Sat, Jul 12, 2025
[Archive]
Volume 5, Issue 4 (7-2007)
IJRM 2007, 5(4): 121-126 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Peirouvi T, Farjah G, Soleimani Rad J, Ghaffari Novin M. Vitrification induced apoptosis in spermatozoa from fertile and subfertile men. IJRM 2007; 5 (4) :121-126
URL: http://ijrm.ir/article-1-79-en.html
URL: http://ijrm.ir/article-1-79-en.html
1- Department of Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran , tpeirouvi@yahoo.co.uk
2- Department of Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
3- Depatment of Histology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz , Iran
4- Department of Reproductive Endocrinology and Embryology, Avesina Research Center, Shahid Beheshti University, Tehran, Iran
2- Department of Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
3- Depatment of Histology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz , Iran
4- Department of Reproductive Endocrinology and Embryology, Avesina Research Center, Shahid Beheshti University, Tehran, Iran
Full-Text [PDF 305 kb]
(602 Downloads)
| Abstract (HTML) (3648 Views)
Full-Text: (438 Views)
Introduction
Human sperm vitrification is made possible for several years (1). Cryopreservation of sperm in patients with testicular tumor, whom undergoing chemotherapy and radiotherapy and in cases which sperms are obtained through testicular biopsy is the main part of infertility treatment protocol (2, 3). However, recovery of functional sperm in freeze-thawed samples varies among patients (4). Sperm vitrification result in various structural and functional alterations (5). Such alterations are Human sperm vitrification is made possible for several years (1). Cryopreservation of sperm in patients with testicular tumor, whom undergoing chemotherapy and radiotherapy and in cases which sperms are obtained through testicular biopsy is the main part of infertility treatment protocol (2, 3). However, recovery of functional sperm in freeze-thawed samples varies among patients (4). Sperm vitrification result in various structural and functional alterations (5). Such alterations are partially due to disturbances of membrane integrity (6) and include changes in; viability, motility and fertilization potential of sperms. The involving factors include: temperature, maturity of the cell, cryoprotectant and rate of cooling (7). However, little is known about the effect of vitrification on sperms from fertile and subfertile men.
Apoptosis or programmed cell death is a phenomenon which leads to in situ cell death without any changes on surrounding cells (9). It is shown that apoptosis occurs during normal spermatogenesis and play an important role in controlling sperm population (5). On the other hand, apoptosis may induce by several environmental, chemical and physical factors including; UV irradiation, anticancer drugs and temperature changes (10). While it is shown that slow rate freezing and thawing is associated with apoptosis in human ejaculated sperm, little is known about the effect of vitrification on induction of apoptosis in sperms. Apoptosis is associated with demolishing of membrane phospholipids asymmetry. Phospholipids are distributed asymmetrically between inner and outer leaflets of the plasma membrane of live cells (10). Early during apoptosis this asymmetry is disrupted and phosphatidylserine becomes exposed on the outside surface of the plasma membrane (11).
Annexin V binding measures the translocation of phosphatidyleserine (PS) of the plasma membrane (5). PS translocation also occurs during necrosis. Annexin V is not an absolute marker of apoptosis (12). Therefore, it is often used in conjunction with propidium iodide (PI), which bind to nucleic acids, that can penetrate the plasma membrane when membrane integrity is disturbed (13).
Since vitrification-thawing could demolish membrane phospholipid asymmetry (11), and it can be postulated that, in general, sperms are more susceptible to damage in subfertile men (8), the aim of the present study was to evaluate the effect of the vitrification on apoptosis of sperms in semen from fertile and subfertile men.
Materials and methods
Semen samples
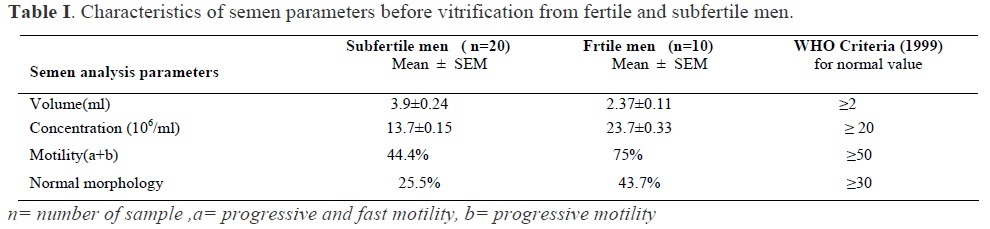
In this case-control and interventional study, semen samples were collected from 20 subfertile and 10 fertile men by masturbation following 48 hours sexual abstinence. According to World Health Organization (WHO) standard, fertile men were defined as whom had at least one child and subfertile men were from patients who referred to infertility center for ART, due to oligospermia (14).
Semen analysis
After semen liquefaction for 30 min at 37ºC, semen analysis was performed by semen analyzer system (Sperm Quality Analyzer, Medical Electronics System, USA) to determine concentration, motility and morphology according to WHO guidelines (14). Each sample was divided into two portions, one for pre freeze semen analysis and evaluation of apoptosis and the other for cryopreservation.
Vitrification-Thawing of spermatozoa
For vitrification, 500 micro liter of semen were pipetted into 1.8 ml cryovials and mixed with Human Sperm Preservation Medium (Fertipro, Belgium) as 1:0.7, containing Glycerol, HSA, and Hepes according to company instruction. The mixture was exposed to evaporated nitrogen at 20 cm over the liquid nitrogen for 30 minutes and then placed in liquid nitrogen tank.
For thawing, two weeks after freezing, specimens were removed from liquid nitrogen tank, put in a container of liquid nitrogen for 30 seconds and then transferred to a water bath at 37ºC for 5 minutes. The thawed semen used for detection of apoptosis as in fresh semen (3).
Detection of apoptosis
Apoptosis detection was performed based on translocation of membrane phosphatidyleserine and using of Annexin-V-Fluos kit and propidium iodid (PI) (Roche, Penzberg, Germany)(13).
Semen samples containing 1×106 spermatozoa were washed with PBS and centrifugated at 200× g for 5 min. Cell pellet was resuspended in 100 μL of Annexin V-flous labeling solution (containing Annexin V-flous labeling reagent in 1ml incubation buffer and propidium iodide (PI) in Hepes buffer). After incubation at 15 to 25°C for 10-15 min, apoptotic cells were detected immediately by fluorescence microscopy (Olympus BX5, Japan). Apoptotic cells were differentiated from necrotic cells based on staining with PI.
Statistical analysis
The statistical analysis was performed using SPSS software for windows. Paired t-test was applied for the comparison of parameters in the same group pre and post freezing semen. Unpaired t test were used for comparison of data from two different groups. Results are expressed as Mean ± SEM. The difference was considered statistically significant when p<0.05.
Results
A total of 30 semen samples were evaluated in this study (10 semen samples from fertile and 20 semen samples from subfertile men).
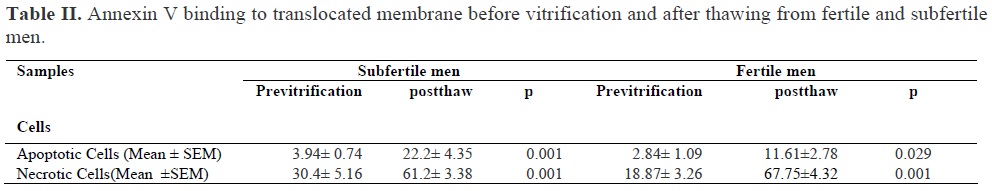
Semen parameters in fertile and subfertile men before vitrification are shown in Table I. As it is clear from the table, the concentration of sperm, sperm motility and morphology were significantly lower in subfertile men in comparison to fertile men. The comparison of pre-vitrification and post-thaw results of annexin-V binding assay for both groups are shown in Table II. In this study, Annexin V staining were applied for determination of PS exposure. The mean percentage of early apoptotic cells after vitrification and thawing increased significantly in both study and control groups. In addition, the mean percentage of late apoptotic or necrotic cells after vitrification and thaw increased significantly in both groups. The early apoptotic cells based on translocation of PS was demonstrated by the staining of annexin V conjugated FITC green fluorescence around the head, midpiece, and certain parts of the tail, while the staining of the sperm head by PI red fluorescence indicated the damage of membrane and necrotic or late apoptotic cells. Figure 1 shows early apoptotic cell with Annexin V-positive but PI negative. Figure 2 shows late apoptotic or necrotic cell with both Annexin and PI positive.

Discussion
Binding of annexin V to human ejaculated spermatozoa was determined in fresh and vitrified-thawed semen samples. Annexin-V is a calcium dependent binding protein with high affinity for phosphatidylserine, a negatively charged phospholipids (15). Phosphatidyleserine, as a ligand of annexin V, is located on the inner leaflet of plasma membrane normally. It is translocated to the outer leaflet of plasma membrane in the beginning of apoptotic processes (16). Hence, acts as a biomarker for apoptosis by facilitating annexin V binding to cell surface receptors. However, it is insufficient in late apoptosis when integrity of cell membrane is lost and the status of cell is similar to those in necrotic cells. At present, the Annexin V-staining assay in conjugation with a vital dye (Propidium Iodide), has been developed as a sensitive method for detection of progressive stage of early apoptosis (Annexin-V-positive and PI negative) and late apoptosis or necrosis (both Annexin and PI-positive) (13). The annexin V-binding assay is more sensitive than PI staining at detecting the early alteration of membrane structure.
In our study, both early apoptotic cells with phosphatidyleserin translocation (Annexin-V-positive and PI negative) and late apoptotic or necrotic cells (Annexin V and PI-positive) were observed. We also found that annexin V stains; head, mid-piece and tail of the spermatozoa. The result showed that apoptotic and necrotic cells are increased significantly (p ≤ 0.001) in both fertile and subfertile men after vitrification, however, this rate was higher in subfertile men than fertile men. Our findings are in agreement with previous findings in that cryopreservation –thawing is associated with an increase in the rate of apoptotic sperms from fertile (5,6) and subfertile (13) men. They have shown that freezing of sperms for 24 and 48 hours in both fertile and infertile men increases the number of apoptotic and necrotic cells. Our findings show that vitrification, similar to slow rate freezing increases the occurrence of apoptotic sperms in semen from fertile and subfertile men. Based on a three years vitrification of zygote (17) it is concluded that vitrification is a reliable method for cryopreservation of human embryos.
According to our findings, the comparison between slow rate freezing and vitrification of sperms is not possible. However, it appears that both slow rate and vitrification could result in apoptosis of sperms. Therefore, improvement of cryopreservation techniques would improve quality of sperm preservation in both fertile and subfertile men.
Conclusion
It is concluded that phosphatidylserine translocation to the outer leaflet occurs at different parts of the cell membrane of the sperm. Vitrification induced alterations on sperm, as apoptosis and necrosis, are similar in both fertile and subfertile men, but it proportionally, is much higher in semen from subfertile men than fertile men.
Human sperm vitrification is made possible for several years (1). Cryopreservation of sperm in patients with testicular tumor, whom undergoing chemotherapy and radiotherapy and in cases which sperms are obtained through testicular biopsy is the main part of infertility treatment protocol (2, 3). However, recovery of functional sperm in freeze-thawed samples varies among patients (4). Sperm vitrification result in various structural and functional alterations (5). Such alterations are Human sperm vitrification is made possible for several years (1). Cryopreservation of sperm in patients with testicular tumor, whom undergoing chemotherapy and radiotherapy and in cases which sperms are obtained through testicular biopsy is the main part of infertility treatment protocol (2, 3). However, recovery of functional sperm in freeze-thawed samples varies among patients (4). Sperm vitrification result in various structural and functional alterations (5). Such alterations are partially due to disturbances of membrane integrity (6) and include changes in; viability, motility and fertilization potential of sperms. The involving factors include: temperature, maturity of the cell, cryoprotectant and rate of cooling (7). However, little is known about the effect of vitrification on sperms from fertile and subfertile men.
Apoptosis or programmed cell death is a phenomenon which leads to in situ cell death without any changes on surrounding cells (9). It is shown that apoptosis occurs during normal spermatogenesis and play an important role in controlling sperm population (5). On the other hand, apoptosis may induce by several environmental, chemical and physical factors including; UV irradiation, anticancer drugs and temperature changes (10). While it is shown that slow rate freezing and thawing is associated with apoptosis in human ejaculated sperm, little is known about the effect of vitrification on induction of apoptosis in sperms. Apoptosis is associated with demolishing of membrane phospholipids asymmetry. Phospholipids are distributed asymmetrically between inner and outer leaflets of the plasma membrane of live cells (10). Early during apoptosis this asymmetry is disrupted and phosphatidylserine becomes exposed on the outside surface of the plasma membrane (11).
Annexin V binding measures the translocation of phosphatidyleserine (PS) of the plasma membrane (5). PS translocation also occurs during necrosis. Annexin V is not an absolute marker of apoptosis (12). Therefore, it is often used in conjunction with propidium iodide (PI), which bind to nucleic acids, that can penetrate the plasma membrane when membrane integrity is disturbed (13).
Since vitrification-thawing could demolish membrane phospholipid asymmetry (11), and it can be postulated that, in general, sperms are more susceptible to damage in subfertile men (8), the aim of the present study was to evaluate the effect of the vitrification on apoptosis of sperms in semen from fertile and subfertile men.
Materials and methods
Semen samples
In this case-control and interventional study, semen samples were collected from 20 subfertile and 10 fertile men by masturbation following 48 hours sexual abstinence. According to World Health Organization (WHO) standard, fertile men were defined as whom had at least one child and subfertile men were from patients who referred to infertility center for ART, due to oligospermia (14).
Semen analysis
After semen liquefaction for 30 min at 37ºC, semen analysis was performed by semen analyzer system (Sperm Quality Analyzer, Medical Electronics System, USA) to determine concentration, motility and morphology according to WHO guidelines (14). Each sample was divided into two portions, one for pre freeze semen analysis and evaluation of apoptosis and the other for cryopreservation.
Vitrification-Thawing of spermatozoa
For vitrification, 500 micro liter of semen were pipetted into 1.8 ml cryovials and mixed with Human Sperm Preservation Medium (Fertipro, Belgium) as 1:0.7, containing Glycerol, HSA, and Hepes according to company instruction. The mixture was exposed to evaporated nitrogen at 20 cm over the liquid nitrogen for 30 minutes and then placed in liquid nitrogen tank.
For thawing, two weeks after freezing, specimens were removed from liquid nitrogen tank, put in a container of liquid nitrogen for 30 seconds and then transferred to a water bath at 37ºC for 5 minutes. The thawed semen used for detection of apoptosis as in fresh semen (3).
Detection of apoptosis
Apoptosis detection was performed based on translocation of membrane phosphatidyleserine and using of Annexin-V-Fluos kit and propidium iodid (PI) (Roche, Penzberg, Germany)(13).
Semen samples containing 1×106 spermatozoa were washed with PBS and centrifugated at 200× g for 5 min. Cell pellet was resuspended in 100 μL of Annexin V-flous labeling solution (containing Annexin V-flous labeling reagent in 1ml incubation buffer and propidium iodide (PI) in Hepes buffer). After incubation at 15 to 25°C for 10-15 min, apoptotic cells were detected immediately by fluorescence microscopy (Olympus BX5, Japan). Apoptotic cells were differentiated from necrotic cells based on staining with PI.
Statistical analysis
The statistical analysis was performed using SPSS software for windows. Paired t-test was applied for the comparison of parameters in the same group pre and post freezing semen. Unpaired t test were used for comparison of data from two different groups. Results are expressed as Mean ± SEM. The difference was considered statistically significant when p<0.05.
Results
A total of 30 semen samples were evaluated in this study (10 semen samples from fertile and 20 semen samples from subfertile men).
Semen parameters in fertile and subfertile men before vitrification are shown in Table I. As it is clear from the table, the concentration of sperm, sperm motility and morphology were significantly lower in subfertile men in comparison to fertile men. The comparison of pre-vitrification and post-thaw results of annexin-V binding assay for both groups are shown in Table II. In this study, Annexin V staining were applied for determination of PS exposure. The mean percentage of early apoptotic cells after vitrification and thawing increased significantly in both study and control groups. In addition, the mean percentage of late apoptotic or necrotic cells after vitrification and thaw increased significantly in both groups. The early apoptotic cells based on translocation of PS was demonstrated by the staining of annexin V conjugated FITC green fluorescence around the head, midpiece, and certain parts of the tail, while the staining of the sperm head by PI red fluorescence indicated the damage of membrane and necrotic or late apoptotic cells. Figure 1 shows early apoptotic cell with Annexin V-positive but PI negative. Figure 2 shows late apoptotic or necrotic cell with both Annexin and PI positive.



Discussion
Binding of annexin V to human ejaculated spermatozoa was determined in fresh and vitrified-thawed semen samples. Annexin-V is a calcium dependent binding protein with high affinity for phosphatidylserine, a negatively charged phospholipids (15). Phosphatidyleserine, as a ligand of annexin V, is located on the inner leaflet of plasma membrane normally. It is translocated to the outer leaflet of plasma membrane in the beginning of apoptotic processes (16). Hence, acts as a biomarker for apoptosis by facilitating annexin V binding to cell surface receptors. However, it is insufficient in late apoptosis when integrity of cell membrane is lost and the status of cell is similar to those in necrotic cells. At present, the Annexin V-staining assay in conjugation with a vital dye (Propidium Iodide), has been developed as a sensitive method for detection of progressive stage of early apoptosis (Annexin-V-positive and PI negative) and late apoptosis or necrosis (both Annexin and PI-positive) (13). The annexin V-binding assay is more sensitive than PI staining at detecting the early alteration of membrane structure.
In our study, both early apoptotic cells with phosphatidyleserin translocation (Annexin-V-positive and PI negative) and late apoptotic or necrotic cells (Annexin V and PI-positive) were observed. We also found that annexin V stains; head, mid-piece and tail of the spermatozoa. The result showed that apoptotic and necrotic cells are increased significantly (p ≤ 0.001) in both fertile and subfertile men after vitrification, however, this rate was higher in subfertile men than fertile men. Our findings are in agreement with previous findings in that cryopreservation –thawing is associated with an increase in the rate of apoptotic sperms from fertile (5,6) and subfertile (13) men. They have shown that freezing of sperms for 24 and 48 hours in both fertile and infertile men increases the number of apoptotic and necrotic cells. Our findings show that vitrification, similar to slow rate freezing increases the occurrence of apoptotic sperms in semen from fertile and subfertile men. Based on a three years vitrification of zygote (17) it is concluded that vitrification is a reliable method for cryopreservation of human embryos.
According to our findings, the comparison between slow rate freezing and vitrification of sperms is not possible. However, it appears that both slow rate and vitrification could result in apoptosis of sperms. Therefore, improvement of cryopreservation techniques would improve quality of sperm preservation in both fertile and subfertile men.
Conclusion
It is concluded that phosphatidylserine translocation to the outer leaflet occurs at different parts of the cell membrane of the sperm. Vitrification induced alterations on sperm, as apoptosis and necrosis, are similar in both fertile and subfertile men, but it proportionally, is much higher in semen from subfertile men than fertile men.
Type of Study: Original Article |
References
1. Sherman LK. Freezing and freeze-drying of human spermatozoa. Fertil Steril 1954;5:357-371. [DOI:10.1016/S0015-0282(16)31685-5]
2. Giraud M, Motta C, Boucher D, Grizard G: Membrane fluidity predicts the outcome of cryopreservation of human spermatozoa. Hum Reprod 2000; 15:2160-2164. [DOI:10.1093/humrep/15.10.2160]
3. Donnelly ET, Steele EK, McClure N, Lewis SE. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod 2001; 16:1191-1199. [DOI:10.1093/humrep/16.6.1191]
4. Centola GM, Raubertas RF, Mattox JH: Cryopreservation of humansemen: comparison of cryopreservation, sources of variability, and prediction of post-thaw survival. J Androl 1992; 13:283-288.
5. Duru NK, Morshedi M, Schuffner A, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa and plasma membrane translocation of phosphatidylserine. Fertil Steril 2001; 75:263-268. [DOI:10.1016/S0015-0282(00)01694-0]
6. Schuffner A, Morshedi M, Oehninger S. Cryopreservation of fractionated, highly motile human spermatozoa: Effect on membrane phosphatidylserine externalization and lipid peroxidation. Hum Reprod 2001; 16:2148-2153. [DOI:10.1093/humrep/16.10.2148]
7. Paasch U, Sharma RK, Gupta KA, Grunewald S, Mascha EJ, Thomas AJ, et al: Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol Reprod 2004; 71:1828-1837. [DOI:10.1095/biolreprod.103.025627]
8. Vayena E, Rowe PJ, Griffin PD. Report of a WHO meeting Current practices and controversies in assisted reproduction. WHO, Geneva 2002; 156.
9. Ortiz A, Cuadrado SG, Lorz C, Egido J. Apoptosis in renal diseases. Front Biosci 1996; 1:30-47. [DOI:10.2741/A114]
10. Nagata S. Apoptosis by death factor. Cell 1997; 88:355-365. [DOI:10.1016/S0092-8674(00)81874-7]
11. Glander HJ, Schaller J. Binding of annexin V to plasma membranes of human spermatozoa: A rapid assay for detection of membrane changes after cryostorage. Mol Hum Reprod 1999 ; 5:109-115. [DOI:10.1093/molehr/5.2.109]
12. Schwartz LM, Ashwell JD: Apoptosis. Volume 66, USA, Academic press, 2001; 33-84
13. Shen HM, Dai J, Chia SE, Lim A, Ong CN: Detection of apoptotic alterations in sperm in subfertile patients and their correlations with sperm quality. Hum Reprod 2002;17:1266-1273. [DOI:10.1093/humrep/17.5.1266]
14. World Health Organization (WHO) laboratory manual for the examination of human semen and semen-servical mucus interaction. 4th ed, Cambridge :Cambridge University Press;1999: 6-23.
15. Vermes I, Haanen C, Steffens-Nakken H, Reutelinqsperger C: A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995;184:39-51. [DOI:10.1016/0022-1759(95)00072-I]
16. Barroso G, Morshedi M, Oehninger S: Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod 2000; 15:1338-1344. [DOI:10.1093/humrep/15.6.1338]
17. Alhasani S, Ozmen B, Kouttaki N, Schoepper B, Diedrich K, Schultze-Mosgau A: Three years of routin vitrification of human zygotes: is still fair to advocate slow rate freezing? Biomedicine Online 2007; 14: 288-293. [DOI:10.1016/S1472-6483(10)60869-3]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




