Sat, Jul 12, 2025
[Archive]
Volume 18, Issue 8 (August 2020)
IJRM 2020, 18(8): 591-596 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Davar R, Hoseini M, Saeed L. Vaginal compared oral administration of estradiol in women with thin endometrium: A cross-sectional study. IJRM 2020; 18 (8) :591-596
URL: http://ijrm.ir/article-1-1396-en.html
URL: http://ijrm.ir/article-1-1396-en.html
1- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,masroorehoseini@yahoo.com
2- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,
Full-Text [PDF 264 kb]
(1590 Downloads)
| Abstract (HTML) (32394 Views)
1. Introduction
Due to the advancement of laboratory equipment and protocols of ovarian stimulation, infertile patients who receive in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment can bear the potential for extra healthy embryos frozen for future transfer. As a major part of IVF/ICSI techniques, frozen-thawed embryo transfer (FET) enjoys some advantages including i) the prevention of IVF/ICSI complications such as ovarian hyperstimulation syndrome, ii) a rise in the rate of cumulative pregnancy, and iii) better endometrium synchronism (1). Endometrial thickness is regarded as an indicator of the receptivity of the endometrium and as a prognostic factor in the transfer of an embryo in the course of IVF/ICSI treatment. Appropriate endometrial thickness is crucial in the success of pregnancy, and many studies report low rates of pregnancy due to thin endometrium.
Clinicians have varying perspectives on the ideal thickness of the endometrium for conception (2). It has been verified that the rate of pregnancy and implantation is negatively correlated with endometrial thickness < 7 mm and responsible for a higher probability of miscarriage (3). Many protocols exist for the preparation of the endometrium in FET cycles, the most popular one of which is hormonal replacement treatment utilizing exogenous estradiol and progesterone (1). Patients preparing for FET need the supplementation of extra estrogen or some other interventions in case their endometrium is thin (4).
This study was designed to investigate the clinical outcomes of endometrial preparation by oral compared with vaginal administration of estradiol valerate in women with inadequate endometrial thickness after 12 days of taking estrogen supplementation.
2. Materials and Methods
In this cross-sectional study, the medical records of 79 women underwent endometrial preparation for FET treatment in the Research and Clinical Center for Infertility, Yazd, Iran between June 2018 and January 2019 were reviewed. Our inclusion criteria were endometrial thickness < 7 mm measured by vaginal ultrasonography on day 13 cycle, after administration of 6 mg oral estradiol valerate/day from the second day of the menstrual cycle. The exclusion criteria comprised of donor oocyte recipients and gestational carriers.
The preparation of the endometrium in all participants started with the oral administration of the estradiol valerate tablet 6 mg/day (Aburaihan Co., Tehran, Iran) from the second day of the menstrual cycle. On the 13th day, vaginal ultrasonography was employed in order to measure the thickness of the endometrium. In case the thickness of the endometrium was < 7 mm, the women would be administered with 10 mg/day estradiol valerate. The oral or vaginal administration of the estradiol valerate depended on the clinician’s decision; 36 women (cycles) have been treated with the oral estradiol valerate (OE group) and 43 women (cycles) with estradiol valerate tablet vaginally (VE group). When the endometrial thickness reached ≥ 8mm, all women were administered with 400 mg of Cyclogest®vaginal pessaries (Cox Pharmaceuticals, Barnstaple, UK) twice a day. The embryo transfer was conducted three days after the administration of progesterone for cleavage embryos and five days after for blastocyst. After embryo transfer, all the patients received estradiol valerate orally and progesterone suppository. In the cases pregnancy occurred, administration continued until 10th week of the gestational age. Twelve days following the embryo transfer, chemical pregnancy was determined using serum βhCG > 50 IU/L. Moreover, 14 days following a positive βhCG, clinical pregnancy was verified by identifying fetal heartbeats in sonography. Early miscarriage was defined as the loss of pregnancy before 12th week of gestation.
2.1. Ethical consideration
All women signed the informed consent to participate in this study. The study protocol was approved by the Ethics Committee of Yazd Reproductive Sciences Institute, Yazd, Iran (Code: IR.SSU.RSI.REC.1397.026).
2.2. Statistical analysis
SPSS software (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Illinois, USA) version 20.0, Students t test, and Chi-square test were used for the statistical calculations. A p-value < 0.05 was considered as significant.
3. Results
There were no significant differences between the two groups in the mean of age, body mass index, duration of infertility, and type of infertility (Table I). Total cancelation due to inappropriate endometrium thickness was three cycles in the VE group, while it was four cycles in the OE group (7.0% vs 11.1%; p = 0.696). The duration of estradiol supplementation (p = 0.73) and total dose of estradiol (p = 0.847) were similar in both groups. There was no significant difference between the two groups in the terms of endometrial thickness on day 13 and endometrial thickness on the day of transfer (Table II).
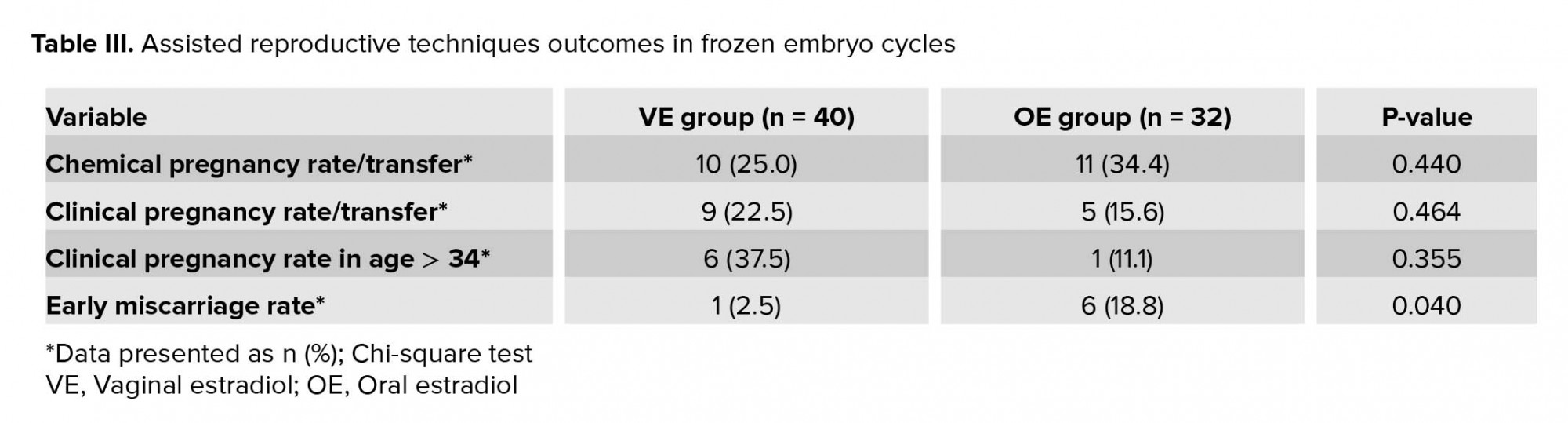
Although women in the VE group obtained lower chemical pregnancy rate compared with the OE group (p = 0.440), yet clinical pregnancy rate was higher for them (p = 0.464), especially for the women who were older than 34 yr (p = 0.355). The early miscarriage rate was lower in the VE group in comparison with the OE group (p = 0.040) (Table III).


4. Discussion
This study identified that vaginal administration of estradiol in women with thin endometrium leads to a lower rate of early miscarriage. In order to induce endometrial receptivity in the cycles of the cryopreserved embryo transfer, progesterone and estrogen are required. Scores of methods and routes of administration of exogenous estradiol are available to reproductive health providers (5). Evidenced by the current published literature, recommending an ideal method for endometrial preparation in the FET is still a matter of controversy (6).
For the assessment of endometrial proliferation, the measurement of the endometrial thickness using ultrasound is regarded as a reproducible and simple method. The lowest rates of pregnancy were relevant to the endometrial thickness of > 14 mm and < 7 mm (7). The incidence of thin endometrium in ART has been stated to be 1.5 to 9.1% (8). As a first-line agent, oral administration of estradiol between day 10th and 14th may be the simplest route, at either an increasing or a constant dose. If insufficient endometrialb thickness is observed, this course can be extended by changing the route of administration to percutaneous or vaginal (5).
In order to improve ET in patients with unresponsive endometrium, several methods have been used till date, including pentoxifylline-vitamin E, aspirin, G-CSF, autologous platelet-rich-plasma, sildenafil, and human chorionic gonadotropin (3, 9-12). However, none of these methods has been reported to be superior to the others.
Parenteral routes of administration, including intramuscular, vaginal, and transdermal circumvent the liver first-pass metabolism of E2 (8, 13). The excessive exposure of the liver due to liver first-pass effect inherent to oral administration can lead to a higher risk of veno-thromboembolism accidents, especially in susceptible women. Vaginal E2, especially in patients with poor endometrial response to E2, can be a valuable alternative. Although creams with special designs are available, oral tablets are administered vaginally by many professionals (14). In the present study, we also administered oral estradiol tablets vaginally to the VE group. In a study conducted by Zolghadri and colleagues, Vagifem was compared with vaginal Premarin (15). However, oral estradiol tablets are more cost-effective and convenient. E2, administered vaginally, can be absorbed into the circulation adequately and by the endometrium selectively. High levels of serum‑approximately eight times higher than the oral administration-are obtained; this seems to be remarkably a highly efficient method for the delivery of E2 directly to the intended tissue with even higher endometrial tissue levels observed (80 times higher) (5). Despite the higher levels of serum E2, it can be as safe as the equal oral dose. There is no difference in the levels of sex hormone-binding globulin or in the patients’ cholesterol profile (16). Following embryo transfer in both groups of the study, estradiol tablets were administered orally. Estrogen concentrations were reported to be positively correlated with the frequency of uterine contraction (17).
In this study, VE and OE groups demonstrated comparable rates of clinical and chemical pregnancies. Correspondingly in another study, Liao and colleagues identified pregnancy outcomes being comparable in females bearing thin endometria administered with estradiol orally or vaginally (4). Fanchin and colleagues compared vaginal and oral routes in 39 infertile females by observing uterine perfusion and ET on day 14th following the E2 administration. They concluded that the vaginal route can trigger better ET and uterine perfusion than the oral route (18).
Contrary to the above, Doyle and colleagues reported that adding vaginal E2 can induce a rapid increase in the levels of serum E2 and thus contribute to a lower rate of live birth as well as a higher rate of miscarriage. The reason, as they concluded, is that an increase in the level of serum E2 over the threshold of 200 pg/ml of the natural menstrual cycle can produce negative effects arising from supraphysiologic E2 levels (19). In contrast, the present study demonstrated a lower rate of early miscarriage in the VE group (p < 0.05).
For implantation to occur successfully, the endometrium needs to undergo key alterations to receive the growing embryo within a defined period known as the "implantation window". This complicated process is adjusted by the interaction of adhesion molecules, ovarian hormones, growth factors, and cytokines (7). Accordingly, one may deduce that vaginal estradiol can improve the endometrial development by affecting not only the thickness of the endometrium but also the microenvironment of the endometrium (4).
5. Conclusion
The findings of this study revealed that women with age>34yr had more pregnancy rates in the VE group, although the differences were not statistically significant. Due to no significant differences in pregnancy rates between two study groups the lower risk of thromboembolism in the vaginal route because lack of hepatic metabolism compared to the oral route, so this method is recommended especially in women at higher risk of thromboembolism. Also, vaginal administration of estradiol tablet in women with thin endometrium leads to a lower rate of early miscarriage.
Acknowledgments
This study was performed with no financial support.
Conflict of Interest
There is no conflict of interest.
Full-Text: (761 Views)
1. Introduction
Due to the advancement of laboratory equipment and protocols of ovarian stimulation, infertile patients who receive in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment can bear the potential for extra healthy embryos frozen for future transfer. As a major part of IVF/ICSI techniques, frozen-thawed embryo transfer (FET) enjoys some advantages including i) the prevention of IVF/ICSI complications such as ovarian hyperstimulation syndrome, ii) a rise in the rate of cumulative pregnancy, and iii) better endometrium synchronism (1). Endometrial thickness is regarded as an indicator of the receptivity of the endometrium and as a prognostic factor in the transfer of an embryo in the course of IVF/ICSI treatment. Appropriate endometrial thickness is crucial in the success of pregnancy, and many studies report low rates of pregnancy due to thin endometrium.
Clinicians have varying perspectives on the ideal thickness of the endometrium for conception (2). It has been verified that the rate of pregnancy and implantation is negatively correlated with endometrial thickness < 7 mm and responsible for a higher probability of miscarriage (3). Many protocols exist for the preparation of the endometrium in FET cycles, the most popular one of which is hormonal replacement treatment utilizing exogenous estradiol and progesterone (1). Patients preparing for FET need the supplementation of extra estrogen or some other interventions in case their endometrium is thin (4).
This study was designed to investigate the clinical outcomes of endometrial preparation by oral compared with vaginal administration of estradiol valerate in women with inadequate endometrial thickness after 12 days of taking estrogen supplementation.
2. Materials and Methods
In this cross-sectional study, the medical records of 79 women underwent endometrial preparation for FET treatment in the Research and Clinical Center for Infertility, Yazd, Iran between June 2018 and January 2019 were reviewed. Our inclusion criteria were endometrial thickness < 7 mm measured by vaginal ultrasonography on day 13 cycle, after administration of 6 mg oral estradiol valerate/day from the second day of the menstrual cycle. The exclusion criteria comprised of donor oocyte recipients and gestational carriers.
The preparation of the endometrium in all participants started with the oral administration of the estradiol valerate tablet 6 mg/day (Aburaihan Co., Tehran, Iran) from the second day of the menstrual cycle. On the 13th day, vaginal ultrasonography was employed in order to measure the thickness of the endometrium. In case the thickness of the endometrium was < 7 mm, the women would be administered with 10 mg/day estradiol valerate. The oral or vaginal administration of the estradiol valerate depended on the clinician’s decision; 36 women (cycles) have been treated with the oral estradiol valerate (OE group) and 43 women (cycles) with estradiol valerate tablet vaginally (VE group). When the endometrial thickness reached ≥ 8mm, all women were administered with 400 mg of Cyclogest®vaginal pessaries (Cox Pharmaceuticals, Barnstaple, UK) twice a day. The embryo transfer was conducted three days after the administration of progesterone for cleavage embryos and five days after for blastocyst. After embryo transfer, all the patients received estradiol valerate orally and progesterone suppository. In the cases pregnancy occurred, administration continued until 10th week of the gestational age. Twelve days following the embryo transfer, chemical pregnancy was determined using serum βhCG > 50 IU/L. Moreover, 14 days following a positive βhCG, clinical pregnancy was verified by identifying fetal heartbeats in sonography. Early miscarriage was defined as the loss of pregnancy before 12th week of gestation.
2.1. Ethical consideration
All women signed the informed consent to participate in this study. The study protocol was approved by the Ethics Committee of Yazd Reproductive Sciences Institute, Yazd, Iran (Code: IR.SSU.RSI.REC.1397.026).
2.2. Statistical analysis
SPSS software (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Illinois, USA) version 20.0, Students t test, and Chi-square test were used for the statistical calculations. A p-value < 0.05 was considered as significant.
3. Results
There were no significant differences between the two groups in the mean of age, body mass index, duration of infertility, and type of infertility (Table I). Total cancelation due to inappropriate endometrium thickness was three cycles in the VE group, while it was four cycles in the OE group (7.0% vs 11.1%; p = 0.696). The duration of estradiol supplementation (p = 0.73) and total dose of estradiol (p = 0.847) were similar in both groups. There was no significant difference between the two groups in the terms of endometrial thickness on day 13 and endometrial thickness on the day of transfer (Table II).
Although women in the VE group obtained lower chemical pregnancy rate compared with the OE group (p = 0.440), yet clinical pregnancy rate was higher for them (p = 0.464), especially for the women who were older than 34 yr (p = 0.355). The early miscarriage rate was lower in the VE group in comparison with the OE group (p = 0.040) (Table III).



4. Discussion
This study identified that vaginal administration of estradiol in women with thin endometrium leads to a lower rate of early miscarriage. In order to induce endometrial receptivity in the cycles of the cryopreserved embryo transfer, progesterone and estrogen are required. Scores of methods and routes of administration of exogenous estradiol are available to reproductive health providers (5). Evidenced by the current published literature, recommending an ideal method for endometrial preparation in the FET is still a matter of controversy (6).
For the assessment of endometrial proliferation, the measurement of the endometrial thickness using ultrasound is regarded as a reproducible and simple method. The lowest rates of pregnancy were relevant to the endometrial thickness of > 14 mm and < 7 mm (7). The incidence of thin endometrium in ART has been stated to be 1.5 to 9.1% (8). As a first-line agent, oral administration of estradiol between day 10th and 14th may be the simplest route, at either an increasing or a constant dose. If insufficient endometrialb thickness is observed, this course can be extended by changing the route of administration to percutaneous or vaginal (5).
In order to improve ET in patients with unresponsive endometrium, several methods have been used till date, including pentoxifylline-vitamin E, aspirin, G-CSF, autologous platelet-rich-plasma, sildenafil, and human chorionic gonadotropin (3, 9-12). However, none of these methods has been reported to be superior to the others.
Parenteral routes of administration, including intramuscular, vaginal, and transdermal circumvent the liver first-pass metabolism of E2 (8, 13). The excessive exposure of the liver due to liver first-pass effect inherent to oral administration can lead to a higher risk of veno-thromboembolism accidents, especially in susceptible women. Vaginal E2, especially in patients with poor endometrial response to E2, can be a valuable alternative. Although creams with special designs are available, oral tablets are administered vaginally by many professionals (14). In the present study, we also administered oral estradiol tablets vaginally to the VE group. In a study conducted by Zolghadri and colleagues, Vagifem was compared with vaginal Premarin (15). However, oral estradiol tablets are more cost-effective and convenient. E2, administered vaginally, can be absorbed into the circulation adequately and by the endometrium selectively. High levels of serum‑approximately eight times higher than the oral administration-are obtained; this seems to be remarkably a highly efficient method for the delivery of E2 directly to the intended tissue with even higher endometrial tissue levels observed (80 times higher) (5). Despite the higher levels of serum E2, it can be as safe as the equal oral dose. There is no difference in the levels of sex hormone-binding globulin or in the patients’ cholesterol profile (16). Following embryo transfer in both groups of the study, estradiol tablets were administered orally. Estrogen concentrations were reported to be positively correlated with the frequency of uterine contraction (17).
In this study, VE and OE groups demonstrated comparable rates of clinical and chemical pregnancies. Correspondingly in another study, Liao and colleagues identified pregnancy outcomes being comparable in females bearing thin endometria administered with estradiol orally or vaginally (4). Fanchin and colleagues compared vaginal and oral routes in 39 infertile females by observing uterine perfusion and ET on day 14th following the E2 administration. They concluded that the vaginal route can trigger better ET and uterine perfusion than the oral route (18).
Contrary to the above, Doyle and colleagues reported that adding vaginal E2 can induce a rapid increase in the levels of serum E2 and thus contribute to a lower rate of live birth as well as a higher rate of miscarriage. The reason, as they concluded, is that an increase in the level of serum E2 over the threshold of 200 pg/ml of the natural menstrual cycle can produce negative effects arising from supraphysiologic E2 levels (19). In contrast, the present study demonstrated a lower rate of early miscarriage in the VE group (p < 0.05).
For implantation to occur successfully, the endometrium needs to undergo key alterations to receive the growing embryo within a defined period known as the "implantation window". This complicated process is adjusted by the interaction of adhesion molecules, ovarian hormones, growth factors, and cytokines (7). Accordingly, one may deduce that vaginal estradiol can improve the endometrial development by affecting not only the thickness of the endometrium but also the microenvironment of the endometrium (4).
5. Conclusion
The findings of this study revealed that women with age>34yr had more pregnancy rates in the VE group, although the differences were not statistically significant. Due to no significant differences in pregnancy rates between two study groups the lower risk of thromboembolism in the vaginal route because lack of hepatic metabolism compared to the oral route, so this method is recommended especially in women at higher risk of thromboembolism. Also, vaginal administration of estradiol tablet in women with thin endometrium leads to a lower rate of early miscarriage.
Acknowledgments
This study was performed with no financial support.
Conflict of Interest
There is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. He W, Lv J, Lin H, Ou J, Tao X, Xing W, et al. Are the estrogen levels on the day of frozen-thawed embryo transfer related to the outcomes in hormonal replacement treatment cycles? Int J Clin Exp Med 2018; 11: 7200-7207.
2. Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet 2006; 23: 337-342. [DOI:10.1007/s10815-006-9053-1] [PMID]
3. Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol 2018; 57: 810-813. [DOI:10.1016/j.tjog.2018.10.007] [PMID]
4. Liao X, Li Z, Dong X, Zhang H. Comparison between oral and vaginal estrogen usage in inadequate endometrial patients for frozen-thawed blastocysts transfer. Int J Clin Exp Pathol 2014; 7: 69926997.
5. Burks H, Paulson R. Cryopreserved embryo transfer: endometrial preparation and timing. Seminars in reproductive medicine. California: Thieme Medical Publishers; 2015. [DOI:10.1055/s-0035-1546302] [PMID]
6. Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 2017; 23: 255-261. [DOI:10.1093/humupd/dmw046] [PMID]
7. El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril 2008; 89: 832-839. [DOI:10.1016/j.fertnstert.2007.04.031] [PMID]
8. Liu K, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod 2018; 33: 1883-1888. [DOI:10.1093/humrep/dey281] [PMID] [PMCID]
9. Wada I, Hsu CC, Williams G, Macnamee MC, Brinsden PR. The benefits of low-dose aspirin therapy in women with impaired uterine perfusion during assisted conception. Hum Reprod 1994; 9: 1954-1957. [DOI:10.1093/oxfordjournals.humrep.a138366] [PMID]
10. Letur-Könirsch H, Guis F, Delanian S. Uterine restoration by radiation sequelae regression with combined pentoxifylline-tocopherol: a phase II study. Fertil Steril 2002; 77: 1219-1226. [DOI:10.1016/S0015-0282(02)03120-5]
11. Davar R, Miraj S, Farid Mojtahedi M. Effect of adding human chorionic gonadotropin to frozen thawed embryo transfer cycles with history of thin endometrium. Int J Reprod BioMed 2016; 14: 53-56. [DOI:10.29252/ijrm.14.1.53]
12. Dehghani Firouzabadi R, Davar R, Hojjat F, Mahdavi M. Effect of sildenafil citrate on endometrial preparation and outcome of frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J Reprod Med 2013; 11: 151-158.
13. Davar R, Janati S, Mohseni F, Khabazkhoob M, Asgari S. A comparison of the effects of transdermal estradiol and estradiol valerate on endometrial receptivity in frozen-thawed embryo transfer cycles: a randomized clinical trial. J Reprod Infertil 2016; 17: 97-103.
14. Gardner DK, Weissman A, Howles CM, Shoham Z. Text book of assisted reproductive techniques: CRC Press. New York: Tylor & Francis group; 2018.
15. Zolghadri J, Haghbin H, Dadras N, Behdin S. Vagifem is superior to vaginal Premarin in induction of endometrial thickness in the frozen-thawed cycle patients with refractory endometria: A randomized clinical trial. Iran J Reprod Med 2014; 12: 415-420.
16. Tourgeman DE, Slater CC, Stanczyk FZ, Paulson RJ. Endocrine and clinical effects of micronized estradiol administered vaginally or orally. Fertil Steril 2001; 75: 200-202. [DOI:10.1016/S0015-0282(00)01640-X]
17. Oike K, Ishihara K, Kikushi S. A study on the endometrial movement and serum hormonal level in connection with uterine contraction. Nihon Sanka Fujinka Gakkai Zasshi 1990; 42: 86-92.
18. Fanchin R, Righini C, Schönauer LM, Olivennes F, Cunha Filho JS, Frydman R. Vaginal versus oral E2 administration: effects on endometrial thickness, uterine perfusion, and contractility. Fertil Steril 2001; 76: 994-998. [DOI:10.1016/S0015-0282(01)02841-2]
19. Doyle N, Parikh T, Eubanks AA, DeCherney A, Healy MW, Yauger B, et al. The effect of vaginal estradiol on live birth in preparation of the endometrium in FET cycles. Fertil Steril 2017; 108 (Suppl.): e170-e171. [DOI:10.1016/j.fertnstert.2017.07.509]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








