanen H, Blauer M, Laipala P, Tuohimaa P, Kujansuu E. Prognostic factors in controlled ovarian hyperstimulation. Fertil Steril 1999; 27: 932- 936. [DOI:10.1016/S0015-0282(99)00397-0]
29. Creus M, Penarrubia A, Fabregues F, Vidal E, Carmona F, Casamitjana R, et al. Day 3 serum inhibin B and FSH and age as predictors of assisted reproduction treatment outcome. Hum Reprod 2000;15: 2341- 2346. [DOI:10.1093/humrep/15.11.2341] [PMID]
30. Revhon A, Lavery S, Michel S, Donaldson M, Margara R, Trew G, Winston R. Dynamic assays of inhibin B and osetradiol following buserelin acetate administrastion as predictors of ovarian response in IVF. Hum Reprod 2000; 15: 2297- 2301. [DOI:10.1093/humrep/15.11.2297] [PMID]
31. Dumesic D, Damario A, Session R, Famuyide A, Lesnick TG, Thornhill R, McNeilly AS. Ovarian morpholgy and serum hormone markers as predictors of ovarian follicle recruiment by gonadotropins for in vitro fertilization. J Clin Endocrinol Metab 2001; 86: 2538- 2543. [DOI:10.1210/jcem.86.6.7605] [PMID]
32. Fawzy M, Lambert A, Harrison RF, Knight PG, Groome N, Hennelly B, Robertson WR. Day 5 inhibin B levels in a treatment cycle are predictive of IVF outcome. Hum Reprod 2002; 17: 1535-1543. [DOI:10.1093/humrep/17.6.1535] [PMID]
33. Lockwood G. The diagnostic value of Inhibin in infertility Evaluation. Semin Reprod Med 2004:22(3):195-208. [DOI:10.1055/s-2004-831895] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




