Sun, Jul 13, 2025
[Archive]
Volume 5, Issue 5 (7-2007)
IJRM 2007, 5(5): 165-170 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khosravi Farsani S, Mahmoudi R, Abdolvahhabi M A, Abbasi M, Malek F, Sobhani A. Comparing the viability and in vitro maturation of cumulus germinal vesicle break down (GVBD) oocyte complexes using two vitrification techniques in mice. IJRM 2007; 5 (5) :165-170
URL: http://ijrm.ir/article-1-92-en.html
URL: http://ijrm.ir/article-1-92-en.html
Somaye Khosravi Farsani1 
 , Reza Mahmoudi2
, Reza Mahmoudi2 
 , Mir Abbas Abdolvahhabi1
, Mir Abbas Abdolvahhabi1 
 , Mehdi Abbasi1
, Mehdi Abbasi1 
 , Fateme Malek1
, Fateme Malek1 
 , Aligholi Sobhani *3
, Aligholi Sobhani *3 


 , Reza Mahmoudi2
, Reza Mahmoudi2 
 , Mir Abbas Abdolvahhabi1
, Mir Abbas Abdolvahhabi1 
 , Mehdi Abbasi1
, Mehdi Abbasi1 
 , Fateme Malek1
, Fateme Malek1 
 , Aligholi Sobhani *3
, Aligholi Sobhani *3 

1- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
2- Department of Anatomy, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran
3- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran ,Sobhania@tums.ac.ir
2- Department of Anatomy, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran
3- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran ,
Full-Text [PDF 147 kb]
(555 Downloads)
| Abstract (HTML) (2970 Views)
Full-Text: (408 Views)
Introduction
Cryopreservation of oocytes has important roles in the preservation and management of genetic resources, genetic engineering and nuclear transfer procedure. It has the potential to become an important technique for preserving gamets for female whose fertility may become compromised by medical treatment (such as chemotherapy and Radiotherapy ) . Oocyte preservation has been successfully applied to livestock including Cattle, Goats, Sheep, and other animals, but it is still an open challenge in most mammalian species, due to the extreme sensitivity of gamets to chilling injuries (1, 2).
Oocyte cryopreservation accompanied with in vitro maturation is important in women either are at risk of ovarian hyper stimulation, or fail to hormonal stimulation response and finally in those patients with polycystic ovarian syndrome (3). Over one decade after the first pregnancy was achieved using frozen and thawed oocyte (4), Fabbri et al (2001) reported a modified slow freezing and rapid thawing method to freeze human oocytes that gave higher rates of oocyte survival and embryo development (5) than before (6,7). The results obtained using this method revealed that frozen- thawed oocytes had significantly lower cleavage rate than non- frozen controls (8-10). Vitrification can be used to freeze mammalian oocytes and embryos (11-13). It is an alternative to traditional freezing methods (slow freezing) to avoid chilling injury and ice crystal formation (14, 15). This method is simple and rapid compared with slow freezing; however, it still needs more studies, including animal research (16). Since the major concerns about spindle damage in oocyte freezing are all linked to the use of mature oocytes (MII) (17,18), several attempts were made to freeze immature oocytes (GV), in which the meiotic spindle is not yet formed. Unfortunately not the nucleus, but the cytoplasm appears to be the main problem in immature oocytes in cryopreservation procedure (19-21). Therefore, choosing an intermediate stage, such as germinal vesicle breakdown (GVBD), may circumvent some of the problems associated with the cryopreservation of GV and MII oocytes. With regard to this fact that the oocytes have distinct properties in each species, our research has been done on GVBD oocytes of mouse. Since 1998, there have done limited appropriate researches on GVBD oocyte stage; on the other hand some researches have only been done on bovine GVBD oocyte (20-23). Therefor, the aim of the present study was to evaluate the effects of step-wise and single step vitrification on survival and maturation rates on GVBD oocytes.
Materials and methods
All chemicals were obtained from sigma (St.Louis, MO) unless indicated otherwise.
Experimental design
Two experimental and one control groups were used in the present study. Experimental groups were vitrified immediately after getting to GVBD in step-wise or single step methods. While non-vitrified GV oocytes in control group were opposed to maturation protocols only (for 24 hr).
Animals and collection of GV oocytes
Female NMRI virgin mice aged 3 to 4 weeks old were prepared from pasture institute (Iran) and maintained for adaptation in anatomical laboratory of Tehran Medical Sciences University for 2 weeks. The animals were injected intra-peritoneal (IP) with 10 IU of pregnant mare serum gonadotropin (PMSG) for ovulation induction. The animals were killed by cervical dislocation 48 hr after PMSG administration, and the ovaries were removed and transferred into a holding medium, which consisted of TCM199 (Gibco, UK) supplemented with 10% fetal bovine serum. GV oocytes were obtained as cumulus–oocyte complexes (COCs) by puncturing antral follicles in the ovaries with a 29-G needle in the holding medium. In all experiments, only full-grown COCs, surrounded by at least two layers of cumulus cells, were selected, whereas partly or completely naked oocytes were discarded.
In vitro maturation of GV oocytes
Fresh GV oocytes were cultured in 100-µl droplets of TCM-199 supplemented with 10% fetal bovine serum (FBS), 0.23 mM sodium pyruvate , 10 ng/mL epidermal growth factor , 100 mIU FSH (GONAL- F serono), 75 µg/ml of penicillin G-K salts , and 50µg/ml of streptomycin sulfate (Sigma) under liquid paraffin oil at 37°C in an atmosphere of 5% CO2 in humidified air, as described by Albarracin et al. (23), with some modifications. In experimental groups the maturation duration for getting to GVBD oocytes before vitrification was 3 to 4 hr.
Oocytes vitrification and thawing
Vitrification: The GVBD oocytes were vitrified by solid surface vitrification, as described by Aono et al. (10), with some modifications. The holding medium used for handling oocytes during vitrification was TCM199 containing 10% FBS. All vitrification solutions were prepared using this holding medium. Manipulation of oocytes and vitrification process were performed at room temperature (25◦C). In the single step group, oocytes were exposed to %20 ethylene glycol (EG) +0.5 M sucrose in a holding medium for 40-60 sec. Oocyte in the stepwise group was exposed to vitrification solution of %2 EG for 5 min (step one), %5 EG for 3 min (step two), %10 EG for 2 min (step three) and %20 EG +0.5 M sucrose for 40-60 sec (step four). Oocytes were loaded into straws by aspiration and the straws were plunged directly into liquid nitrogen. The straws were stored for 7 days in liquid nitrogen.
Thawing: The straws were thawed in air for 10 sec, and immediately plunged into a water bath at 37◦C for 10 sec.
Thawing was carried out in four steps using sucrose solution in a holding medium containing %10 FBS at 37◦C, after which the oocytes were exposed to decreasing concentrations of sucrose (0.5, 0.2, 0.1 and 0.05 M) for 1 minute. GVBD oocytes were washed three times at 37◦C in maturation medium before being transferred for maturation protocol. After thawing, GVBD oocytes were matured for additional 21 hr to fulfill the 24 hr maturation requirement.
Assessment of oocyte viability
Oocyte survival was evaluated morphologically based on the integrity of the oolema and zona pellucida; Oocytes were also assessed for viability based on oolema integrity by propidium iodide (PI) and Hoechst.
For this purpose oocytes were stained with PI (10μg/ml) and Hoechst 33342 (10μg/ml) for 10 minutes, washed, and then observed under a fluorescence microscope.The dead cells showed red fluorescence (PI-positive) for disruption of cellular membrane. The viable cells showed blue fluorescence without red fluorescence (PI-negative) for the intact cell membrane (24).
Statistical analysis
Collected data were analyzed by chi-square test. The differences in the values of survival and maturation rates, were considered significant when p<0.05.
Results
A total of 422 GV oocytes with cumulus cells were obtained from 20 ovaries that were used for 2 vitrified and 1 non-vitrified (control) groups. The number of oocytes collected averaged 21.1 per mouse ovary.
Morphology of GV oocytes
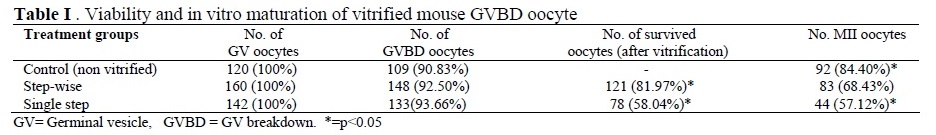
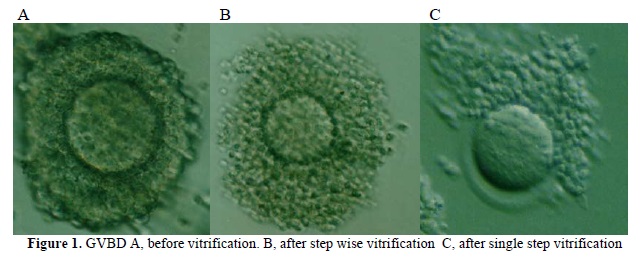
Morphological figures of vitrified GVBD oocytes using non-vitrified (control) and either single step or step-wise method is shown in figure 1 (1A, 1B and 1C). Cumulus-oocyte complexes in the single step method show dispersed cumulus cells (Figure 1C). In step-wise method (Figure 1B) cumulus-oocyte complexes were almost similar to those in the control group (Fig. 1A). Also, viability assessment of oocytes based on oolema integrity by Hoechst and PI showed red fluorescence (Figure 2A) for dead cells for disruption of cellular membrane (PI-positive) and blue fluorescence (Figure 2B) for viable cells for the intact cell membrane (PI-negative).
Viability and in vitro maturation of vitrified GV oocytes
The survival and maturation rates of GV oocytes after different treatments including exposure to 2 type of vitrification and non-vitrified oocytes shown in Table I. The survival and maturation rate in the step-wise group (81.97% and 68.43% respectively) were significantly (p<0.05) higher than single step group (58.04% and 57.12% respectively). But maturation rate in control group was significantly higher than single step and stepwise vitrified groups (p<0.05). The vitrifying/warming process in two experimental groups were greatly reduced the developmental capacity of GVBD oocytes compared to control as assessed by in vitro maturation (p<0.05).
Discussion
In this study, we showed that the cryopreservation of mouse GVBD oocytes by step-wise and single step vitrification enabled oocytes to survive and mature . Successful cryopreservation of GVBD oocytes has been reported only in bovine and calf (20,22,23), but there were low rates of survival capacity after cryopreservation. As some of researcher reported, oocytes at different maturation stages respond to cryopreservation differently (19, 25). In fact, several authors have reported that GV oocytes are just as sensitive to chilling injury (26) or more sensitive to chilling or cryopreservation than MII oocytes (27,28).Bovine oocytes at the GVBD stage have been described as more resistant to cooling than GV or MII oocytes. However, when cryopreservation was attempted, Men et al (2002) observed that a significantly higher proportion of cleaved bovine embryos from vitrified MII oocytes developed into blastocysts than those derived from vitrified GVBD oocytes (20).
In addition to GVBD oocytes, attempts have also been made on vitrifying oocytes at other maturation stages. Thus when bovine oocytes were vitrified 0, 6, 12 or 24 hr after the onset of maturation, Hochi et al (1998) found the best stage for vitrification was that of oocytes matured for 12 hr (22). The reason for the high sensitive nature of bovine oocytes at GVBD oocytes to cryopreservation is unknown. In bovine and other mammalian oocytes, as Hunter and Moor (1987) reported, active transcription and translation occur at GVBD and later stages of meiotic maturation (28,30).Therefore, in addition to the detrimental effect on cytology, the biochemical process with the oocytes may also be affected by cryopreservation. Consequently, the impaired biochemical process will negatively influence the cytoplasmic maturation of oocytes. In addition to meiotic stages, the protocol of vitrification is important in survival, in vitro maturation and cleavage rate of oocytes after thawing. In the present study, we used step-wise and single-step vitrification methods to cryopreserve GVBD oocyte of mice. We found high rates of survival and IVM rates when the oocytes were vitrified after step-wise method. The survival and in vitro maturation in single step was lower in comparison to step-wise. Our results in step-wise group in survival rate are consistent with Men et al (20) that reported vitrification of bovine GVBD oocytes after a two-step vitrification (81.97% versus 79.59%). Four-step vitrification in our study might be responsible for higher survival rate than the study of Men et al, in where they used two-step vitrification. Other reasons could explain such differences between their results and ours: the different size of oocyte in bovine and mouse and the different cryoprotectant agents (CPAs) in it.
Mahmmoudi et al (2005) reported that intact mouse oocytes had a higher developmental competence than denuded oocytes (31) and damage to connection between oocyte and cumulus cells after exposure to cryopreservation has adverse effect on in vitro maturation after thawing. Hurt et al (2000) reported that vitrification with EG would lead to expansion of cumulus cells probably due to damage of the gap junction between cumulus and oocytes (32). In this study, as Cetin and Bastan stated (33), contrary to Hurt et al. (32) in step-wise vitrification with EG, distribution of cumulus cells did not occur after dissolution.
Finally, as reported by other investigators, the cryodamage of the oocytes is the major determinant to the significant low maturation rate in cryopreserved oocytes. Therefor, we do not regard present vitrification protocol as a definitive protocol. Anyway, further research will help clarify the cellular and molecular mechanisms of cryopreservation-indused injury.
Conclusion
Our results suggest that step-wise vitrification of GVBD oocytes as compared to single step vitrification was better in rate of survival and in vitro maturation of oocytes.
Acknowledgements
This project financially supported by research deputy of Tehran University of Medical Sciences and Health services grant No. 85-04-30-4669. So, we would like to thank for their helps.
Cryopreservation of oocytes has important roles in the preservation and management of genetic resources, genetic engineering and nuclear transfer procedure. It has the potential to become an important technique for preserving gamets for female whose fertility may become compromised by medical treatment (such as chemotherapy and Radiotherapy ) . Oocyte preservation has been successfully applied to livestock including Cattle, Goats, Sheep, and other animals, but it is still an open challenge in most mammalian species, due to the extreme sensitivity of gamets to chilling injuries (1, 2).
Oocyte cryopreservation accompanied with in vitro maturation is important in women either are at risk of ovarian hyper stimulation, or fail to hormonal stimulation response and finally in those patients with polycystic ovarian syndrome (3). Over one decade after the first pregnancy was achieved using frozen and thawed oocyte (4), Fabbri et al (2001) reported a modified slow freezing and rapid thawing method to freeze human oocytes that gave higher rates of oocyte survival and embryo development (5) than before (6,7). The results obtained using this method revealed that frozen- thawed oocytes had significantly lower cleavage rate than non- frozen controls (8-10). Vitrification can be used to freeze mammalian oocytes and embryos (11-13). It is an alternative to traditional freezing methods (slow freezing) to avoid chilling injury and ice crystal formation (14, 15). This method is simple and rapid compared with slow freezing; however, it still needs more studies, including animal research (16). Since the major concerns about spindle damage in oocyte freezing are all linked to the use of mature oocytes (MII) (17,18), several attempts were made to freeze immature oocytes (GV), in which the meiotic spindle is not yet formed. Unfortunately not the nucleus, but the cytoplasm appears to be the main problem in immature oocytes in cryopreservation procedure (19-21). Therefore, choosing an intermediate stage, such as germinal vesicle breakdown (GVBD), may circumvent some of the problems associated with the cryopreservation of GV and MII oocytes. With regard to this fact that the oocytes have distinct properties in each species, our research has been done on GVBD oocytes of mouse. Since 1998, there have done limited appropriate researches on GVBD oocyte stage; on the other hand some researches have only been done on bovine GVBD oocyte (20-23). Therefor, the aim of the present study was to evaluate the effects of step-wise and single step vitrification on survival and maturation rates on GVBD oocytes.
Materials and methods
All chemicals were obtained from sigma (St.Louis, MO) unless indicated otherwise.
Experimental design
Two experimental and one control groups were used in the present study. Experimental groups were vitrified immediately after getting to GVBD in step-wise or single step methods. While non-vitrified GV oocytes in control group were opposed to maturation protocols only (for 24 hr).
Animals and collection of GV oocytes
Female NMRI virgin mice aged 3 to 4 weeks old were prepared from pasture institute (Iran) and maintained for adaptation in anatomical laboratory of Tehran Medical Sciences University for 2 weeks. The animals were injected intra-peritoneal (IP) with 10 IU of pregnant mare serum gonadotropin (PMSG) for ovulation induction. The animals were killed by cervical dislocation 48 hr after PMSG administration, and the ovaries were removed and transferred into a holding medium, which consisted of TCM199 (Gibco, UK) supplemented with 10% fetal bovine serum. GV oocytes were obtained as cumulus–oocyte complexes (COCs) by puncturing antral follicles in the ovaries with a 29-G needle in the holding medium. In all experiments, only full-grown COCs, surrounded by at least two layers of cumulus cells, were selected, whereas partly or completely naked oocytes were discarded.
In vitro maturation of GV oocytes
Fresh GV oocytes were cultured in 100-µl droplets of TCM-199 supplemented with 10% fetal bovine serum (FBS), 0.23 mM sodium pyruvate , 10 ng/mL epidermal growth factor , 100 mIU FSH (GONAL- F serono), 75 µg/ml of penicillin G-K salts , and 50µg/ml of streptomycin sulfate (Sigma) under liquid paraffin oil at 37°C in an atmosphere of 5% CO2 in humidified air, as described by Albarracin et al. (23), with some modifications. In experimental groups the maturation duration for getting to GVBD oocytes before vitrification was 3 to 4 hr.
Oocytes vitrification and thawing
Vitrification: The GVBD oocytes were vitrified by solid surface vitrification, as described by Aono et al. (10), with some modifications. The holding medium used for handling oocytes during vitrification was TCM199 containing 10% FBS. All vitrification solutions were prepared using this holding medium. Manipulation of oocytes and vitrification process were performed at room temperature (25◦C). In the single step group, oocytes were exposed to %20 ethylene glycol (EG) +0.5 M sucrose in a holding medium for 40-60 sec. Oocyte in the stepwise group was exposed to vitrification solution of %2 EG for 5 min (step one), %5 EG for 3 min (step two), %10 EG for 2 min (step three) and %20 EG +0.5 M sucrose for 40-60 sec (step four). Oocytes were loaded into straws by aspiration and the straws were plunged directly into liquid nitrogen. The straws were stored for 7 days in liquid nitrogen.
Thawing: The straws were thawed in air for 10 sec, and immediately plunged into a water bath at 37◦C for 10 sec.
Thawing was carried out in four steps using sucrose solution in a holding medium containing %10 FBS at 37◦C, after which the oocytes were exposed to decreasing concentrations of sucrose (0.5, 0.2, 0.1 and 0.05 M) for 1 minute. GVBD oocytes were washed three times at 37◦C in maturation medium before being transferred for maturation protocol. After thawing, GVBD oocytes were matured for additional 21 hr to fulfill the 24 hr maturation requirement.
Assessment of oocyte viability
Oocyte survival was evaluated morphologically based on the integrity of the oolema and zona pellucida; Oocytes were also assessed for viability based on oolema integrity by propidium iodide (PI) and Hoechst.
For this purpose oocytes were stained with PI (10μg/ml) and Hoechst 33342 (10μg/ml) for 10 minutes, washed, and then observed under a fluorescence microscope.The dead cells showed red fluorescence (PI-positive) for disruption of cellular membrane. The viable cells showed blue fluorescence without red fluorescence (PI-negative) for the intact cell membrane (24).
Statistical analysis
Collected data were analyzed by chi-square test. The differences in the values of survival and maturation rates, were considered significant when p<0.05.
Results
A total of 422 GV oocytes with cumulus cells were obtained from 20 ovaries that were used for 2 vitrified and 1 non-vitrified (control) groups. The number of oocytes collected averaged 21.1 per mouse ovary.
Morphology of GV oocytes
Morphological figures of vitrified GVBD oocytes using non-vitrified (control) and either single step or step-wise method is shown in figure 1 (1A, 1B and 1C). Cumulus-oocyte complexes in the single step method show dispersed cumulus cells (Figure 1C). In step-wise method (Figure 1B) cumulus-oocyte complexes were almost similar to those in the control group (Fig. 1A). Also, viability assessment of oocytes based on oolema integrity by Hoechst and PI showed red fluorescence (Figure 2A) for dead cells for disruption of cellular membrane (PI-positive) and blue fluorescence (Figure 2B) for viable cells for the intact cell membrane (PI-negative).
Viability and in vitro maturation of vitrified GV oocytes
The survival and maturation rates of GV oocytes after different treatments including exposure to 2 type of vitrification and non-vitrified oocytes shown in Table I. The survival and maturation rate in the step-wise group (81.97% and 68.43% respectively) were significantly (p<0.05) higher than single step group (58.04% and 57.12% respectively). But maturation rate in control group was significantly higher than single step and stepwise vitrified groups (p<0.05). The vitrifying/warming process in two experimental groups were greatly reduced the developmental capacity of GVBD oocytes compared to control as assessed by in vitro maturation (p<0.05).

Discussion
In this study, we showed that the cryopreservation of mouse GVBD oocytes by step-wise and single step vitrification enabled oocytes to survive and mature . Successful cryopreservation of GVBD oocytes has been reported only in bovine and calf (20,22,23), but there were low rates of survival capacity after cryopreservation. As some of researcher reported, oocytes at different maturation stages respond to cryopreservation differently (19, 25). In fact, several authors have reported that GV oocytes are just as sensitive to chilling injury (26) or more sensitive to chilling or cryopreservation than MII oocytes (27,28).Bovine oocytes at the GVBD stage have been described as more resistant to cooling than GV or MII oocytes. However, when cryopreservation was attempted, Men et al (2002) observed that a significantly higher proportion of cleaved bovine embryos from vitrified MII oocytes developed into blastocysts than those derived from vitrified GVBD oocytes (20).
In addition to GVBD oocytes, attempts have also been made on vitrifying oocytes at other maturation stages. Thus when bovine oocytes were vitrified 0, 6, 12 or 24 hr after the onset of maturation, Hochi et al (1998) found the best stage for vitrification was that of oocytes matured for 12 hr (22). The reason for the high sensitive nature of bovine oocytes at GVBD oocytes to cryopreservation is unknown. In bovine and other mammalian oocytes, as Hunter and Moor (1987) reported, active transcription and translation occur at GVBD and later stages of meiotic maturation (28,30).Therefore, in addition to the detrimental effect on cytology, the biochemical process with the oocytes may also be affected by cryopreservation. Consequently, the impaired biochemical process will negatively influence the cytoplasmic maturation of oocytes. In addition to meiotic stages, the protocol of vitrification is important in survival, in vitro maturation and cleavage rate of oocytes after thawing. In the present study, we used step-wise and single-step vitrification methods to cryopreserve GVBD oocyte of mice. We found high rates of survival and IVM rates when the oocytes were vitrified after step-wise method. The survival and in vitro maturation in single step was lower in comparison to step-wise. Our results in step-wise group in survival rate are consistent with Men et al (20) that reported vitrification of bovine GVBD oocytes after a two-step vitrification (81.97% versus 79.59%). Four-step vitrification in our study might be responsible for higher survival rate than the study of Men et al, in where they used two-step vitrification. Other reasons could explain such differences between their results and ours: the different size of oocyte in bovine and mouse and the different cryoprotectant agents (CPAs) in it.
Mahmmoudi et al (2005) reported that intact mouse oocytes had a higher developmental competence than denuded oocytes (31) and damage to connection between oocyte and cumulus cells after exposure to cryopreservation has adverse effect on in vitro maturation after thawing. Hurt et al (2000) reported that vitrification with EG would lead to expansion of cumulus cells probably due to damage of the gap junction between cumulus and oocytes (32). In this study, as Cetin and Bastan stated (33), contrary to Hurt et al. (32) in step-wise vitrification with EG, distribution of cumulus cells did not occur after dissolution.
Finally, as reported by other investigators, the cryodamage of the oocytes is the major determinant to the significant low maturation rate in cryopreserved oocytes. Therefor, we do not regard present vitrification protocol as a definitive protocol. Anyway, further research will help clarify the cellular and molecular mechanisms of cryopreservation-indused injury.
Conclusion
Our results suggest that step-wise vitrification of GVBD oocytes as compared to single step vitrification was better in rate of survival and in vitro maturation of oocytes.


Acknowledgements
This project financially supported by research deputy of Tehran University of Medical Sciences and Health services grant No. 85-04-30-4669. So, we would like to thank for their helps.
Type of Study: Original Article |
References
1. Kuleshova LL, Lopata A. Vitrification can be more favorable than slow cooling. Fertil Steril 2002; 78: 449-454. [DOI:10.1016/S0015-0282(02)03305-8]
2. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update 2003; 9:463-470. [DOI:10.1093/humupd/dmg032]
3. Cooper A, Paynter SJ, Fuller BJ, Shaw RW. Differential effects of cryopreservation on nuclear or cytoplasmic maturation in vitro in immature mouse oocytes from stimulated ovaries. Hum Reprod 1998; 13: 971-978. [DOI:10.1093/humrep/13.4.971]
4. Chen C. Pregnancy after human oocyte cryopreservation. Lancet 1986; 1: 884-886 [DOI:10.1016/S0140-6736(86)90989-X]
5. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod 2001; 16:411-416. [DOI:10.1093/humrep/16.3.411]
6. Porcu E, Ciotti PM, Fabbri R, Magrini O, Seracchioli R , Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril 1997; 68:724-726. [DOI:10.1016/S0015-0282(97)00268-9]
7. Tucker MJ, Morton PC, Wright G, Sweitzer CL, Massey JB. Clinical application of human egg cryopreservation. Hum Reprod 1998; 13:3156-3159. [DOI:10.1093/humrep/13.11.3156]
8. Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Cryopreservation of mature bovine oocytes by vitrification in straws. Cryobiology 1998; 37:77-85. [DOI:10.1006/cryo.1998.2103]
9. Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev 2001; 58:342-347.
https://doi.org/10.1002/1098-2795(200103)58:3<342::AID-MRD13>3.0.CO;2-X [DOI:10.1002/1098-2795(200103)58:33.0.CO;2-X]
10. Aono N, Naganuma T, Abe Y, Hara K, Sasada H, Sato E, Yoshida H. Successful production of blastocysts following ultra rapid vitrification with step-wise equilibriation of germinal vesicle-stage mouse oocytes. J Reprod Dev 2003; 49:501-506. [DOI:10.1262/jrd.49.501]
11. Hong SW, Chung HM, Lim JM, Ko JJ, Joon TK, Yee B, et al. Improved human oocyte development after vitrification: a comparison of thawing methods. Fertil Steril 1999; 72:142-146. [DOI:10.1016/S0015-0282(99)00199-5]
12. Lane M, Bavister BD, Lyons EA, Forest KT. Containerless vitrification of mammalian oocytes and embryos. Nat Biotechnol 1999; 17: 1234-1236. [DOI:10.1038/70795]
13. Yoon TK, Chung HM, Lim JM, Han SY, Ko JJ, Cha KY. Pregnancy and delivery of healthy infants developed from vitrified oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril 2000; 74: 180-181. [DOI:10.1016/S0015-0282(00)00572-0]
14. Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at _196 8C by vitrification. Nature 1985; 313: 573-375. [DOI:10.1038/313573a0]
15. Gupta MK, Uhm SJ, Lee HT. Cryopreservation of immature and in vitro matured porcine oocytes by solid surface vitrification. Theriogenology 2007; 67: 238-248. [DOI:10.1016/j.theriogenology.2006.07.015]
16. Cai XY, Chen GA, Lian Y, Zheng XY, Peng HM. Cryoloop vitrification of rabbit oocytes. Human Reprod 2005; 20: 1969-1974. [DOI:10.1093/humrep/deh805]
17. Mandelbaum J, Anastasiou O, Lévy R, Guérin JF, de Larouziére V, Antoine JM. Effects of cryopreservation on the meiotic spindle of human oocytes. Eur J Obstet Gynecol Reprod Biol 2004; 113:17-23. [DOI:10.1016/j.ejogrb.2003.11.005]
18. Hyttel P, Vajta G, Callesen H. Vitrification of bovine oocytes with the open pulled straw method: Ultra structural consequences. Mol Reprod Dev 2000; 56: 80-88.
https://doi.org/10.1002/(SICI)1098-2795(200005)56:1<80::AID-MRD10>3.0.CO;2-U [DOI:10.1002/(SICI)1098-2795(200005)56:13.0.CO;2-U]
19. Otoi T, Yamamoto K, Koyama N, Suzuki T. In vitro fertilization and development of immature and mature bovine oocytes cryopreserved by ethylene glycol with sucrose.Cryobiology 1995; 32: 455-460. [DOI:10.1006/cryo.1995.1045]
20. Men H, Monson RL, Rutledge JJ. Effect of meiotic stages and maturation protocols on bovine oocyte's resistance to cryopreservation. Theriogenology 2002; 57:1095-1103. [DOI:10.1016/S0093-691X(01)00679-3]
21. Ambrosini G, Andrisani A, Porcu E, Rebellato E, Revelli A, Caserta D, et al. Oocyte's cryopreservation: state of art. Reprod Toxicol 2006; 22: 250-262. [DOI:10.1016/j.reprotox.2006.04.024]
22. Hochi S, Ito K, Hirabayashi M, Ueda M, Kimura K, Hanada A. Effect of nuclear stages during IVM on the survival of vitrified-warmed bovine oocytes. Theriogenology. 1998; 49:787-796. [DOI:10.1016/S0093-691X(98)00028-4]
23. Albarracin JL, Morato R, Izquierdo D, Mogas T. Vitrification of calf oocytes: effects of maturation stage and prematuration treatment on the nuclear and cytoskeletal components of oocytes and their subsequent development. Mol Reprod Dev 2005; 72:239-249. [DOI:10.1002/mrd.20326]
24. Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig 2006; 13:451-458. [DOI:10.1016/j.jsgi.2006.05.005]
25. Lim JM, Ko JJ, Hwang WS, Chung HM, Niwa K. Development of in vitro matured bovine oocytes after cryopreservation with different cryoprotectants. Theriogenology 1999; 51:1303-1310. [DOI:10.1016/S0093-691X(99)00074-6]
26. Martino A, Pollard JW, Leibo SP. Effect of chilling bovine oocytes on their developmental competence. Mol Reprod Dev 1996; 45:503-512.
https://doi.org/10.1002/(SICI)1098-2795(199612)45:4<503::AID-MRD13>3.0.CO;2-X [DOI:10.1002/(SICI)1098-2795(199612)45:43.0.CO;2-X]
27. Zeron Y, Pearl M, Borochov A, Arav A. Kinetic and temporal factors influence chilling injury to germinal vesicle and mature bovine oocytes. Cryobiology 1999; 38:35-42. [DOI:10.1006/cryo.1998.2139]
28. Goud A, Goud P, Qian C, Van der Elst J, Van Maele G, Dhont M. Cryopreservation of human germinal vesicle stage and in vitro matured M II oocytes: influence of cryopreservation media on the survival, fertilization, and early cleavage divisions. Fertil Steril 2000; 74:487-494. [DOI:10.1016/S0015-0282(00)00672-5]
29. Sirard MA, Florman HM, Leibfried-Rutledge ML, Barnes FL, Sims ML, First NL. Timing of nuclear progression and protein synthesis necessary for meiotic maturation of bovine oocytes. Biol Reprod 1989; 40: 1257-1263. [DOI:10.1095/biolreprod40.6.1257]
30. Hunter AG, Moor RM. Stage-dependent effects of inhibiting ribonucleic acids and protein synthesis on meiotic maturation of bovine oocytes in vitro. J Dairy Sci 1987; 70:1646-1651. [DOI:10.3168/jds.S0022-0302(87)80192-3]
31. Mahmoudi R, Subhani A, Pasbakhsh P, Abolhasani F, Amiri I, Salehnia M, et al. The Effects of cumulus cells on in vitro maturation of mouse germinal vesicle stage oocytes. IJRM 2005; 3:74-78.
32. Hurt AE, Landim F, Seidel GE, Squires EL. Vitrification of immature and mature equine and bovine oocytes in an ethylene glycol, ficoll and sucrose solution using open-pulled straws. Theriogenology 2000; 54:119-128. [DOI:10.1016/S0093-691X(00)00330-7]
33. Cetin Y, Bastan A. Cryopreservation of immature bovine oocytes by vitrification in straws. Anim Reprod Sci 2006; 92:29-36. [DOI:10.1016/j.anireprosci.2005.05.016]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


