Wed, Jan 7, 2026
[Archive]
Volume 19, Issue 11 (November 2021)
IJRM 2021, 19(11): 987-996 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moradi A, Ghasemian F, Mashayekhi F. The reaggregation of normal granulosa-cumulus cells and mouse oocytes with polycystic ovarian syndrome in vitro: An experimental study. IJRM 2021; 19 (11) :987-996
URL: http://ijrm.ir/article-1-1944-en.html
URL: http://ijrm.ir/article-1-1944-en.html
1- Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran.
2- Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran. ,ghasemian.21@gmail.com
2- Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran. ,
Full-Text [PDF 3634 kb]
(1421 Downloads)
| Abstract (HTML) (2161 Views)
1. Introduction
Maturation and developmental competence of oocytes and their quality are important criteria in successful reproductive outcomes of assisted reproduction technologies. Polycystic ovarian syndrome (PCOS) is known as the most common cause of female infertility during reproductive age. It is distinguished by oligomenorrhea, bilateral polycystic ovaries, hyperinsulinemia, biochemical and/or hyperandrogenism, and oligo- or chronic anovulatory (1-3). One of the most important symptoms in PCOS patients is follicular development arrest and dysregulation of paracrine follicle activity (4). As a result, one of the main important concerns in PCOS patients is the poor competence of oocyte development (3, 5) and weak response to ovarian stimulation (6, 7). For this reason, the use of assisted reproductive technologies such as in vitro maturation (IVM) for such patients is suggested.
During the IVM procedure, the maturation medium usually replaces the ovarian follicular environment. Therefore, it is necessary to create an appropriate culture medium for improving the competence of immature oocyte development for fertilization and embryogenesis (7). The development and growth of oocytes are supported by granulosa cells (GCs) from primordial to antral stages (8); therefore, one of the strategies to increase in vitro oocyte maturation is co-culture with GCs (9).
In a study, the developmental rate of porcine oocytes derived from early antral follicles improved using reconstructed oocyte-GC complexes (10). In another study, the maturation and development competence of buffalo oocytes derived from preantral follicles was evaluated in the presence of antral follicles and the survival and growth of the preantral follicles improved in this co-culture system (11).
Many studies have reported oocytes as an avascular environment. Normal folliculogenesis relies on the crosstalk among GCs, cumulus cells, and oocytes via gap junction intercellular communication (12, 13). Two matchable hemichannels (connexin, Cx) in the plasma membrane of adjacent cells are involved in the formation of gap junctions. Cx43 is mainly localized to the membranes of the GCs and cumulus cells (12). It has been reported that Cx43 expression decreases in the presence of high androgen levels and hence gap-junctional intercellular communication between human GCs is impaired (14). GCs in PCOS patients were induced to produce more androgens, which resulted in the enhancement of hyperandrogenemia and anovulation (1). High androgen levels may also impair folliculogenesis in PCOS patients (14). Therefore, the co-culture of PCOS oocytes with normal cumulus GCs may improve the maturation rate and competence of oocyte development.
To the best of our knowledge, there have been no published studies on the use of normal cumulus GCs to improve the maturation of PCOS oocytes in co-culture conditions. Therefore, the purpose of this study was to answer the following questions: (i) Will a connection be made between normal cumulus-GCs and PCOS immature oocytes in a co-culture medium? (ii) How will a connection between PCOS immature oocytes and normal granulosa-cumulus cells (GCCs) affect the maturation rate of PCOS oocytes and their quality?
2. Materials and Methods
2.1. Study animals
This experimental study was conducted over a period of two years (2019-2020) in the University of Guilan, Rasht, Iran. 20 virgin adult Naval Medical Research Institute female mice (30-35 gr, 7-8 wk old) were used for the study. Animals were acquired from the Razi Institute (Karaj, Iran) and housed in a central animal care room with a controlled environment of 22 ± 3°C temperature, 45-55% humidity and a 12 hr light/dark cycle. Mice were kept in a cage in groups of four and fed with a standard diet and water accessed ad libitum. All chemicals and reagents were purchased from Sigma Aldrich Company (Germany), unless otherwise specified.
2.2. PCOS induction
All the experimental animals except for those in the control group were injected with estradiol valerate (Aburaihan Co., Iran) at a dose of 40 mg/kg body weight dissolved in 0.5% sesame oil by intramuscular injection once daily for 60 days. Vaginal epithelia smears were obtained daily and evaluated by light microscope (Olympus, Japan) using Giemsa stain to determine the induction of PCOS. An irregular estrous cycle and occurrence of persistent vaginal cornification phase were the symptoms of PCOS induction. Ovaries were cut through at their longest longitudinal dimension and fixed in alcoholic Bouin's solution. After dehydration, the ovaries were serially sectioned at 5 µm, and stained with hematoxylin and eosin. The sections were used for histologic evaluation of the PCOS ovaries.
In addition, to confirm the PCOS induction, blood samples of PCOS mice were collected transcardially. Then, the separated serum was stored at -20°C. Using immunofluorometric techniques, the levels of serum follicle-stimulating and luteinizing hormones were measured. 2.9% and 2.6% were considered as total assay variation coefficients. In addition, the Coat-A-Count RIA kit was used to analyzed the level of serum testosterone with a sensitivity of 4 ng/dL (0.139 nmol/L).
2.3. Experimental design
To produce a conditioned medium, normal GCCs were collected from the ovaries of normal mice. Following cervical dislocation, the ovaries were placed in α-minimum essential medium (Gibco, Paisley, UK) supplemented with 5% fetal bovine serum (FBS; Gibco, UK), and were mechanically dissected. GCCs were collected and cultured in 24-well plates (1×106 cells/well) containing 500 μl of the same medium supplemented with 5% FBS, 50 μg/ml penicillin, and 50 μg/ml streptomycin for 24-48 hr. The unattached cells were removed following replacement with fresh medium.
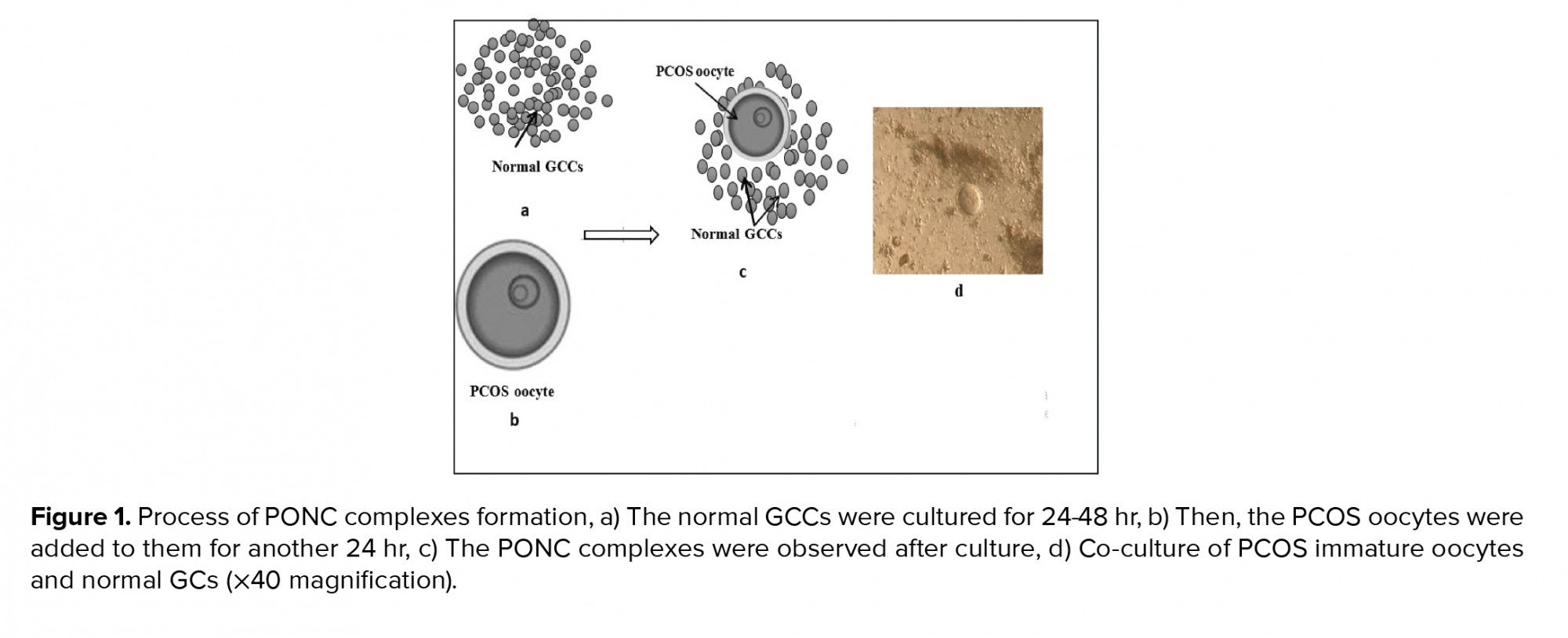
After the confirmation of PCOS induction and following excision of the ovaries, the PCOS ovaries were placed in an α-minimum essential medium supplemented with 5% FBS. The ovaries were mechanically dissected and oocytes at the germinal vesicle stage were collected. The mouse germinal vesicle oocytes showed a large spherical germinal vesicle (30 µm in diameter) and a single nucleolus. After washing three times, the co-culture of PCOS oocytes with cultured GCCs (one oocyte/well) were initiated in the α-minimum essential medium supplemented with 5% FBS, 0.23 mM sodium pyruvate, 75 mU/ml of follicle-stimulating hormone, 7.5 IU/ml human chorionic gonadotropin, 50 μg/ml penicillin, and 50 μg/ml streptomycin (experimental group). After 24 hr co-culture, a complex between PCOS oocytes with normal GCCs was observed and was referred to as a PCOS oocyte-normal GCC complex (PONC) (Figure 1). Therefore, the groups of study were as follows: 1) experimental group - PCOS oocytes cultured in the presence of normal GCCs; and 2) control group - PCOS oocytes cultured in the absence of normal GCCs.
The development of PONC complexes and the properties of the oocytes in these complexes were evaluated 24 hr after co-culture and compared to the control group. The maturation rate of the reconstructed complexes (the presence of the first polar body extrusion) was also examined 24 hr after co-culture (15). The GCCs’ viability was assessed by trypan blue staining. The experiment was repeated five times.
2.4. Scanning electron microscopy (SEM) evaluation
For each experimental group, the fixation of PONC complexes was performed in 2.5% glutaraldehyde/0.1 M phosphate-buffered saline (PBS) and prepared for conventional SEM evaluation. After washing of fixed samples in 0.1 M PBS, postfixation was done in 1% osmium tetroxide/0.1 M PBS. Finally, the samples were washed in 0.1 M PBS, and dehydrated in ascending ethanol series. The dried samples were put on aluminum stubs and, then, covered with gold. The SEM images were observed at low accelerating voltage (3-10 kV) using a SEM microscope (Philips XL-30-CP).
2.5. RNA isolation and real-time polymerase chain reaction (real-time PCR)
At the end of the culture period, the PONC complexes and PCOS oocytes (experimental and control groups, respectively) were collected. Total RNA was extracted using the RNeasy Mini Kit (Roche Molecular Biochemicals, Mannheim, Germany) and stored at -80oC. The cDNA was synthesized using the cDNA kit (Thermo Scientific, EU) in accordance with the manufacturer’s instructions at 42oC for 60 min, and stored at -20oC.
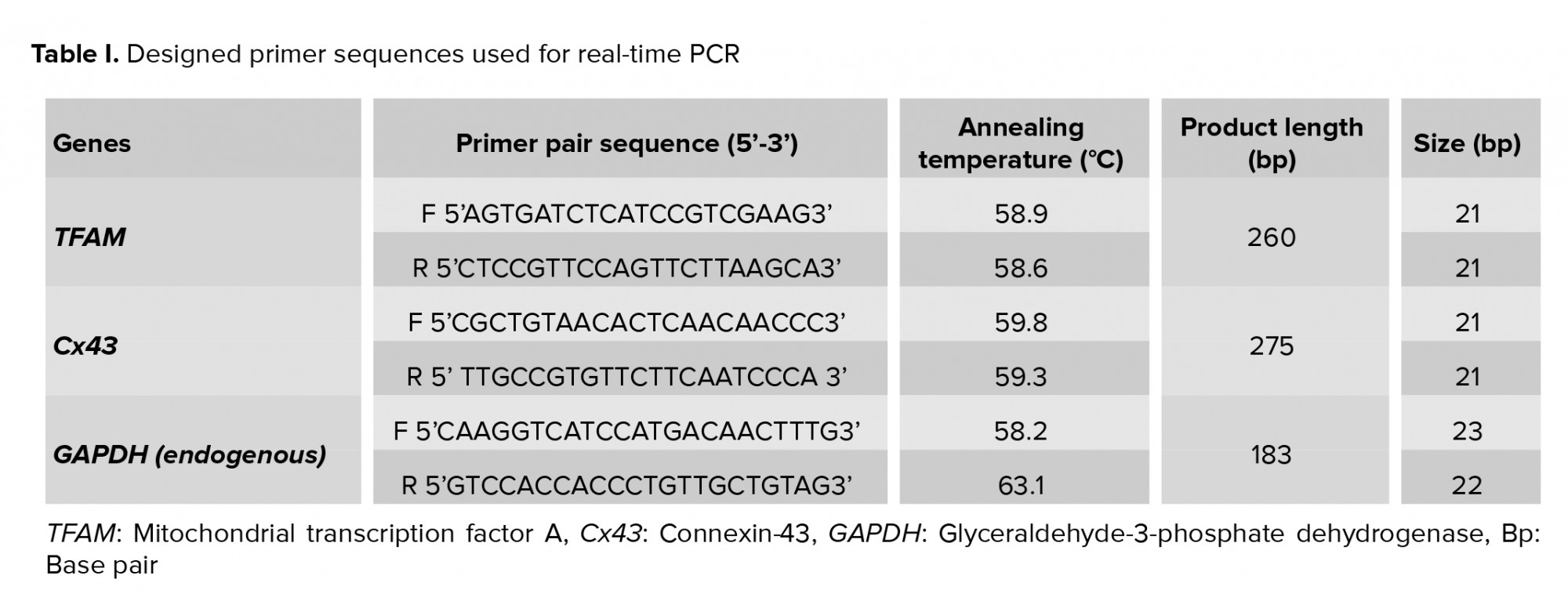
Real time qRT-PCR was used to quantify the mRNA transcript levels of the mitochondrial transcription factor A (TFAM) and Cx43 genes. Primer pairs for amplifying the TFAM and Cx43 genes were designed using GenBank from the National Center for Biotechnology Information. The primer sequences are shown in table I. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Real time thermal cycler (Applied Biosystems, Foster City, USA) was used for analyzing gene expression. QuantiTect SYBR Green RT-PCR kit (Applied Biosystems, USA) was also employed for amplifying the targeted genes. Amplification of the reference and target genes was performed in the same run, for each sample. The thermal protocol of real time-PCR was programmed as: the holding step at 95oC for 5 min, cycling step at 95oC for 15 sec, 58oC for 30 sec, and 72oC for 15 sec. The melt curve step was performed as 95oC (15 sec), 60oC (1 min), and 95oC (15 sec). The method of ΔΔCT was used to determine the relative quantity of the target genes. All experiments of real time-PCR were done five times.
2.6. Ethical considerations
All investigations conformed to the ethical and humane principles of research and were approved by Guilan University of Medical Science Committee on the Use and Care of Animals (Code: IR.GUMS.REC.1399.255).
2.7. Statistical analysis
All experiments were repeated five times and data were expressed as mean ± S.D. χ2, one-way ANOVA, and Tukey’s post-hoc tests were used to analyze differences among the groups and gene expression. The statistical analysis was performed using the Statistical Package for the Social Sciences version 20 (SPSS, IBM, Armonk, NY, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. PCOS ovaries evaluation
In the PCOS mice, the irregular estrous cycles were confirmed along with the restriction to estrous stages upon estradiol valerate treatment. The histological examinations showed that the number of pre-antral follicles was higher in the PCOS mice. In addition, atretic and cystic follicles were observed in these mice (n = 6 ovaries) and their ovaries contained fewer corpora lutea (Figure 2). The evaluation of steroid hormones showed that the serum testosterone and luteinizing hormone levels were higher in the estradiol valerate-treated mice (p = 0.04) at 60 days. The serum follicle-stimulating hormone level did not change after treatment with estradiol valerate.
In the experimental group, following co-culture of PCOS oocytes with normal GCCs, significant reaggregation of cells and oocytes was observed. These reconstructed complexes were formed as PONC complexes. The rate of reconstruction was 79.8%. The evaluation of the maturation rate of the PONC complex PCOS oocytes in the experimental group and of the PCOS oocytes from the control group showed that the nuclear maturation rate of PONC complexes was significantly higher compared to the control group (Table II, p = 0.01).
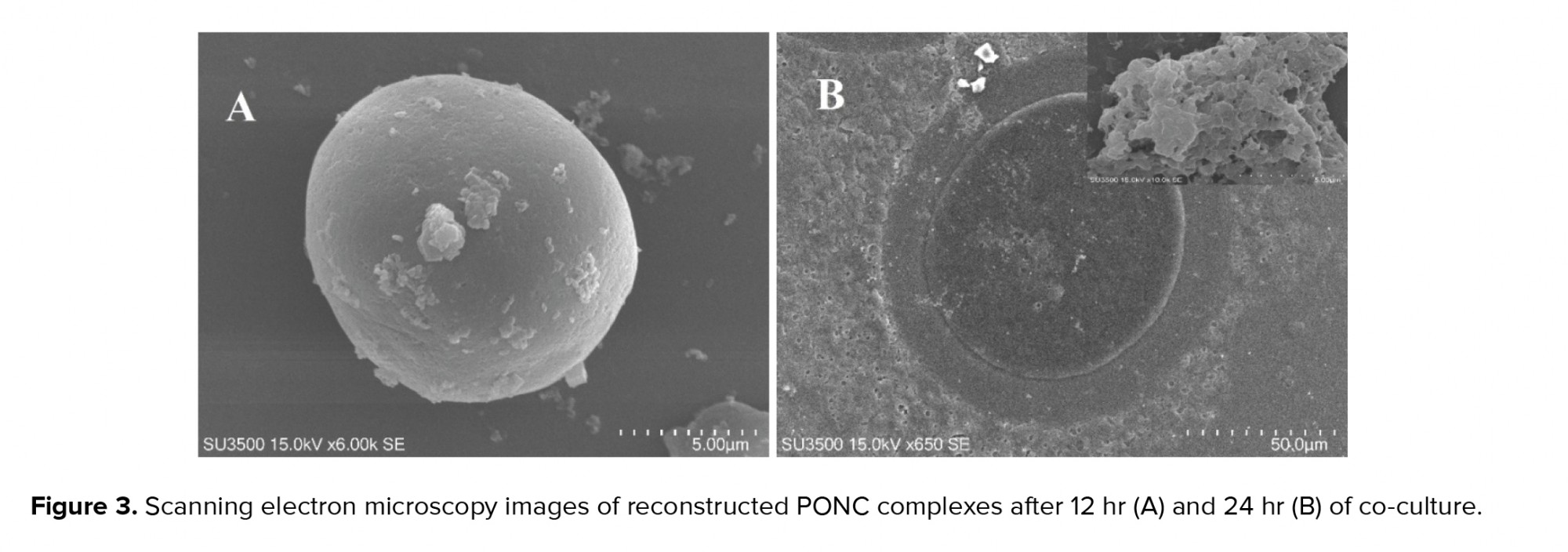
After co-culture, the PCOS oocytes were surrounded by multiple layers of strictly packaged GCCs, as shown in the SEM images (Figure 3).
The molecular analysis showed that the expression rates of the TFAM gene in the experimental and control groups were 0.460 ± 0.090 and 0.025 ± 0.005, respectively. The rate of TFAM gene expression was significantly higher in the experimental group (PONC complexes) in comparison to the control group (p = 0.03). In addition, Cx43 gene expression was also higher in the experimental group (PONC complexes) (4.28 ± 0.57) compared with the control group (0.96 ± 0.12, p < 0.01) (Table III). The high expression of the Cx43 gene could confirm the connection between PCOS oocytes and normal GCCs.



4. Discussion
The maturation medium and IVM procedure strongly affected the oocyte quality and maturation. The present study shows that normal GCCs could interact with PCOS oocytes, with a gap junction created among them, and that as a result, PCOS oocyte maturation rates can improve. It seems that co-culturing with normal GCCs provides a suitable ‘niche’ for PCOS oocyte maturation like the ovary. Therefore, after co-culture, the maturation rate of PCOS oocytes and the profile of gene expression improved. These results are reasonable because the dialogue between oocytes and the surrounding cells plays a major role in progressing oocyte meiosis and their developmental potential (14).
It is possible that some molecules from normal GCCs contribute to the growth potential of PCOS oocytes under in vitro conditions. Given that females with PCOS have a weak response to ovarian stimulation and have lower oocyte maturation competence (7), improving the medium efficiency for IVM protocols is important. The abnormal function of GCs in women with PCOS has been reported (16). In addition, the expression of the growth differentiation factor-9 gene is lower in GCCs from patients with PCOS (16). Growth differentiation factor-9 plays a critical role in promoting GC mitosis (17), maintaining and developing the gap junction (increased Cx43 gene expression) between oocytes and adjacent GCs (18), generating luteinizing hormone, and synthesizing cyclic adenosine monophosphate (16, 19). Therefore, as the results of the present study show, co-culture with normal GCCs can improve in vitro maturation of PCOS oocytes. In accordance with previous studies, the results of this study show that Cx43 gene expression was higher in the co-culture system between normal GCCs and PCOS oocytes. In addition, the expression of the TFAM gene, which is one of the genes representing the quality of the oocyte, was higher in the PCOS oocytes during co-culture. It has been observed that cumulus cells are difficult to detach (20); therefore, in this study they were only cultured for 24-48 hr before adding the PCOS oocytes. This prevented the attachment of the GCCs to the plate bottom and allowed interaction with and connection to the PCOS oocytes.
It has been reported that the cleavage rate of embryos resulting from matured oocytes in a co-cultured condition with cumulus cells can be higher in comparison to matured denuded oocytes (21). In one study, a novel co-culture system was described regarding preantral follicles along with antral follicles. This efficacious co-culture system promoted the development of small preantral follicles (11). This research was in line with the present study’s results that the PCOS oocyte quality and meiotic progress was improved in the co-culture with normal GCCs. It has also been reported that the rate of mouse blastocyst formation can be improved in co-culture with cumulus cells (20). However, in a study by Lin and colleagues co-culture of oocytes with GCs did not have a positive effect on mice oocyte maturation (22).
It has been found that some substances are produced by GCs cultured in vitro. These substances could inhibit or delay the meiotic maturation of oocytes (23). The inhibitory effects of GCs on oocyte maturation were observed in a study on cows (24). Another study found that gap junctions among oocytes and cumulus cells could transfer cAMP among cells and the accumulation of cAMP in the oocytes resulted in meiosis inhibition (23). Therefore, there are still contradictory results in relation to the co-culture effects of GCs on oocyte maturation.
It seems plausible that soluble factors, such as the extra-cytoplasmic matrix or extracellular medium, can affect the formation of an oocyte-cumulus cell complex (25). The co-culture system, and consequently the connection between oocytes and GCs, may allow better coordination between nuclear and cytoplasmic maturation, which promotes maturation potential. Therefore, the proportion of matured oocytes is higher compared with ones in maturation media without GCs (26). It has also been demonstrated that an extensive production and reorganization of organelles and the replication of the mitochondrial genome occur during oocyte maturation. These alterations are vital for oocyte cytoplasmic maturation (25). Mitochondrial function is correlated with the mitochondrial DNA. During oocyte maturation, the amount of mitochondrial DNA becomes significantly larger. TFAM is known as an important factor in regulating mitochondrial DNA transcription and replication. Therefore, the relative expression of the TFAM gene and the developmental competence of oocytes are associated (25). In the present study, TFAM gene expression was significantly higher in PCOS oocytes co-cultured with normal GCCs. Therefore, it could be concluded that co-culture with normal GCCs may promote cytoplasmic maturation and developmental competence of PCOS oocytes.
5. Conclusion
The co-culture of PCOS oocytes with normal GCCs appears to improve PCOS-related abnormal follicular development. In addition, the connection among PCOS oocytes and GCCs, the higher levels of Cx43 and TFAM gene expression, and the improved maturation of PCOS oocytes after co-culture suggest that the co-culture system using normal GCCs might be a better method for IVM protocols than adding different external factors. This may result in the promotion of assisted reproduction techniques.
Acknowledgements
We thank Ms. Mirzanezhad for her skillful technical assistance (Genetic laboratory, University of Guilan, Rasht, Iran). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to disclose.
Full-Text: (522 Views)
1. Introduction
Maturation and developmental competence of oocytes and their quality are important criteria in successful reproductive outcomes of assisted reproduction technologies. Polycystic ovarian syndrome (PCOS) is known as the most common cause of female infertility during reproductive age. It is distinguished by oligomenorrhea, bilateral polycystic ovaries, hyperinsulinemia, biochemical and/or hyperandrogenism, and oligo- or chronic anovulatory (1-3). One of the most important symptoms in PCOS patients is follicular development arrest and dysregulation of paracrine follicle activity (4). As a result, one of the main important concerns in PCOS patients is the poor competence of oocyte development (3, 5) and weak response to ovarian stimulation (6, 7). For this reason, the use of assisted reproductive technologies such as in vitro maturation (IVM) for such patients is suggested.
During the IVM procedure, the maturation medium usually replaces the ovarian follicular environment. Therefore, it is necessary to create an appropriate culture medium for improving the competence of immature oocyte development for fertilization and embryogenesis (7). The development and growth of oocytes are supported by granulosa cells (GCs) from primordial to antral stages (8); therefore, one of the strategies to increase in vitro oocyte maturation is co-culture with GCs (9).
In a study, the developmental rate of porcine oocytes derived from early antral follicles improved using reconstructed oocyte-GC complexes (10). In another study, the maturation and development competence of buffalo oocytes derived from preantral follicles was evaluated in the presence of antral follicles and the survival and growth of the preantral follicles improved in this co-culture system (11).
Many studies have reported oocytes as an avascular environment. Normal folliculogenesis relies on the crosstalk among GCs, cumulus cells, and oocytes via gap junction intercellular communication (12, 13). Two matchable hemichannels (connexin, Cx) in the plasma membrane of adjacent cells are involved in the formation of gap junctions. Cx43 is mainly localized to the membranes of the GCs and cumulus cells (12). It has been reported that Cx43 expression decreases in the presence of high androgen levels and hence gap-junctional intercellular communication between human GCs is impaired (14). GCs in PCOS patients were induced to produce more androgens, which resulted in the enhancement of hyperandrogenemia and anovulation (1). High androgen levels may also impair folliculogenesis in PCOS patients (14). Therefore, the co-culture of PCOS oocytes with normal cumulus GCs may improve the maturation rate and competence of oocyte development.
To the best of our knowledge, there have been no published studies on the use of normal cumulus GCs to improve the maturation of PCOS oocytes in co-culture conditions. Therefore, the purpose of this study was to answer the following questions: (i) Will a connection be made between normal cumulus-GCs and PCOS immature oocytes in a co-culture medium? (ii) How will a connection between PCOS immature oocytes and normal granulosa-cumulus cells (GCCs) affect the maturation rate of PCOS oocytes and their quality?
2. Materials and Methods
2.1. Study animals
This experimental study was conducted over a period of two years (2019-2020) in the University of Guilan, Rasht, Iran. 20 virgin adult Naval Medical Research Institute female mice (30-35 gr, 7-8 wk old) were used for the study. Animals were acquired from the Razi Institute (Karaj, Iran) and housed in a central animal care room with a controlled environment of 22 ± 3°C temperature, 45-55% humidity and a 12 hr light/dark cycle. Mice were kept in a cage in groups of four and fed with a standard diet and water accessed ad libitum. All chemicals and reagents were purchased from Sigma Aldrich Company (Germany), unless otherwise specified.
2.2. PCOS induction
All the experimental animals except for those in the control group were injected with estradiol valerate (Aburaihan Co., Iran) at a dose of 40 mg/kg body weight dissolved in 0.5% sesame oil by intramuscular injection once daily for 60 days. Vaginal epithelia smears were obtained daily and evaluated by light microscope (Olympus, Japan) using Giemsa stain to determine the induction of PCOS. An irregular estrous cycle and occurrence of persistent vaginal cornification phase were the symptoms of PCOS induction. Ovaries were cut through at their longest longitudinal dimension and fixed in alcoholic Bouin's solution. After dehydration, the ovaries were serially sectioned at 5 µm, and stained with hematoxylin and eosin. The sections were used for histologic evaluation of the PCOS ovaries.
In addition, to confirm the PCOS induction, blood samples of PCOS mice were collected transcardially. Then, the separated serum was stored at -20°C. Using immunofluorometric techniques, the levels of serum follicle-stimulating and luteinizing hormones were measured. 2.9% and 2.6% were considered as total assay variation coefficients. In addition, the Coat-A-Count RIA kit was used to analyzed the level of serum testosterone with a sensitivity of 4 ng/dL (0.139 nmol/L).
2.3. Experimental design
To produce a conditioned medium, normal GCCs were collected from the ovaries of normal mice. Following cervical dislocation, the ovaries were placed in α-minimum essential medium (Gibco, Paisley, UK) supplemented with 5% fetal bovine serum (FBS; Gibco, UK), and were mechanically dissected. GCCs were collected and cultured in 24-well plates (1×106 cells/well) containing 500 μl of the same medium supplemented with 5% FBS, 50 μg/ml penicillin, and 50 μg/ml streptomycin for 24-48 hr. The unattached cells were removed following replacement with fresh medium.
After the confirmation of PCOS induction and following excision of the ovaries, the PCOS ovaries were placed in an α-minimum essential medium supplemented with 5% FBS. The ovaries were mechanically dissected and oocytes at the germinal vesicle stage were collected. The mouse germinal vesicle oocytes showed a large spherical germinal vesicle (30 µm in diameter) and a single nucleolus. After washing three times, the co-culture of PCOS oocytes with cultured GCCs (one oocyte/well) were initiated in the α-minimum essential medium supplemented with 5% FBS, 0.23 mM sodium pyruvate, 75 mU/ml of follicle-stimulating hormone, 7.5 IU/ml human chorionic gonadotropin, 50 μg/ml penicillin, and 50 μg/ml streptomycin (experimental group). After 24 hr co-culture, a complex between PCOS oocytes with normal GCCs was observed and was referred to as a PCOS oocyte-normal GCC complex (PONC) (Figure 1). Therefore, the groups of study were as follows: 1) experimental group - PCOS oocytes cultured in the presence of normal GCCs; and 2) control group - PCOS oocytes cultured in the absence of normal GCCs.
The development of PONC complexes and the properties of the oocytes in these complexes were evaluated 24 hr after co-culture and compared to the control group. The maturation rate of the reconstructed complexes (the presence of the first polar body extrusion) was also examined 24 hr after co-culture (15). The GCCs’ viability was assessed by trypan blue staining. The experiment was repeated five times.
2.4. Scanning electron microscopy (SEM) evaluation
For each experimental group, the fixation of PONC complexes was performed in 2.5% glutaraldehyde/0.1 M phosphate-buffered saline (PBS) and prepared for conventional SEM evaluation. After washing of fixed samples in 0.1 M PBS, postfixation was done in 1% osmium tetroxide/0.1 M PBS. Finally, the samples were washed in 0.1 M PBS, and dehydrated in ascending ethanol series. The dried samples were put on aluminum stubs and, then, covered with gold. The SEM images were observed at low accelerating voltage (3-10 kV) using a SEM microscope (Philips XL-30-CP).
2.5. RNA isolation and real-time polymerase chain reaction (real-time PCR)
At the end of the culture period, the PONC complexes and PCOS oocytes (experimental and control groups, respectively) were collected. Total RNA was extracted using the RNeasy Mini Kit (Roche Molecular Biochemicals, Mannheim, Germany) and stored at -80oC. The cDNA was synthesized using the cDNA kit (Thermo Scientific, EU) in accordance with the manufacturer’s instructions at 42oC for 60 min, and stored at -20oC.
Real time qRT-PCR was used to quantify the mRNA transcript levels of the mitochondrial transcription factor A (TFAM) and Cx43 genes. Primer pairs for amplifying the TFAM and Cx43 genes were designed using GenBank from the National Center for Biotechnology Information. The primer sequences are shown in table I. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Real time thermal cycler (Applied Biosystems, Foster City, USA) was used for analyzing gene expression. QuantiTect SYBR Green RT-PCR kit (Applied Biosystems, USA) was also employed for amplifying the targeted genes. Amplification of the reference and target genes was performed in the same run, for each sample. The thermal protocol of real time-PCR was programmed as: the holding step at 95oC for 5 min, cycling step at 95oC for 15 sec, 58oC for 30 sec, and 72oC for 15 sec. The melt curve step was performed as 95oC (15 sec), 60oC (1 min), and 95oC (15 sec). The method of ΔΔCT was used to determine the relative quantity of the target genes. All experiments of real time-PCR were done five times.


2.6. Ethical considerations
All investigations conformed to the ethical and humane principles of research and were approved by Guilan University of Medical Science Committee on the Use and Care of Animals (Code: IR.GUMS.REC.1399.255).
2.7. Statistical analysis
All experiments were repeated five times and data were expressed as mean ± S.D. χ2, one-way ANOVA, and Tukey’s post-hoc tests were used to analyze differences among the groups and gene expression. The statistical analysis was performed using the Statistical Package for the Social Sciences version 20 (SPSS, IBM, Armonk, NY, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. PCOS ovaries evaluation
In the PCOS mice, the irregular estrous cycles were confirmed along with the restriction to estrous stages upon estradiol valerate treatment. The histological examinations showed that the number of pre-antral follicles was higher in the PCOS mice. In addition, atretic and cystic follicles were observed in these mice (n = 6 ovaries) and their ovaries contained fewer corpora lutea (Figure 2). The evaluation of steroid hormones showed that the serum testosterone and luteinizing hormone levels were higher in the estradiol valerate-treated mice (p = 0.04) at 60 days. The serum follicle-stimulating hormone level did not change after treatment with estradiol valerate.
In the experimental group, following co-culture of PCOS oocytes with normal GCCs, significant reaggregation of cells and oocytes was observed. These reconstructed complexes were formed as PONC complexes. The rate of reconstruction was 79.8%. The evaluation of the maturation rate of the PONC complex PCOS oocytes in the experimental group and of the PCOS oocytes from the control group showed that the nuclear maturation rate of PONC complexes was significantly higher compared to the control group (Table II, p = 0.01).
After co-culture, the PCOS oocytes were surrounded by multiple layers of strictly packaged GCCs, as shown in the SEM images (Figure 3).
The molecular analysis showed that the expression rates of the TFAM gene in the experimental and control groups were 0.460 ± 0.090 and 0.025 ± 0.005, respectively. The rate of TFAM gene expression was significantly higher in the experimental group (PONC complexes) in comparison to the control group (p = 0.03). In addition, Cx43 gene expression was also higher in the experimental group (PONC complexes) (4.28 ± 0.57) compared with the control group (0.96 ± 0.12, p < 0.01) (Table III). The high expression of the Cx43 gene could confirm the connection between PCOS oocytes and normal GCCs.




4. Discussion
The maturation medium and IVM procedure strongly affected the oocyte quality and maturation. The present study shows that normal GCCs could interact with PCOS oocytes, with a gap junction created among them, and that as a result, PCOS oocyte maturation rates can improve. It seems that co-culturing with normal GCCs provides a suitable ‘niche’ for PCOS oocyte maturation like the ovary. Therefore, after co-culture, the maturation rate of PCOS oocytes and the profile of gene expression improved. These results are reasonable because the dialogue between oocytes and the surrounding cells plays a major role in progressing oocyte meiosis and their developmental potential (14).
It is possible that some molecules from normal GCCs contribute to the growth potential of PCOS oocytes under in vitro conditions. Given that females with PCOS have a weak response to ovarian stimulation and have lower oocyte maturation competence (7), improving the medium efficiency for IVM protocols is important. The abnormal function of GCs in women with PCOS has been reported (16). In addition, the expression of the growth differentiation factor-9 gene is lower in GCCs from patients with PCOS (16). Growth differentiation factor-9 plays a critical role in promoting GC mitosis (17), maintaining and developing the gap junction (increased Cx43 gene expression) between oocytes and adjacent GCs (18), generating luteinizing hormone, and synthesizing cyclic adenosine monophosphate (16, 19). Therefore, as the results of the present study show, co-culture with normal GCCs can improve in vitro maturation of PCOS oocytes. In accordance with previous studies, the results of this study show that Cx43 gene expression was higher in the co-culture system between normal GCCs and PCOS oocytes. In addition, the expression of the TFAM gene, which is one of the genes representing the quality of the oocyte, was higher in the PCOS oocytes during co-culture. It has been observed that cumulus cells are difficult to detach (20); therefore, in this study they were only cultured for 24-48 hr before adding the PCOS oocytes. This prevented the attachment of the GCCs to the plate bottom and allowed interaction with and connection to the PCOS oocytes.
It has been reported that the cleavage rate of embryos resulting from matured oocytes in a co-cultured condition with cumulus cells can be higher in comparison to matured denuded oocytes (21). In one study, a novel co-culture system was described regarding preantral follicles along with antral follicles. This efficacious co-culture system promoted the development of small preantral follicles (11). This research was in line with the present study’s results that the PCOS oocyte quality and meiotic progress was improved in the co-culture with normal GCCs. It has also been reported that the rate of mouse blastocyst formation can be improved in co-culture with cumulus cells (20). However, in a study by Lin and colleagues co-culture of oocytes with GCs did not have a positive effect on mice oocyte maturation (22).
It has been found that some substances are produced by GCs cultured in vitro. These substances could inhibit or delay the meiotic maturation of oocytes (23). The inhibitory effects of GCs on oocyte maturation were observed in a study on cows (24). Another study found that gap junctions among oocytes and cumulus cells could transfer cAMP among cells and the accumulation of cAMP in the oocytes resulted in meiosis inhibition (23). Therefore, there are still contradictory results in relation to the co-culture effects of GCs on oocyte maturation.
It seems plausible that soluble factors, such as the extra-cytoplasmic matrix or extracellular medium, can affect the formation of an oocyte-cumulus cell complex (25). The co-culture system, and consequently the connection between oocytes and GCs, may allow better coordination between nuclear and cytoplasmic maturation, which promotes maturation potential. Therefore, the proportion of matured oocytes is higher compared with ones in maturation media without GCs (26). It has also been demonstrated that an extensive production and reorganization of organelles and the replication of the mitochondrial genome occur during oocyte maturation. These alterations are vital for oocyte cytoplasmic maturation (25). Mitochondrial function is correlated with the mitochondrial DNA. During oocyte maturation, the amount of mitochondrial DNA becomes significantly larger. TFAM is known as an important factor in regulating mitochondrial DNA transcription and replication. Therefore, the relative expression of the TFAM gene and the developmental competence of oocytes are associated (25). In the present study, TFAM gene expression was significantly higher in PCOS oocytes co-cultured with normal GCCs. Therefore, it could be concluded that co-culture with normal GCCs may promote cytoplasmic maturation and developmental competence of PCOS oocytes.
5. Conclusion
The co-culture of PCOS oocytes with normal GCCs appears to improve PCOS-related abnormal follicular development. In addition, the connection among PCOS oocytes and GCCs, the higher levels of Cx43 and TFAM gene expression, and the improved maturation of PCOS oocytes after co-culture suggest that the co-culture system using normal GCCs might be a better method for IVM protocols than adding different external factors. This may result in the promotion of assisted reproduction techniques.
Acknowledgements
We thank Ms. Mirzanezhad for her skillful technical assistance (Genetic laboratory, University of Guilan, Rasht, Iran). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to disclose.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Azziz R. Polycystic ovary syndrome. Obstet Gynecol 2018; 132: 321-336. [DOI:10.1097/AOG.0000000000002698] [PMID]
2. Shah D, Rasool S. Polycystic ovary syndrome and metabolic syndrome: The worrisome twosome? Climacteric 2016; 19: 7-16. [DOI:10.3109/13697137.2015.1116505] [PMID]
3. Hassani F, Oryan S, Eftekhari-Yazdi P, Bazrgar M, Moini A, Nasiri N, et al. Association between the number of retrieved mature oocytes and insulin resistance or sensitivity in infertile women polycystic ovary syndrome. Int J Fertil Steril 2019; 12: 310-315.
4. Xi W, Yang Y, Mao H, Zhao X, Liu M, Fu S. Circulating anti-mullerian hormone as predictor of ovarian response to clomiphene citrate in women with polycystic ovary syndrome. J Ovarian Res 2016; 9: 3. [DOI:10.1186/s13048-016-0214-2] [PMID] [PMCID]
5. Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab 2017; 28: 186-198. [DOI:10.1016/j.tem.2016.11.008] [PMID]
6. Amer SA, Mahran A, Abdelmaged A, El-Adawy AR, Eissa MK, Shaw RW. The influence of circulating anti-Müllerian hormone on ovarian responsiveness to ovulation induction with gonadotrophins in women with polycystic ovarian syndrome: A pilot study. Reprod Biol Endocrinol 2013; 11: 115. [DOI:10.1186/1477-7827-11-115] [PMID] [PMCID]
7. Tahaei LS, Eimani H, Eftekhar-Yazdi P, Ebrahimi B, Fathi R. Effects of retinoic acid on maturation of immature mouse oocytes in the presence and absence of a granulosa cell co-culture system. J Assist Reprod Genet 2011; 28: 553-558. [DOI:10.1007/s10815-011-9579-8] [PMID] [PMCID]
8. Jafari Atrabi M, Akbarinejad V, Khanbabaee R, Dalman A, Amorim CA, Najar-Asl M, et al. Formation and activation induction of primordial follicles using granulosa and cumulus cells conditioned media. J Cell Physiol 2019; 234: 10148-10156. [DOI:10.1002/jcp.27681] [PMID]
9. Palmerini MG, Nottola SA, Tunjung WA, Kadowaki A, Bianchi S, Cecconi S, et al. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes. Biochem Biophys Res Commun 2016; 481: 159-164. [DOI:10.1016/j.bbrc.2016.10.151] [PMID]
10. Oi A, Tasaki H, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Effects of reaggregated granulosa cells and oocytes derived from early antral follicles on the properties of oocytes grown in vitro. J Reprod Dev 2015; 61: 191-197. [DOI:10.1262/jrd.2014-123] [PMID] [PMCID]
11. Sharma GT, Dubey PK, Nath A, Saikumar G. Co-culture of buffalo (Bubalus bubalis) preantral follicles with antral follicles: A comparative study of developmental competence of oocytes derived from in vivo developed and in vitro cultured antral follicles. Zygote 2013; 21: 286-294. [DOI:10.1017/S0967199411000700] [PMID]
12. Zhang Y, Xu Y, Kuai Y, Wang S, Xue Q, Shang J. Effect of testosterone on the Connexin37 of sexual mature mouse cumulus oocyte complex. J Ovarian Res 2016; 9: 82. [DOI:10.1186/s13048-016-0290-3] [PMID] [PMCID]
13. Russell DL, Robker RL. Molecular mechanisms of ovulation: Co-ordination through the cumulus complex. Hum Reprod Update 2007; 13: 289-312. [DOI:10.1093/humupd/dml062] [PMID]
14. Wu CH, Yang JG, Yang JJ, Lin YM, Tsai HD, Lin CY, et al. Androgen excess down-regulates connexin43 in a human granulosa cell line. Fertil Steril 2010; 94: 2938-2941. [DOI:10.1016/j.fertnstert.2010.06.077] [PMID]
15. Bahadori MH, Azarnia M, Ghasemian F. [The effect of hepatocyte growth factor on mouse oocyte in vitro maturation and subsequent fertilization and embryo development]. Zahedan J Res Med Sci 2011; 13: 26-30. (in Persian)
16. Cheraghi E, Soleimani Mehranjani M, Shariatzadeh SMA, Nasr Esfahani MH, Alani B. N-acetylcysteine compared to metformin, improves the expression profile of growth differentiation factor-9 and receptor tyrosine kinase c-Kit in the oocytes of patients with polycystic ovarian syndrome. Int J Fertil Steril 2018; 11: 270-278.
17. Li J, Luo W, Huang T, Gong Y. Growth differentiation factor 9 promotes follicle-stimulating hormone-induced progesterone production in chicken follicular granulosa cells. Gen Comp Endocrinol 2019; 276: 69-76. [DOI:10.1016/j.ygcen.2019.03.005] [PMID]
18. Yamamoto N, Christenson LK, McAllister JM, Strauss JF. Growth differentiation factor-9 inhibits 3'5'-adenosine monophosphate-stimulated steroidogenesis in human granulosa and theca cells. J Clin Endocrinol Metab 2002; 87: 2849-2856.
https://doi.org/10.1210/jcem.87.6.8551 [DOI:10.1210/jc.87.6.2849] [PMID]
19. Omar Farouk FN, Stott D, Vlad M. Mouse embryo co-culture with autologous cumulus cells and fetal development post-embryo transfer. Anim Sci J 2011; 82: 420-427. [DOI:10.1111/j.1740-0929.2010.00869.x] [PMID]
20. Sowinska N, Müller K, Niżański W, Jewgenow K. Mitochondrial characteristics in oocytes of the domestic cat (Felis catus) after in vitro maturation and vitrification. Reprod Domest Anim 2017; 52: 806-813.
https://doi.org/10.1111/rda.12895 [DOI:10.1111/rda.12982]
21. Mizumachi S, Aritomi T, Sasaki K, Matsubara K, Hirao Y. Macromolecular crowded conditions strengthen contacts between mouse oocytes and companion granulosa cells during in vitro growth. J Reprod Dev 2018; 64: 153-160.
https://doi.org/10.1262/jrd.2017-162 [DOI:10.1262/jrd.2017-162e] [PMID] [PMCID]
22. Lin YH, Hwang JL, Seow KM, Huang LW, Chen HJ, Tzeng CR. Effects of growth factors and granulosa cell co-culture on in-vitro maturation of oocytes. Reprod Biomed Online 2009; 19: 165-170. [DOI:10.1016/S1472-6483(10)60068-5]
23. Adeldust H, Zeinoaldini S, Kohram H, Amiri Roudbar M, Daliri Joupari M. In vitro maturation of ovine oocyte in a modified granulosa cells co-culture system and alpha-tocopherol supplementation: Effects on nuclear maturation and cleavage. J Anim Sci Technol 2015; 57: 27. [DOI:10.1186/s40781-015-0061-5] [PMID] [PMCID]
24. Sirard MA, Bilodeau S. Granulosa cells inhibit the resumption of meiosis in bovine oocytes in vitro. Biol Reprod 1990; 43: 777-783. [DOI:10.1095/biolreprod43.5.777] [PMID]
25. Ghaffari Novin M, Noruzinia M, Allahveisi A, Saremi A, Fadaei Fathabadi F, Mastery Farahani R, et al. Comparison of mitochondrial-related transcriptional levels of TFAM, NRF1 and MT-CO1 genes in single human oocytes at various stages of the oocyte maturation. Iran Biomed J 2015; 19: 23-28.
26. Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 2000; 226: 167-179. [DOI:10.1006/dbio.2000.9863] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








