Thu, Jan 8, 2026
[Archive]
Volume 1, Issue 1 (1-2003)
IJRM 2003, 1(1): 7-11 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khalili M A, G Rabchevsky A. Restoration of Spermatogenesis by Adenoviral Gene Transfer into Injured Spinal Cords of Rats. IJRM 2003; 1 (1) :7-11
URL: http://ijrm.ir/article-1-8-en.html
URL: http://ijrm.ir/article-1-8-en.html
Full-Text [PDF 445 kb]
(661 Downloads)
| Abstract (HTML) (3106 Views)
Full-Text: (427 Views)
Introduction
At present, spinal cord injury (SCI) is one of the major public ealth problem worldwide. In the United States alone, over 10,000 new cases of SCI occur annually. Eighty-two percent of the victims are males, and the majority are in their prime reproductive years. Infertility due to SCI is a common problem which result from a combination of ejaculatory dysfunction and abnormal semen parameters of sperm count, progressive motility, and morphology (Rajasekaran and Monga, 1999). With advancements of the assisted reproductive technology (ART), some spinal cord injured men have become the biological father of their children. Despite these clinical advances in recent years, there are still a large number of victims suffering from prolonged infertility. Therefore, a significant amount of basic research has been directed towards potential strategies for improving axonal regeneration following SCI which may
subsequently improve the fertility potential of victims (Romero and Smith, 1998).
The application of gene therapy for SCI has become a relatively recent development. Less than a decade ago, gene of the environment through which axons must regenerate. This is allowed therapy was considered only for the treatment of genetic disorders. Today, gene therapy is being considered for both neurological and reproductive disorders that are not due to genetic abnormalities. Gene therapy will become an intrinsic part of spinal cord therapy of the future for the following reasons: First, spinal cord regeneration requires manipulation through manipulation of cellular environment by changing the genetic expression of spinal cord cells. Second, regeneration takes a long time, probably years in humans. It may be more efficient to administer factors through gene therapy rather than through drug administration which may indirectly influence the male reproductive system (Stribley et al., 2002; Romero and Smith ,1998).

In conclusion, gene therapy is another effective tool which can be applied non-invasively. It can be used to augment or alter the expression of many factors in the target cells. Finally, gene therapy will greatly accelerate progress towards an effective "cure" of SCI in human (Stribley et al.,2002; Robbins and Ghivizzani,1998; Romero and Smith, 1998). At present, the adenovirus is the most frequently used vector for in vivo trasfections of the CNS. It has been used successfully to transfect the cellular neurotrophins, and the anti-inflammatory cytokines. Since, adenovirus is a common cold virus, some human subjects have pre-existing immunity against them. In general, adenoviruses produce more efficient trasfection than retrovirus vectors, because the virus is easy to manipulate, and it can be grown at high titers. Also, transgene capacity in adenovirus is high compared to other vectors (Wickham, 2000; Robbins and Ghivizzani, 1998). In view of these findings, the present investigation was undertaken to examine the role of adenovirus- mediated gene transfer to experimentally injured spinal cords in rats on possible restoration of spermatogenesis.
Materials and Methods
Young adult male Sprague-Dawley rats (200-235g) were assigned to one of the three groups of control, SCI and Ad (n=3/ group).
Control Group
Three rats served as non-surgical, non-injected controls.
SCI Group
Rats were first anesthetized with ketamine (80mg/ kg) and xylazine (10mg/ kg) before a laminectomy was performed at T10. The exposed vertebral column was stabilized by clamping both T9 and T11 vertebral bodies with Adson delicate forceps (Miltex Instruments Co., NY) using the NYU impactor, a 10g brass rod with a tip diameter of 2mm was dropped from a height of 12.5mm onto the exposed, intact dura overlying the dorsal spinal cord. The impact rod was immediately removed, the wound irrigated with saline, and the muscle and skin openings sutured together in layers (Robchevsky etal.,2000). Following surgery, rats were injected with 10 cc sterile saline s.c. and 33.3mg/ kg cefazolin antibiotic s.c. (Solopak Laboratories, IL) before being placed on a heating pad during recovery. The animals were housed two per cage and postoperative care took place. This included the manual expression of bladders twice a day until bladder function returned, as well as injections of cefazolin twice a day for up to 1 week. Animals surviving for 43 days were overdosed with sodium pentobarbital and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, PH 7.4) followed by 3% glutaraldehyde in 0.1 M PBS. Each spinal cord was then transected rostrally at T6 and a 3 cm segment of the cord was disected and post fixed for 2 h at 4°C before croyprotecting in 20% sucrose/ PBS at 4°C. Spinal cords were then placed side by side into plastic cryomolds containing embedding medium consisting of gum tragcanth (Sigma Co., MO) in 20% sucrose/ PBS. The entire mold was snap-frozen in acetone chilled to -40°C and stored at -80°C until sectioning on a Cryostat (Micom Laboratorate, Germany). Serial 20 µm cryosections seperated by 80 µm (discarded) were then stored at -20°C till histology was performed. In addition, following careful dissection, a small piece of seminiferous tubules were dissected out and place in fresh 3% glutaraldehyde for TEM study.
Ad Group
Animals were first injured as descried for the aforementioned SCI group. Following SCI, Ad microinjection was performed. All spinal cord microinjections were done as described by Romero and Smith, 1998 (Romero and Smith, 1998). Before, the Ad administration, the animals received 100 µg intraperitonealy of combined solution of rat CD-4 (W3/25) and CD-45 (MRC OX-22) anti-sera to suppress the immune response. Each rat then received eight bilateral injections (4 µl; 0.5 mm apart and 0.5 mm deep) of adenoviral vectors of encoding LacZ (5x106 p.f.u./ µl) along the T6 - T10 dorsal root entry zone (DREZ). Each injection was done with a nano injector 2000 attached to a beveled micropipette. Just before injection, the micropipette was filled with colored mineral oil followed by viral suspension (Romero and Smith, 1998). Following injections, post-operative care was done for each animal. Animals were sacrificed 43 days post surgery, and both testicular and spinal cord specimens were collected for further assessment.
Tissue Processing for TEM
The testicular samples were cut in small pieces and stored in 0.1 M PBS in 10% sucrose at 4°C. The specimens were washed in 0.1 M PBS, and then post- fixed in 2% aqueous osmium tetroxide in above buffer.


The specimens were subsequently dehydrated in a graded series of ethanol solutions, and embedded in Araldite. Ultra-thin sections were cut on a Reichert Ultramicrotome (OMU3). The ultra-thin sections were picked up on 200 mesh copper grids and stained with uranyl acetate for 12 min in dark, and lead citrate for 2 min in a co2 free atmosphere. The micrographs were finally taken using a Philips TEM at an accelerating voltage of 60 kv.
Histology for Spinal Cord
A modified eriochrome cyanine (EC) staining protocol for differentiation of white matter and cell bodies was used to calculate the amount of spared tissue in sections of injured cords. Briefly, air-dried sections were cleared and hydrated before being placed for 10 min into a solution consisting of 2 ml 10% FeCl3 and 40 ml of 0.2% EC (Sigma Co. OM) in 0.5% aqueous H2So4 brought to a final volume of 50 ml with dH2O. This was followed by washes in water and differentiation for 2 min in 0.5% aqueous NH4OH. The reaction was terminated with rinses in water before sections were dehydrated and cleared for coverslipping with permount (Fisher Scientific Co., OH). Morphometry: Image analysis was performed on each series of EC stained sections using the Bioquant image analysis system (Bio- metrics Inc., TN). Spared tissue was based on positive
metrics Inc., TN). Spared tissue was based on positive staining for myelin or if the gray matter cyto-architecture approximated that seen in control rats. Area measurements of gray and white matters, and total tissue sparing were each quantified separately as previously described (Rabchevsky et al.,2000). All slides were assessed blindly with respect to treatment. cyto-architecture approximated that seen in control rats. Area measurements of gray and white matters, and total tissue sparing were each quantified separately as previously described (Rabchevsky et al.,2000). All slides were assessed blindly with respect to treatment.
Statistical Analysis
Measurements of the lesion extents were compared using a 2-way ANOVA. The Mann-Whitney test was performed to determine significant differences between the groups. Significance was set at p<0.05.
Results
Spinal Cord Tissue
The morphological evaluation of spinal cord sections from control rats showed a normal appearing cytoarchitecture. However, the injured spinal cord showed an abnormal gray matter area with dramatic reduction in intact tissue. In addition, the group differences in the percentage of spared tissue (white and gray combined) were statistically significant when compared to the controls (P<0.05, Table I).
Testicular Tissue
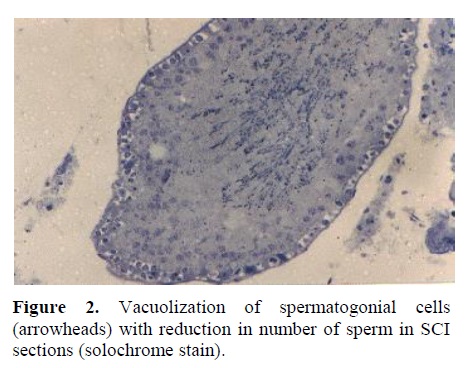
a) Light Microscopy: Complete Spermatogenesis was observed in testis of control rats (Figure 1). Normal appearing spermatogonia cell adjacent to the basement membrane as well as tremendous number of spermatozoa filling the lumen of the seminiferous tubules were observed in testicular sections of controls. However, in SCI animals, vacuolization of the majority of spermatogonial arranged in an abnormal fashion (Figure 2). Figure 3 represent a cross section of seminiferous tubule from Ad group with complete spermatogenesis. Rare vacuolization of spermatogonia
cells were, however, observed. Also, active spermatogenesis with presence of normal sperm were seen in Ad samples.
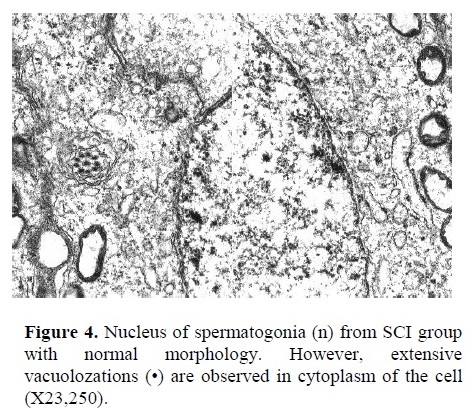
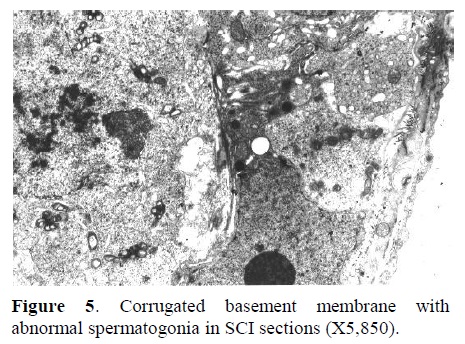
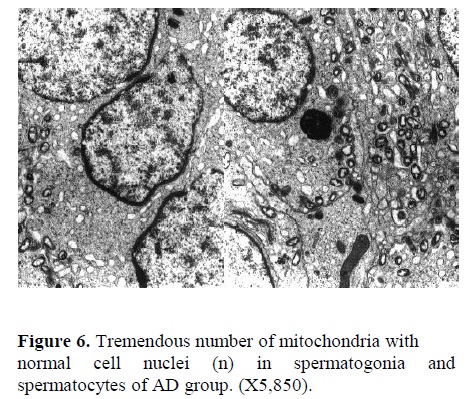
b) Electron Microscopy: In Figure 4, the nucleus of a spermatogonia from SCI group showed to be normal with clear nuclear membrane. However, extensive vacuolization along with vesicular foreign bodies were observed in the cytoplasm of spermatogonial cell (X23,250). Corrugated basement membrane with abnormal spermatogonia cell adjacent to it was another finding. Heterochromatin was located within the nucleus. Also, cellular swelling was observed in SCI specimen (Figure 5, X5,850). Fig. 6 represents the ultrastructure of a few spermatocytes and spermatogonial cells from an Ad testis. Normal nuclei with tremendous number of mitochondria were observed in the section (X5,850).
Discussion
Gene therapy refers to the transfer of genetic material to a target cell to achieve a clinical benefit. Application of gene therapy involves three steps of administration, delivery, and expression. Administration generally refers to introducing DNA into the body. Delivery consists of
the translocation of genetic material from the site of administration to the nucleus of the target cell. Finally, expression determines the production of the therapeutic gene product in the cell (Stribley et al., 2002; Robbins and Ghivizzani,1998; Romero and Smith, 1998).Currently, delivery systems can be divided into viral and non-viral vectors. Among the most used viruses, adenoviruses are usually applied more in neurotrauma diseases such as SCI.
Host cells infected with wild-type adenovirus undergo cell lysis, resulting in viral load release. Therefore, in present study adenoviral vectors of encoding LacZ gene was injected along the DREZ of injured spinal cords (Romero and Smith, 1998). The inflammatory response of the spinal cord to adenovirus depends on viral titer
(dose) and rat strain. Wood et al. (1996) found that administration of high viral titers (>106 p.f.u.) to spinal cord produced severe tissue damage. However, lower viral titers produced lasting expression of the gene that they sought to express, beta-galactosidase, with minimal immune response. They concluded that the immune responses to adenovirus administration are both dose- and strain- dependent (Wood etal.,1996). Therefore, in this study we injected only 5x106 p.f.u. Ad vector into DREZ of Sprague- Dawley rats which are the most common species used in the gene therapy studies on SCI. Also, adenoviruses may encode certain proteins that allow them to evade the immune system.
A sympathetic center, located in spinal cord segment T11-L2 with efferent fibers in hypogastric nerve to seminal vesicles, and prostatic smooth muscle fibers give rise to the peristalsis necessary for ejaculation. Also, a para-sympathetic center located in S2-4 with efferents in nervi perigents supplies the prostate glands leading to formation of seminal fluid. This indicates that the SCI directly influence the reproductive system in men ( lisenmeyer and Perkash,1991). Another reason postulated for poor semen quality after SCI is intristinc damage of the testicles. Bors and associates found abnormal testicular histologies in 31 of 34 men with SCI. The most common finding was atrophy of the seminiferous tubules. In addition, the biopsies revealed abnormalities that varied from absence of spermatogenic
cells to rare spermatids and spermatozoa. Our results indicate that there was a marked reduction in spermatogenesis in spinal cord injured rats at 43 days post injury. If this situation were to persist, we would predict that spinal cord injured animals would eventually become azoospermia. However, it seems that following a complete spermatogenic cycle which takes around 43 days in rats, there are only a few spermatozoa with normal architecture.
Bors showed that spermatogenesis was impaired in 60 to 90% of men with SCI (Bors and Commar,1960). In an experimental SCI model in rats, Linsenmeyer et al. (1994) assessed the seminiferous tubules at 2 weeks and 1month intervals after SCI. A minority of subjects demonstrated distinct spermatogenic abnormalities, including delayed spermiation as well as incomplete spermatogenesis at early phase. However, after 1 month, varying degree of severity, including atrophy was noted
in tubules (Linsenmeyer et al. 1994). In addition, Holstein et al. (1985) noticed malformations of spermatids and sperm in biopsies evaluated using electron microscopy following chronic SCI (Holstein et al.,1985).
In their recent study, Hirsch and colleagues (1999) demonstrated that SD rats that underwent chronic SCI showed significant deficient spermatogenesis which paralleled their clinical experience (Hirsch et al.,1999). Both Hirsch and the present study demonstrated derangement of the tubules in experimentally injured rodents. Additionally, our study carried the role of Ad vectors on spinal cord regeneration which demonstrated the persistence improvement of spermatogenic cell lines. The results from our study is in agreement with the study done by Liu et al (1997) that introducing recombinant adenovirus into injured spinal cord may have transduced cells surrounding the lesion site and induce them to synthesize and release neurotrophins to the nerve fibers and neuronal cells which subsequently could improve the nerve supply to the testis of rats (Liu et al.,1997). Hirsch et al. (1999) suggested that spermatogenic defects may occur soon after SCI (early phase). Altered testicular function following SCI may result from abnormal thermal regulation associated with denervation, resulting in elevated scrotal temperature. Additionally, spermatogenic insult in early phase of SCI may result from endocrine alterations. While, the present study did not consider hormonal parameters, Linsenmeyer et al. (1994) reported lower serum testosterone in injured rats (Lisenmeyer et al.,1994). Moreover, the same group noted a significant alteration in serum gonadotropin and testostrone levels, which resolved in 2 weeks. These findings would not support a hormonal etiology for the histologic abnormalities that persisit in our SCI animals.
Therefore, while its exact etiology remains unclear, spermatogenic deficit following clinical and experimental SCI is a commonly observed sequela. This prospective study investigated the potential role of Ad injections in improving the spermatogenesis which generally alters following SCI. Significant spermatogenic dysfunction occurred in spinal cord injected rats. Therefore, it is concluded that altered spermatogenesis can be reversed following Ad injections in the spinal cord segments and a spermatogenic cycles which takes over forty days in rats.
At present, spinal cord injury (SCI) is one of the major public ealth problem worldwide. In the United States alone, over 10,000 new cases of SCI occur annually. Eighty-two percent of the victims are males, and the majority are in their prime reproductive years. Infertility due to SCI is a common problem which result from a combination of ejaculatory dysfunction and abnormal semen parameters of sperm count, progressive motility, and morphology (Rajasekaran and Monga, 1999). With advancements of the assisted reproductive technology (ART), some spinal cord injured men have become the biological father of their children. Despite these clinical advances in recent years, there are still a large number of victims suffering from prolonged infertility. Therefore, a significant amount of basic research has been directed towards potential strategies for improving axonal regeneration following SCI which may
subsequently improve the fertility potential of victims (Romero and Smith, 1998).
The application of gene therapy for SCI has become a relatively recent development. Less than a decade ago, gene of the environment through which axons must regenerate. This is allowed therapy was considered only for the treatment of genetic disorders. Today, gene therapy is being considered for both neurological and reproductive disorders that are not due to genetic abnormalities. Gene therapy will become an intrinsic part of spinal cord therapy of the future for the following reasons: First, spinal cord regeneration requires manipulation through manipulation of cellular environment by changing the genetic expression of spinal cord cells. Second, regeneration takes a long time, probably years in humans. It may be more efficient to administer factors through gene therapy rather than through drug administration which may indirectly influence the male reproductive system (Stribley et al., 2002; Romero and Smith ,1998).

In conclusion, gene therapy is another effective tool which can be applied non-invasively. It can be used to augment or alter the expression of many factors in the target cells. Finally, gene therapy will greatly accelerate progress towards an effective "cure" of SCI in human (Stribley et al.,2002; Robbins and Ghivizzani,1998; Romero and Smith, 1998). At present, the adenovirus is the most frequently used vector for in vivo trasfections of the CNS. It has been used successfully to transfect the cellular neurotrophins, and the anti-inflammatory cytokines. Since, adenovirus is a common cold virus, some human subjects have pre-existing immunity against them. In general, adenoviruses produce more efficient trasfection than retrovirus vectors, because the virus is easy to manipulate, and it can be grown at high titers. Also, transgene capacity in adenovirus is high compared to other vectors (Wickham, 2000; Robbins and Ghivizzani, 1998). In view of these findings, the present investigation was undertaken to examine the role of adenovirus- mediated gene transfer to experimentally injured spinal cords in rats on possible restoration of spermatogenesis.
Materials and Methods
Young adult male Sprague-Dawley rats (200-235g) were assigned to one of the three groups of control, SCI and Ad (n=3/ group).
Control Group
Three rats served as non-surgical, non-injected controls.
SCI Group
Rats were first anesthetized with ketamine (80mg/ kg) and xylazine (10mg/ kg) before a laminectomy was performed at T10. The exposed vertebral column was stabilized by clamping both T9 and T11 vertebral bodies with Adson delicate forceps (Miltex Instruments Co., NY) using the NYU impactor, a 10g brass rod with a tip diameter of 2mm was dropped from a height of 12.5mm onto the exposed, intact dura overlying the dorsal spinal cord. The impact rod was immediately removed, the wound irrigated with saline, and the muscle and skin openings sutured together in layers (Robchevsky etal.,2000). Following surgery, rats were injected with 10 cc sterile saline s.c. and 33.3mg/ kg cefazolin antibiotic s.c. (Solopak Laboratories, IL) before being placed on a heating pad during recovery. The animals were housed two per cage and postoperative care took place. This included the manual expression of bladders twice a day until bladder function returned, as well as injections of cefazolin twice a day for up to 1 week. Animals surviving for 43 days were overdosed with sodium pentobarbital and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, PH 7.4) followed by 3% glutaraldehyde in 0.1 M PBS. Each spinal cord was then transected rostrally at T6 and a 3 cm segment of the cord was disected and post fixed for 2 h at 4°C before croyprotecting in 20% sucrose/ PBS at 4°C. Spinal cords were then placed side by side into plastic cryomolds containing embedding medium consisting of gum tragcanth (Sigma Co., MO) in 20% sucrose/ PBS. The entire mold was snap-frozen in acetone chilled to -40°C and stored at -80°C until sectioning on a Cryostat (Micom Laboratorate, Germany). Serial 20 µm cryosections seperated by 80 µm (discarded) were then stored at -20°C till histology was performed. In addition, following careful dissection, a small piece of seminiferous tubules were dissected out and place in fresh 3% glutaraldehyde for TEM study.
Ad Group
Animals were first injured as descried for the aforementioned SCI group. Following SCI, Ad microinjection was performed. All spinal cord microinjections were done as described by Romero and Smith, 1998 (Romero and Smith, 1998). Before, the Ad administration, the animals received 100 µg intraperitonealy of combined solution of rat CD-4 (W3/25) and CD-45 (MRC OX-22) anti-sera to suppress the immune response. Each rat then received eight bilateral injections (4 µl; 0.5 mm apart and 0.5 mm deep) of adenoviral vectors of encoding LacZ (5x106 p.f.u./ µl) along the T6 - T10 dorsal root entry zone (DREZ). Each injection was done with a nano injector 2000 attached to a beveled micropipette. Just before injection, the micropipette was filled with colored mineral oil followed by viral suspension (Romero and Smith, 1998). Following injections, post-operative care was done for each animal. Animals were sacrificed 43 days post surgery, and both testicular and spinal cord specimens were collected for further assessment.
Tissue Processing for TEM
The testicular samples were cut in small pieces and stored in 0.1 M PBS in 10% sucrose at 4°C. The specimens were washed in 0.1 M PBS, and then post- fixed in 2% aqueous osmium tetroxide in above buffer.



The specimens were subsequently dehydrated in a graded series of ethanol solutions, and embedded in Araldite. Ultra-thin sections were cut on a Reichert Ultramicrotome (OMU3). The ultra-thin sections were picked up on 200 mesh copper grids and stained with uranyl acetate for 12 min in dark, and lead citrate for 2 min in a co2 free atmosphere. The micrographs were finally taken using a Philips TEM at an accelerating voltage of 60 kv.
Histology for Spinal Cord
A modified eriochrome cyanine (EC) staining protocol for differentiation of white matter and cell bodies was used to calculate the amount of spared tissue in sections of injured cords. Briefly, air-dried sections were cleared and hydrated before being placed for 10 min into a solution consisting of 2 ml 10% FeCl3 and 40 ml of 0.2% EC (Sigma Co. OM) in 0.5% aqueous H2So4 brought to a final volume of 50 ml with dH2O. This was followed by washes in water and differentiation for 2 min in 0.5% aqueous NH4OH. The reaction was terminated with rinses in water before sections were dehydrated and cleared for coverslipping with permount (Fisher Scientific Co., OH). Morphometry: Image analysis was performed on each series of EC stained sections using the Bioquant image analysis system (Bio- metrics Inc., TN). Spared tissue was based on positive
metrics Inc., TN). Spared tissue was based on positive staining for myelin or if the gray matter cyto-architecture approximated that seen in control rats. Area measurements of gray and white matters, and total tissue sparing were each quantified separately as previously described (Rabchevsky et al.,2000). All slides were assessed blindly with respect to treatment. cyto-architecture approximated that seen in control rats. Area measurements of gray and white matters, and total tissue sparing were each quantified separately as previously described (Rabchevsky et al.,2000). All slides were assessed blindly with respect to treatment.
Statistical Analysis
Measurements of the lesion extents were compared using a 2-way ANOVA. The Mann-Whitney test was performed to determine significant differences between the groups. Significance was set at p<0.05.
Results
Spinal Cord Tissue
The morphological evaluation of spinal cord sections from control rats showed a normal appearing cytoarchitecture. However, the injured spinal cord showed an abnormal gray matter area with dramatic reduction in intact tissue. In addition, the group differences in the percentage of spared tissue (white and gray combined) were statistically significant when compared to the controls (P<0.05, Table I).
Testicular Tissue
a) Light Microscopy: Complete Spermatogenesis was observed in testis of control rats (Figure 1). Normal appearing spermatogonia cell adjacent to the basement membrane as well as tremendous number of spermatozoa filling the lumen of the seminiferous tubules were observed in testicular sections of controls. However, in SCI animals, vacuolization of the majority of spermatogonial arranged in an abnormal fashion (Figure 2). Figure 3 represent a cross section of seminiferous tubule from Ad group with complete spermatogenesis. Rare vacuolization of spermatogonia
cells were, however, observed. Also, active spermatogenesis with presence of normal sperm were seen in Ad samples.



b) Electron Microscopy: In Figure 4, the nucleus of a spermatogonia from SCI group showed to be normal with clear nuclear membrane. However, extensive vacuolization along with vesicular foreign bodies were observed in the cytoplasm of spermatogonial cell (X23,250). Corrugated basement membrane with abnormal spermatogonia cell adjacent to it was another finding. Heterochromatin was located within the nucleus. Also, cellular swelling was observed in SCI specimen (Figure 5, X5,850). Fig. 6 represents the ultrastructure of a few spermatocytes and spermatogonial cells from an Ad testis. Normal nuclei with tremendous number of mitochondria were observed in the section (X5,850).
Discussion
Gene therapy refers to the transfer of genetic material to a target cell to achieve a clinical benefit. Application of gene therapy involves three steps of administration, delivery, and expression. Administration generally refers to introducing DNA into the body. Delivery consists of
the translocation of genetic material from the site of administration to the nucleus of the target cell. Finally, expression determines the production of the therapeutic gene product in the cell (Stribley et al., 2002; Robbins and Ghivizzani,1998; Romero and Smith, 1998).Currently, delivery systems can be divided into viral and non-viral vectors. Among the most used viruses, adenoviruses are usually applied more in neurotrauma diseases such as SCI.
Host cells infected with wild-type adenovirus undergo cell lysis, resulting in viral load release. Therefore, in present study adenoviral vectors of encoding LacZ gene was injected along the DREZ of injured spinal cords (Romero and Smith, 1998). The inflammatory response of the spinal cord to adenovirus depends on viral titer
(dose) and rat strain. Wood et al. (1996) found that administration of high viral titers (>106 p.f.u.) to spinal cord produced severe tissue damage. However, lower viral titers produced lasting expression of the gene that they sought to express, beta-galactosidase, with minimal immune response. They concluded that the immune responses to adenovirus administration are both dose- and strain- dependent (Wood etal.,1996). Therefore, in this study we injected only 5x106 p.f.u. Ad vector into DREZ of Sprague- Dawley rats which are the most common species used in the gene therapy studies on SCI. Also, adenoviruses may encode certain proteins that allow them to evade the immune system.
A sympathetic center, located in spinal cord segment T11-L2 with efferent fibers in hypogastric nerve to seminal vesicles, and prostatic smooth muscle fibers give rise to the peristalsis necessary for ejaculation. Also, a para-sympathetic center located in S2-4 with efferents in nervi perigents supplies the prostate glands leading to formation of seminal fluid. This indicates that the SCI directly influence the reproductive system in men ( lisenmeyer and Perkash,1991). Another reason postulated for poor semen quality after SCI is intristinc damage of the testicles. Bors and associates found abnormal testicular histologies in 31 of 34 men with SCI. The most common finding was atrophy of the seminiferous tubules. In addition, the biopsies revealed abnormalities that varied from absence of spermatogenic
cells to rare spermatids and spermatozoa. Our results indicate that there was a marked reduction in spermatogenesis in spinal cord injured rats at 43 days post injury. If this situation were to persist, we would predict that spinal cord injured animals would eventually become azoospermia. However, it seems that following a complete spermatogenic cycle which takes around 43 days in rats, there are only a few spermatozoa with normal architecture.
Bors showed that spermatogenesis was impaired in 60 to 90% of men with SCI (Bors and Commar,1960). In an experimental SCI model in rats, Linsenmeyer et al. (1994) assessed the seminiferous tubules at 2 weeks and 1month intervals after SCI. A minority of subjects demonstrated distinct spermatogenic abnormalities, including delayed spermiation as well as incomplete spermatogenesis at early phase. However, after 1 month, varying degree of severity, including atrophy was noted
in tubules (Linsenmeyer et al. 1994). In addition, Holstein et al. (1985) noticed malformations of spermatids and sperm in biopsies evaluated using electron microscopy following chronic SCI (Holstein et al.,1985).
In their recent study, Hirsch and colleagues (1999) demonstrated that SD rats that underwent chronic SCI showed significant deficient spermatogenesis which paralleled their clinical experience (Hirsch et al.,1999). Both Hirsch and the present study demonstrated derangement of the tubules in experimentally injured rodents. Additionally, our study carried the role of Ad vectors on spinal cord regeneration which demonstrated the persistence improvement of spermatogenic cell lines. The results from our study is in agreement with the study done by Liu et al (1997) that introducing recombinant adenovirus into injured spinal cord may have transduced cells surrounding the lesion site and induce them to synthesize and release neurotrophins to the nerve fibers and neuronal cells which subsequently could improve the nerve supply to the testis of rats (Liu et al.,1997). Hirsch et al. (1999) suggested that spermatogenic defects may occur soon after SCI (early phase). Altered testicular function following SCI may result from abnormal thermal regulation associated with denervation, resulting in elevated scrotal temperature. Additionally, spermatogenic insult in early phase of SCI may result from endocrine alterations. While, the present study did not consider hormonal parameters, Linsenmeyer et al. (1994) reported lower serum testosterone in injured rats (Lisenmeyer et al.,1994). Moreover, the same group noted a significant alteration in serum gonadotropin and testostrone levels, which resolved in 2 weeks. These findings would not support a hormonal etiology for the histologic abnormalities that persisit in our SCI animals.
Therefore, while its exact etiology remains unclear, spermatogenic deficit following clinical and experimental SCI is a commonly observed sequela. This prospective study investigated the potential role of Ad injections in improving the spermatogenesis which generally alters following SCI. Significant spermatogenic dysfunction occurred in spinal cord injected rats. Therefore, it is concluded that altered spermatogenesis can be reversed following Ad injections in the spinal cord segments and a spermatogenic cycles which takes over forty days in rats.
Acknowledgment
We would like to thank Miss Rebecca Herman and Dr. A. Mesbah for their technical assistance with electron microscopy.
Type of Study: Original Article |
References
1. Bors E, Comarr E. Neurological disturbances of sexual function with speical reference to 529 patients with spinal cord injury. Urol Surv 1960; 10: 191-222.
2. Hirsch IH, Huang B, Chancellor MB, Rivas DA, Salzman SK, Jost LK, Evenson DP. Spermatogenesis in early and chronic phases of experimental spinal cord injury in the rodent model. J Androl 1999; 20: 63-71.
3. Holstein AE, Saverwein D, Schirren U. Spermatogenesis in patients with traumatic transverse paralysis. Urologe 1985; 24: 208-11.
4. Linsenmeyer TA, Perkash I. Infertility in men with spinal cord injury. Arch Phys Med Rehabil 1991; 72: 747-54.
5. Linsenmeyer TA, Pogach L, Ottenweller JE, Huang HF. Spermatogenesis and the pituitary testicular hormone axis in rats during acute phase of spinal cord injury. J Urol 1994; 152: 1303-7. [DOI:10.1016/S0022-5347(17)32572-7]
6. Liu Y, Himes BT, Moul J, Huang W, Chow S, Tessler A, Fischer I. Application of recombinant adenovirus for in vivo gene delivery to spinal cord. Brain Research 1997; 768: 19-29. [DOI:10.1016/S0006-8993(97)00587-8]
7. Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factors enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol 2000; 164: 280-91. [DOI:10.1006/exnr.2000.7399] [PMID]
8. Rajasekaran M, Monga M. Cellular and molecular causes of male infertility in spinal cord injury. J Androl 1999; 20: 326-30.
9. Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther 1998; 80: 35-47. [DOI:10.1016/S0163-7258(98)00020-5]
10. Romero MI, Smith GM. Adenoviral gene transfer into the normal and injured spinal cord: enhanced transgene stability by combined administration of temperature-sensitive virus and transient immune blockade. Gene Therapy 1998; 5: 1612-21. [DOI:10.1038/sj.gt.3300774] [PMID]
11. Stribley JM, Rehman KS, Niu H, Christman GM. Gene therapy and reproductive medicine. Fert Steril 2002; 77: 645-57. [DOI:10.1016/S0015-0282(01)03233-2]
12. Wickham Tj. Targeting adenovirus. Gene Therapy 2000; 7: 110-14. [DOI:10.1038/sj.gt.3301115] [PMID]
13. Wood M, Charlton H, Wood K, Kajiwara K, Byrnes A. Immune responses to adenovirus vectors in the nervous system. Trends Neurosci 1996; 19; 497-501. [DOI:10.1016/S0166-2236(96)10060-6]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




