Wed, Jan 28, 2026
[Archive]
Volume 20, Issue 10 (October 2022)
IJRM 2022, 20(10): 831-840 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aledani T H W, Al-Hayder M N, Al-Mayyahi R S. Severe deterioration in sperm parameters and testes of rats administered naproxen and diclofenac at pre-puberty: An experimental study. IJRM 2022; 20 (10) :831-840
URL: http://ijrm.ir/article-1-2352-en.html
URL: http://ijrm.ir/article-1-2352-en.html
1- Department of Clinical Laboratory Sciences, College of Pharmacy, University of Basrah, Basrah, Iraq. , tamadir.wadi@uobasrah.edu.iq
2- Department of Pharmacology and Toxicology, College of Pharmacy, University of Basrah, Basrah, Iraq.
3- Department of Clinical Laboratory Sciences, College of Pharmacy, University of Basrah, Basrah, Iraq.
2- Department of Pharmacology and Toxicology, College of Pharmacy, University of Basrah, Basrah, Iraq.
3- Department of Clinical Laboratory Sciences, College of Pharmacy, University of Basrah, Basrah, Iraq.
Full-Text [PDF 6969 kb]
(1178 Downloads)
| Abstract (HTML) (3245 Views)
1. Introduction
Spermatogenesis is a continuous process to produce sperm during the reproductive lifetime. The spermatozoa generate and develop in the seminiferous tubules of testes from spermatogenic stem cells that are called spermatogonia. The generation of normal and mobile gametes is an imperious necessity for male fertility (1).
Many reasons can impact spermatogenesis and sperm maturation, and these can lead to male infertility. Using therapeutic drugs is among the major causes that can harm spermatogonia and spermatogenesis (2). Nonsteroidal anti-inflammatory drugs are broadly used to treat inflammatory diseases such as osteoarthritis, rheumatoid arthritis, etc. (3).
The wide-ranging risks of using these nonsteroidal drugs are serious cardiovascular, gastrointestinal, and renal damage (4). However, little is known about their effects on the reproductive system. It is noteworthy that nonsteroidal anti-inflammatory drugs act as inhibitors of the cyclooxygenases, which can stimulate prostaglandins production such as prostaglandin E2. These prostaglandins are important for many physiological functions and they regulate diverse processes (5). Many studies refer to the important role of prostaglandins in ovulation; in addition their inhibition may lead to female infertility through the chronic use of nonsteroidal drugs (6-8).
For males, the prostaglandins are produced by 2 somatic cells (Leydig and Sertoli cells) in the testes and they have critical roles in spermatogenesis, steroidogenesis, and regulation of the spermatogenic competence (9). Furthermore, cyclooxygenase-2 is highly expressed in the testis, epididymis, and spermatozoa of boars; it may play an effective role in the sperms quality and fertility (10). Thus, drugs that inhibit cyclooxygenases can impair male fertility. Although naproxen and diclofenac are the most commonly used of nonsteroidal anti-inflammatory drugs, there are no sufficient studies on their toxicity affecting male fertility particularly when they are used in the period before sexual maturation.
Consequently, the current study aims to investigate the toxic effects of naproxen and diclofenac sodium on sperm parameters and testes in male rats when they are administered during their pre-pubertal period.
2. Materials and Methods
2.1. Study design and drugs administration
To achieve the goal of this experimental study, 15 pre-pubertal male albino Wistar-Kyoto rats aged 5 wk (70-80 gr) were used and housed in the animals’ house of Basrah University, Basrah, Iraq where there were controlled suitable conditions for housing the animals (12 hr light/dark cycle, and 25°C).
Rats were divided into 3 groups (n = 5/each) and administered by oral gavage every day (in the morning, once per day): A) control rats were administered with 0.1 ml of the dimethyl sulfoxide (5%) (ROMIL LTD, United Kingdom), B) rats were administered with 50 mg/kg body weight of naproxen (PiONEER, IRAQ), and C) rats were administered with 5 mg/kg of diclofenac sodium (MICRO LABS LIMITED, INDIA) respectively. The dimethyl sulfoxide was used to dissolve naproxen and diclofenac sodium. The drugs administration continued for 3 wk (11). At the end of administration, the animals were firstly weighed and then anaesthetized to prevent pain and distress through refinement. After that they were dissected. Subsequently, the testes and epididymides were carefully removed and also weighed to be used in subsequent investigations.
2.2. Evaluation of the sperm parameters
For sperm analysis, the epididymis of each rat was kept in 10 ml of Hibernate HE-Ca medium (Brain Bit, UK). Then the epididymis was teared to leak the sperms into the medium. The World Health Organization protocol was followed to assess sperm count, viability, and morphology as described before (12) with modifications. Briefly, total sperm number per ml was estimated by counting the heads of sperm manually under a light microscope (Genex, USA) using a hemocytometer after preparing a dilution of the sperm-medium suspension (1 ml) with formaldehyde fixative (9 ml).
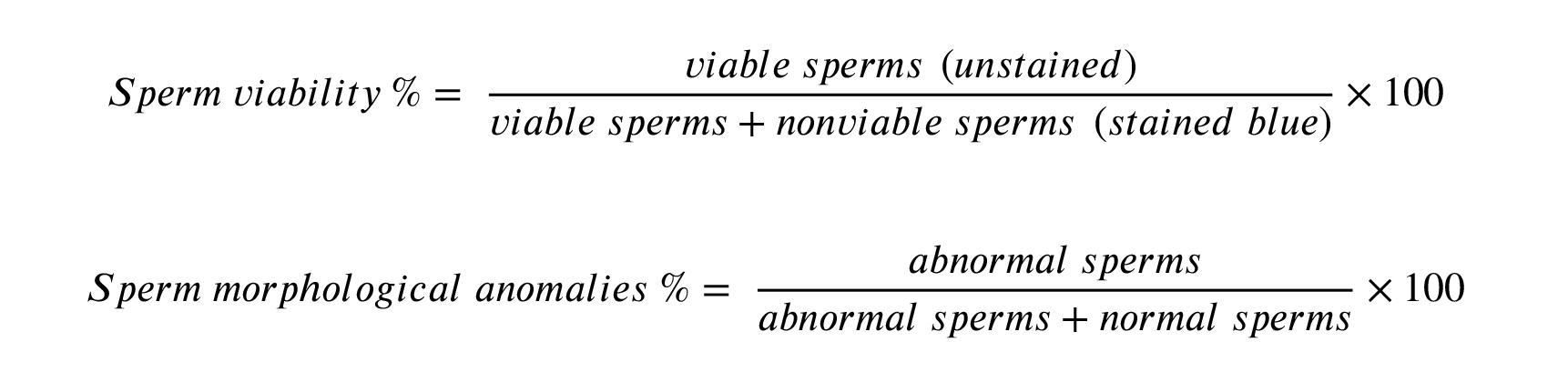
For sperm viability and morphology, trypan blue staining (Beijing Solaria science, China) was utilized and was prepared as a stock solution (0.4% trypan blue in phosphate-buffered saline, pH 7.3). Then, one volume of this stock was mixed with equal volume of sperm-medium suspension to be used immediately for microscopic examination (400×). A total number of 200 spermatozoa were counted for each rat. The trypan blue stained slides were air-dried and then used to detect sperm anomalies. The percentage of sperm viability and morphological abnormalities were calculated as:
2.3. Histopathological and histomorphometric analyses of the testes
After excising the testes from the dissected animals, they were fixed in 10% formalin; then were passed into a series of alcohols and cleared with xylene. Subsequently, they were embedded in paraffin wax. A rotary microtome (Bioevopeak, China) was used to obtain tissue sections thickened at 5 μm. These sections were stained using hematoxylin and eosin stain for histological investigations. For histomorphometric analysis, the average diameter of seminiferous tubular and their lumen were performed by randomly measuring 10 circular tubules in the transverse sections for each animal. In the same sections, the thickness of the seminiferous epithelium (from the basement membrane to the tubular lumen) was measured. All the histomorphometric parameters mentioned above were conducted using digital images of 200× magnification with the software ImageJ (National Institutes of Health, USA).
2.4. Ethical considerations
The current study was approved by Basrah University Ethical Committee for animal research, Basrah, Iraq (Code: 3108003). The ethical guidelines for caring and working with laboratory animals were precisely followed. The origin of the healthy animals (had no genetic modification) was from College of Veterinary Medicine, University of Basrah.
2.5. Statistical analysis
GraphPad software (California, USA) was conducted to statistically analyse the results by using one-way analysis of variance (ANOVA) and Bonferroni’s post-test. P-values < 0.05 were determined as significant values represented by mean ± SEM.
3. Results
3.1. Effect of the drugs on body and testis weights
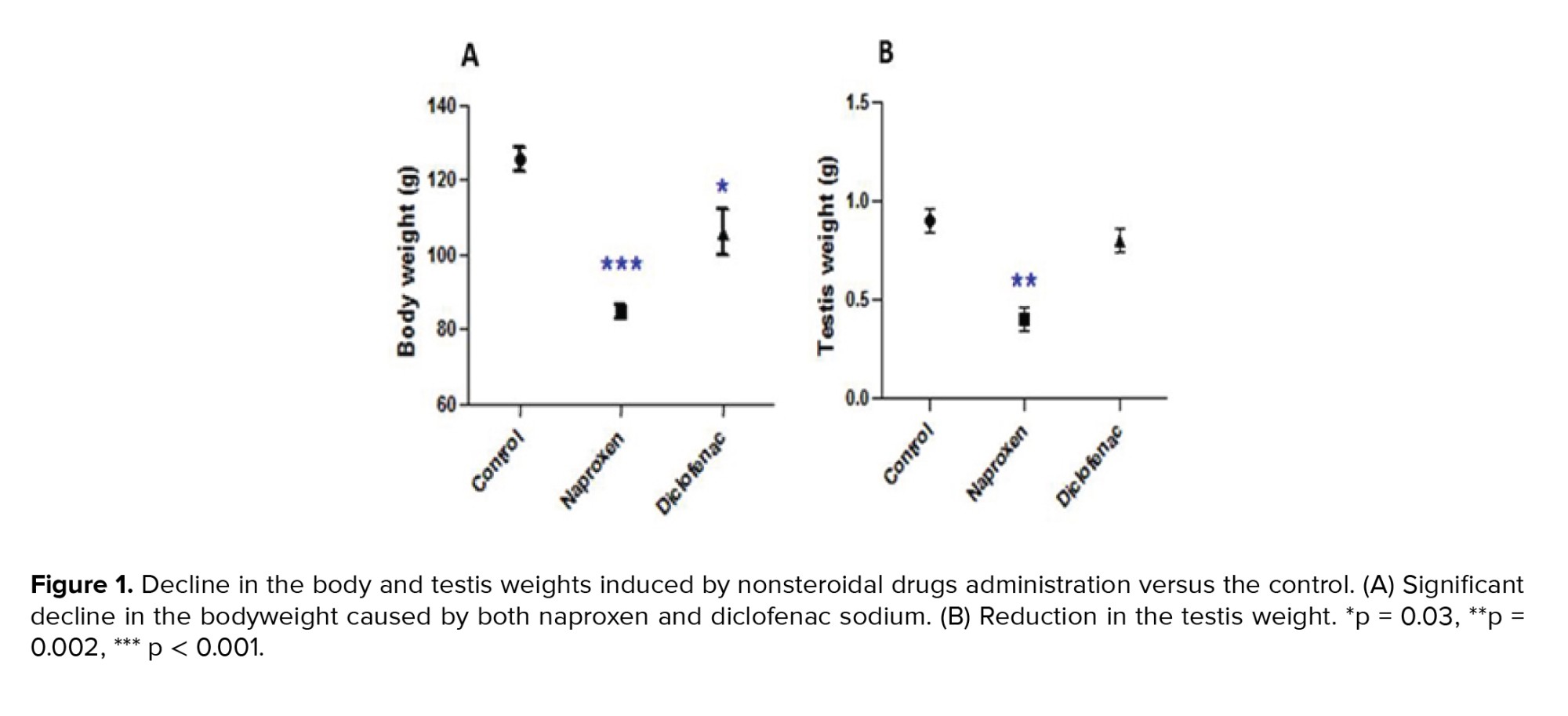
Both naproxen and diclofenac sodium administrations significantly reduced the body weights of the albino rats (p < 0.001 and p = 0.03 respectively) (Figure 1A). The testis weight significantly decreased in the naproxen-administered rats (p = 0.002) compared to the diclofenac sodium-administered rats, which exhibited a nonsignificant decrease in the testis weight (Figure 1B).
3.2. Alteration of the sperm parameters
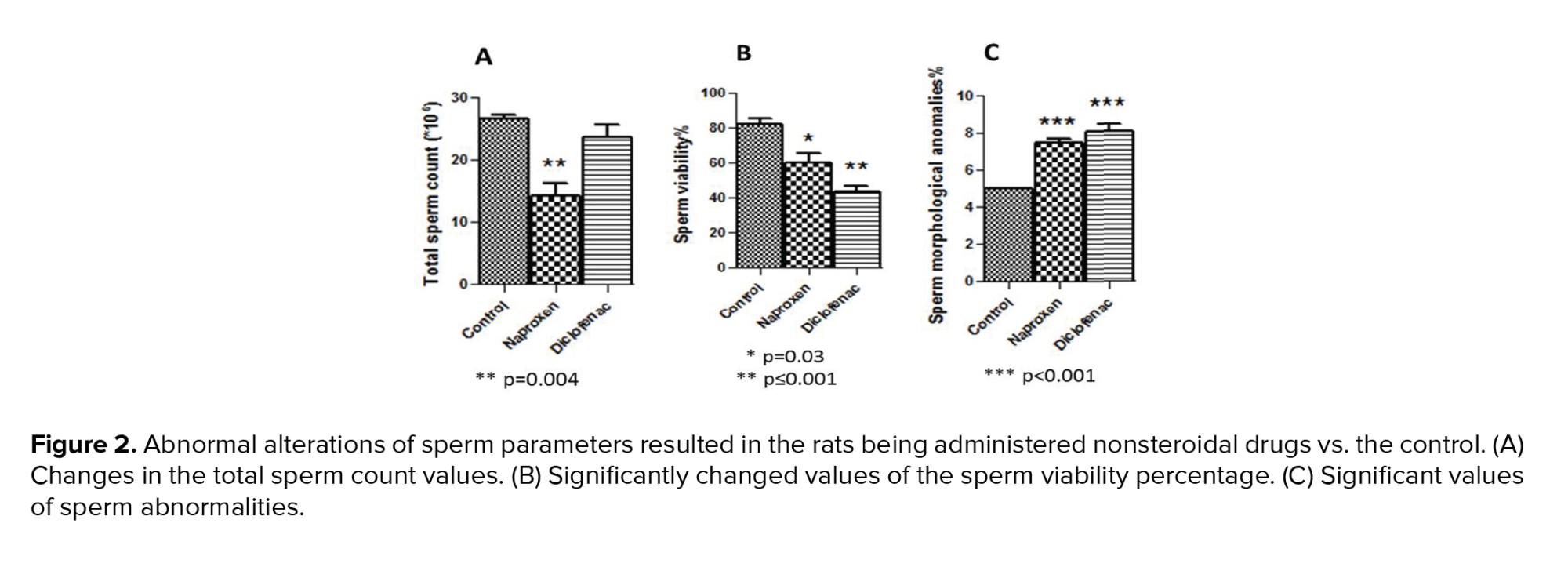
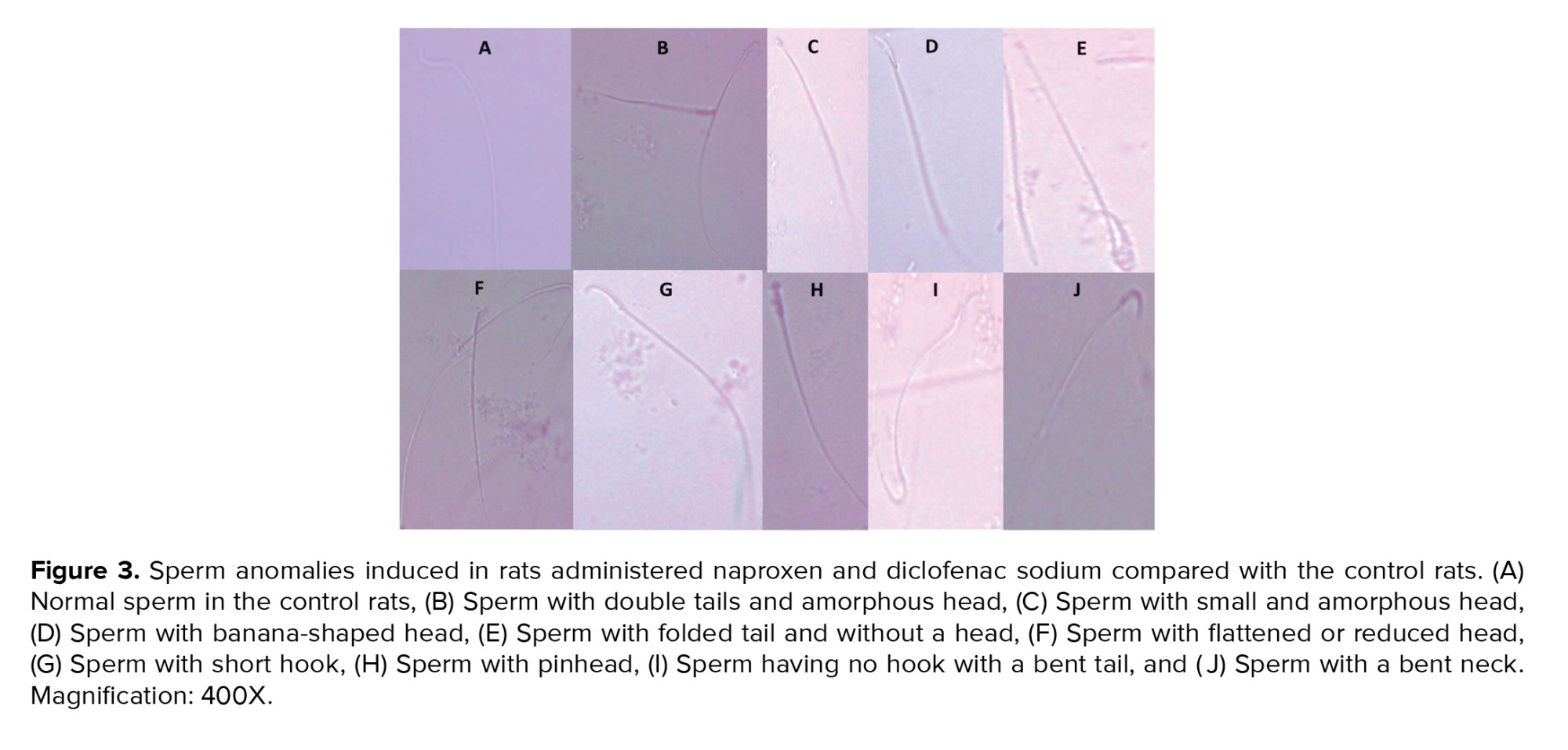
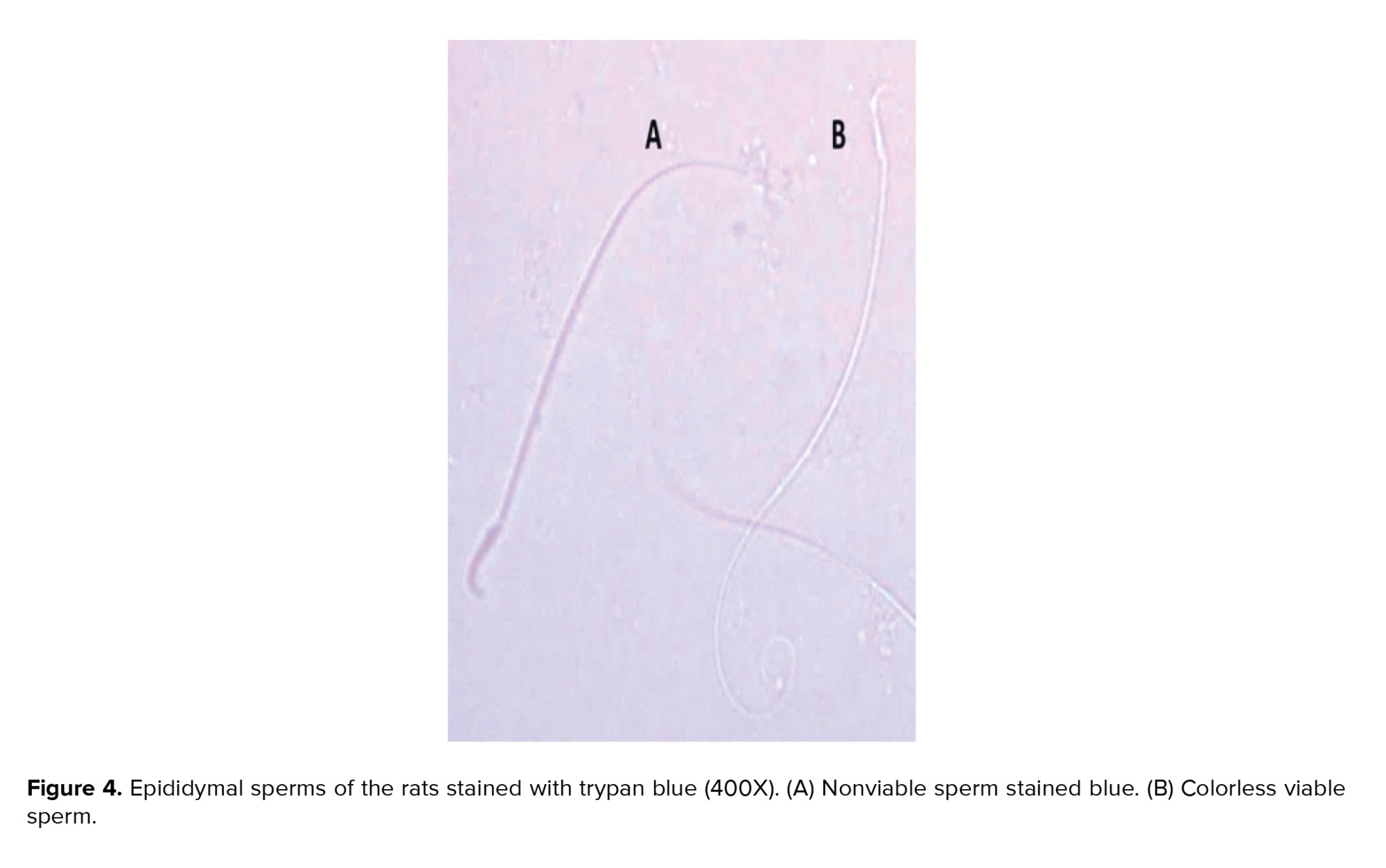
Figure 2 showed a significant decline in the total number of epididymal spermatozoa in the naproxen-administered rats (p = 0.004) in comparison to the control rats. But, sperm count decreased insignificantly in the diclofenac sodium-administered group (Figure 2A). On the other hand, the sperm viability percentage was significantly diminished in both diclofenac and naproxen-administered groups (p ≤ 0.001 and p = 0.03 respectively) (Figure 2B). Also, in both drug-administered groups, there was a significant increase (p < 0.001) in the percentage of sperm morphological anomalies (Figure 2C). These sperm anomalies were shown in figure 3, including sperm with double tails and amorphous head, small and amorphous head, banana-shaped head, folded tail and without a head, flattened or reduced head, short hook, pinhead, no hook with a bent tail, and bent neck. Notably, the nonviable sperms stained blue were differentiated from the viable sperms that were unstained, as shown distinctly in figure 4.
3.3. Testicular histopathology and histomorphometry
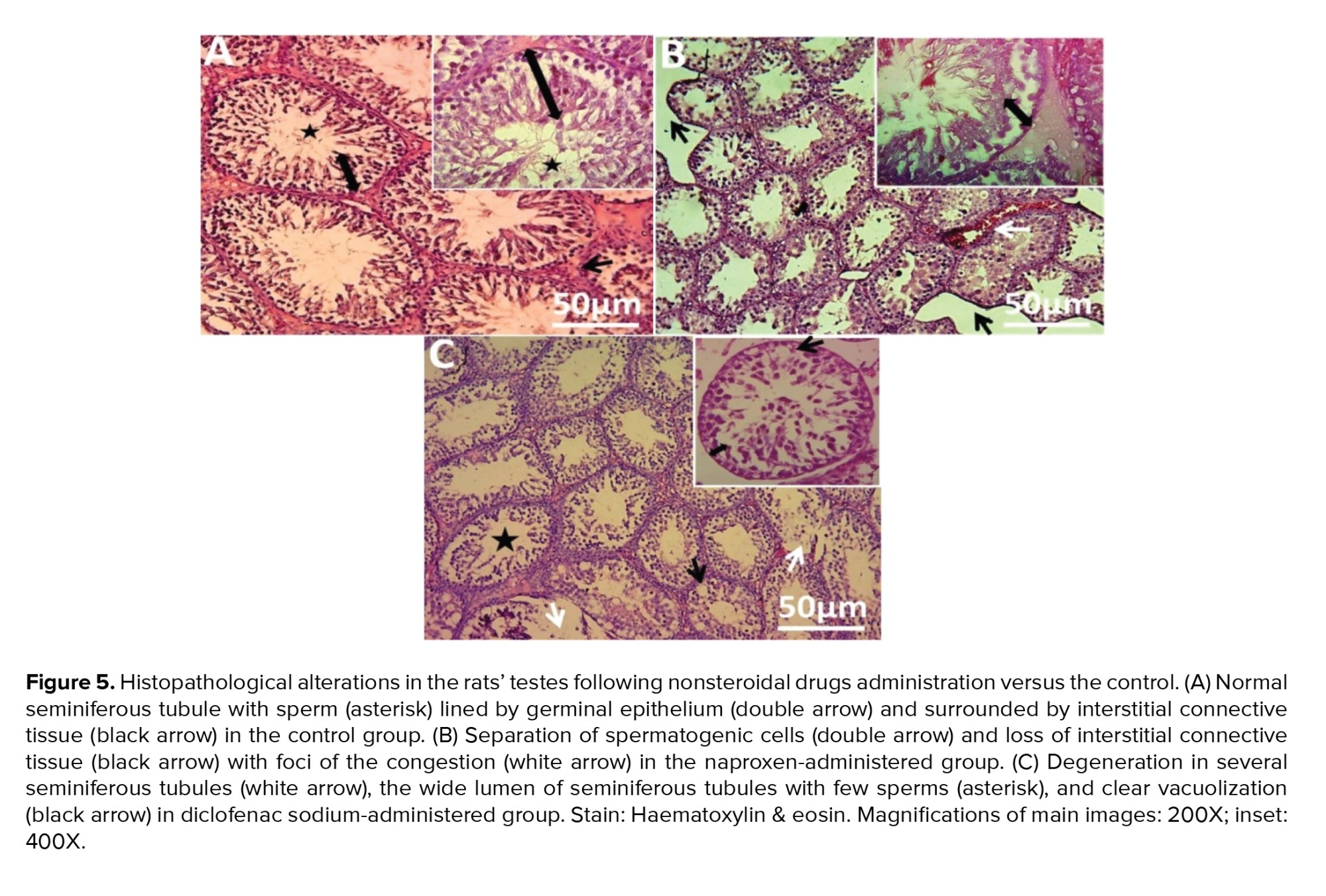
Histological analysis of the present study showed that the control group revealed normal testicular parenchymal architecture with compactly arranged seminiferous tubules at all spermatogenesis stages and interstitial cells (Figure 5A). Naproxen-administered group exhibited clear vacuolization, separation of spermatogenic cells, and loss of interstitial connective tissue, which was replaced with a few foci of congestions (Figure 5B). Also, the diclofenac sodium-administered group showed signs of degeneration in several seminiferous tubules, reduction in the number of spermatogonial cells, and clear vacuolization (Figure 5C).
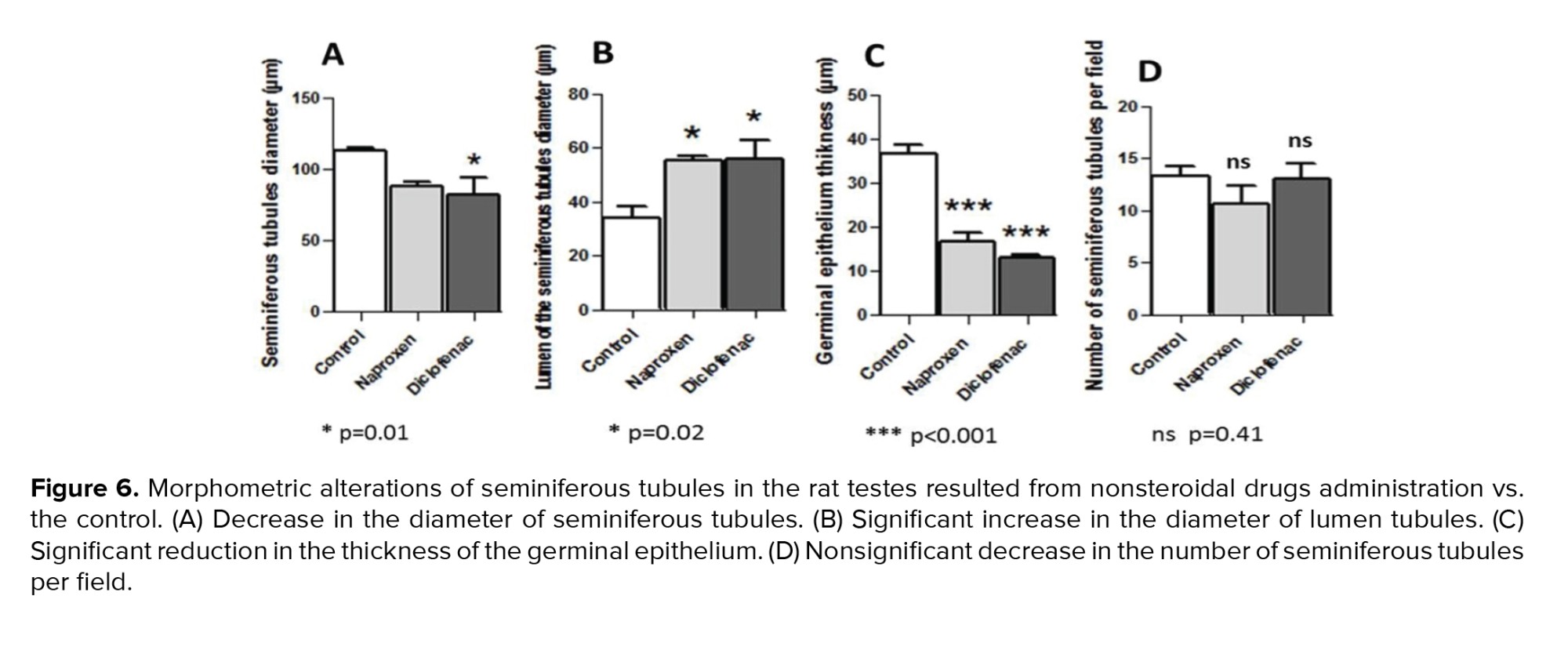
The morphometric findings of seminiferous tubules compared to the control revealed that diameters of the tubules decreased in both drug-administered groups. However, this reduction was significant in the diclofenac sodium-administered group only (p = 0.01) (Figure 6A). Diameters of the tubule lumens, on the other hand, appeared to have a significant increase (p = 0.02) in both drug-administered groups (Figure 6B). Further, in both drug groups, a significant reduction (p < 0.001) was observed in the thickness of the germinal epithelium of the tubules (Figure 6C). Otherwise, the number of the seminiferous tubules per field revealed no significant differences (p = 0.41) between the control and non-steroidal drug-administered groups (Figure 6D).
4. Discussion
Our results demonstrated the harmful effects of both naproxen and diclofenac sodium as nonsteroidal drugs on sperm parameters and testicular histology that may lead to infertility. The drugs administration at the pre-pubertal period of the rats could impact sperm maturation and thereby causing male infertility. Male fertility can be affected when drugs induce harm or damage in the testes and impair spermatogenesis and sperm maturation (2). The harmful effects of naproxen in this study were represented by a significant decrease in the sperm count and seminiferous tubules injuries; such results were similar to those obtained in a previous study (13). Remarkably, there were no more available studies concerning the reproductive toxicity of naproxen in the rats such as the percentage of sperm viability reduction and the percentage of increasing sperm morphological abnormalities. In this context, the previous studies reported a reduction in the total sperm count and sperm viability percentage caused by diclofenac sodium and that corresponds with our results. As for the histopathological effects resulting from diclofenac sodium administration, the aforementioned studies recorded almost similar effects, including degeneration and shrinkage of the seminiferous tubules, vacuolization in the interstitial connective tissue, and depletion of germinal epithelium (14-16).
According to our findings, the degeneration and depletion of the germinal layer mediated by testicular damage might be the reason for the decrease of spermatogonial cells that led to a reduction in sperm number and subsequently affected fertility. The cause of increased sperm anomalies induced by both naproxen and diclofenac sodium might be due to the presence of nuclear DNA damage, or fragmentation in the spermatozoa that is considered the main pathway responsible for sperm morphological defects, as well as chromatin immaturity (17). There is a correlation between the DNA fragmentation and morphological defects of sperms in patients with teratozoospermia (18).
Also, the presence of apoptotic markers in spermatozoa have been strongly related to sperm morphological abnormalities (19). Increasing the apoptosis level during early spermatogenesis results in sperm apoptosis that leads to male infertility (20). Sperm DNA damage and apoptosis can be created by excessive oxidative stress. Oxidative stress has also been correlated with abnormal sperm morphology (19). Moreover, oxidative stress can also reduce sperm viability, and vitamin E administration as an antioxidant leads to reverse this effect significantly (12). It is worth mentioning that our findings of deteriorated sperm parameters (sperm count, viability, and morphology) and injured seminiferous tubules caused by naproxen and diclofenac sodium were in parallel with the toxic effects of other reproductive toxicants such as sodium arsenite and manganese dioxide micro-and nanoparticles (12, 21).
With regards to the morphometric outcomes, the present study showed a decrease in the seminiferous tubules’ diameters and their germinal epithelium thickness in both naproxen and diclofenac sodium-administered groups. These findings were in accordance with the toxic effects of zinc oxide nanoparticles in mouse testis and di-butyl phthalate-induced oxidative stress in the testis of rats (22, 23). The reduction in seminiferous diameter may indicate increased loss of the germ cells through apoptosis (22). Also, the spermatogenesis process's rise results in an expansion of the seminiferous tubules’ diameter and thickness (24). Thus, the histomorphological alterations in our study might be attributed to the reduction in the number of spermatogonial cells and spermatogenesis. Furthermore, the significantly increased diameters of tubule lumens were manifested in both drug-administered groups of the present study. The larger lumen and thinner epithelium of the seminiferous tubules were suggested to be an evidence of increased intra-tubular pressure-mediated spermatogenic damage (25).
It is noteworthy that the body weights of rats administered the non-steroidal drugs significantly declined compared to the control rats. Researchers (14) mentioned that the diclofenac sodium treatment can reduce feed intake and body weight. As for naproxen, it is considered an inhibitor of glycogen synthase kinase-3β, and this pharmacological inhibitor caused a significant reduction in the weights of obese mice (26). Glycogen is the main origin for glucose and plays a crucial role in growing gonads during development (27). Also, inhibition of androgen biosynthesis may be indirectly responsible for significantly reducing the weights of the testis, epididymis, ventral prostate, and seminal vesicle caused by aspirin (nonsteroidal anti-inflammatory drug) treatment; as well as androgen hormones closely regulate the weight, size, and function of testes and other reproductive organs (28). These facts might be involved in the significant drop of testis weight; which was considerable in the naproxen-administered group. Otherwise, diclofenac sodium administration produced a nonsignificant decline in the testis weights. This insignificant decline did not agree with the results of a previous study (16).
5. Conclusion
It can be concluded from the current study that both naproxen and diclofenac sodium exert harms on the sperm parameters (sperm numbers, viability, and morphology), the testicular histology and morphometry in the albino rats when administered before their sexual maturity. These pathological effects are very deleterious to male fertility and can lead to potential infertility.
Acknowledgments
This study has not been supported financially from any organization or agency.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (354 Views)
1. Introduction
Spermatogenesis is a continuous process to produce sperm during the reproductive lifetime. The spermatozoa generate and develop in the seminiferous tubules of testes from spermatogenic stem cells that are called spermatogonia. The generation of normal and mobile gametes is an imperious necessity for male fertility (1).
Many reasons can impact spermatogenesis and sperm maturation, and these can lead to male infertility. Using therapeutic drugs is among the major causes that can harm spermatogonia and spermatogenesis (2). Nonsteroidal anti-inflammatory drugs are broadly used to treat inflammatory diseases such as osteoarthritis, rheumatoid arthritis, etc. (3).
The wide-ranging risks of using these nonsteroidal drugs are serious cardiovascular, gastrointestinal, and renal damage (4). However, little is known about their effects on the reproductive system. It is noteworthy that nonsteroidal anti-inflammatory drugs act as inhibitors of the cyclooxygenases, which can stimulate prostaglandins production such as prostaglandin E2. These prostaglandins are important for many physiological functions and they regulate diverse processes (5). Many studies refer to the important role of prostaglandins in ovulation; in addition their inhibition may lead to female infertility through the chronic use of nonsteroidal drugs (6-8).
For males, the prostaglandins are produced by 2 somatic cells (Leydig and Sertoli cells) in the testes and they have critical roles in spermatogenesis, steroidogenesis, and regulation of the spermatogenic competence (9). Furthermore, cyclooxygenase-2 is highly expressed in the testis, epididymis, and spermatozoa of boars; it may play an effective role in the sperms quality and fertility (10). Thus, drugs that inhibit cyclooxygenases can impair male fertility. Although naproxen and diclofenac are the most commonly used of nonsteroidal anti-inflammatory drugs, there are no sufficient studies on their toxicity affecting male fertility particularly when they are used in the period before sexual maturation.
Consequently, the current study aims to investigate the toxic effects of naproxen and diclofenac sodium on sperm parameters and testes in male rats when they are administered during their pre-pubertal period.
2. Materials and Methods
2.1. Study design and drugs administration
To achieve the goal of this experimental study, 15 pre-pubertal male albino Wistar-Kyoto rats aged 5 wk (70-80 gr) were used and housed in the animals’ house of Basrah University, Basrah, Iraq where there were controlled suitable conditions for housing the animals (12 hr light/dark cycle, and 25°C).
Rats were divided into 3 groups (n = 5/each) and administered by oral gavage every day (in the morning, once per day): A) control rats were administered with 0.1 ml of the dimethyl sulfoxide (5%) (ROMIL LTD, United Kingdom), B) rats were administered with 50 mg/kg body weight of naproxen (PiONEER, IRAQ), and C) rats were administered with 5 mg/kg of diclofenac sodium (MICRO LABS LIMITED, INDIA) respectively. The dimethyl sulfoxide was used to dissolve naproxen and diclofenac sodium. The drugs administration continued for 3 wk (11). At the end of administration, the animals were firstly weighed and then anaesthetized to prevent pain and distress through refinement. After that they were dissected. Subsequently, the testes and epididymides were carefully removed and also weighed to be used in subsequent investigations.
2.2. Evaluation of the sperm parameters
For sperm analysis, the epididymis of each rat was kept in 10 ml of Hibernate HE-Ca medium (Brain Bit, UK). Then the epididymis was teared to leak the sperms into the medium. The World Health Organization protocol was followed to assess sperm count, viability, and morphology as described before (12) with modifications. Briefly, total sperm number per ml was estimated by counting the heads of sperm manually under a light microscope (Genex, USA) using a hemocytometer after preparing a dilution of the sperm-medium suspension (1 ml) with formaldehyde fixative (9 ml).
For sperm viability and morphology, trypan blue staining (Beijing Solaria science, China) was utilized and was prepared as a stock solution (0.4% trypan blue in phosphate-buffered saline, pH 7.3). Then, one volume of this stock was mixed with equal volume of sperm-medium suspension to be used immediately for microscopic examination (400×). A total number of 200 spermatozoa were counted for each rat. The trypan blue stained slides were air-dried and then used to detect sperm anomalies. The percentage of sperm viability and morphological abnormalities were calculated as:

2.3. Histopathological and histomorphometric analyses of the testes
After excising the testes from the dissected animals, they were fixed in 10% formalin; then were passed into a series of alcohols and cleared with xylene. Subsequently, they were embedded in paraffin wax. A rotary microtome (Bioevopeak, China) was used to obtain tissue sections thickened at 5 μm. These sections were stained using hematoxylin and eosin stain for histological investigations. For histomorphometric analysis, the average diameter of seminiferous tubular and their lumen were performed by randomly measuring 10 circular tubules in the transverse sections for each animal. In the same sections, the thickness of the seminiferous epithelium (from the basement membrane to the tubular lumen) was measured. All the histomorphometric parameters mentioned above were conducted using digital images of 200× magnification with the software ImageJ (National Institutes of Health, USA).
2.4. Ethical considerations
The current study was approved by Basrah University Ethical Committee for animal research, Basrah, Iraq (Code: 3108003). The ethical guidelines for caring and working with laboratory animals were precisely followed. The origin of the healthy animals (had no genetic modification) was from College of Veterinary Medicine, University of Basrah.
2.5. Statistical analysis
GraphPad software (California, USA) was conducted to statistically analyse the results by using one-way analysis of variance (ANOVA) and Bonferroni’s post-test. P-values < 0.05 were determined as significant values represented by mean ± SEM.
3. Results
3.1. Effect of the drugs on body and testis weights
Both naproxen and diclofenac sodium administrations significantly reduced the body weights of the albino rats (p < 0.001 and p = 0.03 respectively) (Figure 1A). The testis weight significantly decreased in the naproxen-administered rats (p = 0.002) compared to the diclofenac sodium-administered rats, which exhibited a nonsignificant decrease in the testis weight (Figure 1B).
3.2. Alteration of the sperm parameters
Figure 2 showed a significant decline in the total number of epididymal spermatozoa in the naproxen-administered rats (p = 0.004) in comparison to the control rats. But, sperm count decreased insignificantly in the diclofenac sodium-administered group (Figure 2A). On the other hand, the sperm viability percentage was significantly diminished in both diclofenac and naproxen-administered groups (p ≤ 0.001 and p = 0.03 respectively) (Figure 2B). Also, in both drug-administered groups, there was a significant increase (p < 0.001) in the percentage of sperm morphological anomalies (Figure 2C). These sperm anomalies were shown in figure 3, including sperm with double tails and amorphous head, small and amorphous head, banana-shaped head, folded tail and without a head, flattened or reduced head, short hook, pinhead, no hook with a bent tail, and bent neck. Notably, the nonviable sperms stained blue were differentiated from the viable sperms that were unstained, as shown distinctly in figure 4.
3.3. Testicular histopathology and histomorphometry
Histological analysis of the present study showed that the control group revealed normal testicular parenchymal architecture with compactly arranged seminiferous tubules at all spermatogenesis stages and interstitial cells (Figure 5A). Naproxen-administered group exhibited clear vacuolization, separation of spermatogenic cells, and loss of interstitial connective tissue, which was replaced with a few foci of congestions (Figure 5B). Also, the diclofenac sodium-administered group showed signs of degeneration in several seminiferous tubules, reduction in the number of spermatogonial cells, and clear vacuolization (Figure 5C).
The morphometric findings of seminiferous tubules compared to the control revealed that diameters of the tubules decreased in both drug-administered groups. However, this reduction was significant in the diclofenac sodium-administered group only (p = 0.01) (Figure 6A). Diameters of the tubule lumens, on the other hand, appeared to have a significant increase (p = 0.02) in both drug-administered groups (Figure 6B). Further, in both drug groups, a significant reduction (p < 0.001) was observed in the thickness of the germinal epithelium of the tubules (Figure 6C). Otherwise, the number of the seminiferous tubules per field revealed no significant differences (p = 0.41) between the control and non-steroidal drug-administered groups (Figure 6D).






4. Discussion
Our results demonstrated the harmful effects of both naproxen and diclofenac sodium as nonsteroidal drugs on sperm parameters and testicular histology that may lead to infertility. The drugs administration at the pre-pubertal period of the rats could impact sperm maturation and thereby causing male infertility. Male fertility can be affected when drugs induce harm or damage in the testes and impair spermatogenesis and sperm maturation (2). The harmful effects of naproxen in this study were represented by a significant decrease in the sperm count and seminiferous tubules injuries; such results were similar to those obtained in a previous study (13). Remarkably, there were no more available studies concerning the reproductive toxicity of naproxen in the rats such as the percentage of sperm viability reduction and the percentage of increasing sperm morphological abnormalities. In this context, the previous studies reported a reduction in the total sperm count and sperm viability percentage caused by diclofenac sodium and that corresponds with our results. As for the histopathological effects resulting from diclofenac sodium administration, the aforementioned studies recorded almost similar effects, including degeneration and shrinkage of the seminiferous tubules, vacuolization in the interstitial connective tissue, and depletion of germinal epithelium (14-16).
According to our findings, the degeneration and depletion of the germinal layer mediated by testicular damage might be the reason for the decrease of spermatogonial cells that led to a reduction in sperm number and subsequently affected fertility. The cause of increased sperm anomalies induced by both naproxen and diclofenac sodium might be due to the presence of nuclear DNA damage, or fragmentation in the spermatozoa that is considered the main pathway responsible for sperm morphological defects, as well as chromatin immaturity (17). There is a correlation between the DNA fragmentation and morphological defects of sperms in patients with teratozoospermia (18).
Also, the presence of apoptotic markers in spermatozoa have been strongly related to sperm morphological abnormalities (19). Increasing the apoptosis level during early spermatogenesis results in sperm apoptosis that leads to male infertility (20). Sperm DNA damage and apoptosis can be created by excessive oxidative stress. Oxidative stress has also been correlated with abnormal sperm morphology (19). Moreover, oxidative stress can also reduce sperm viability, and vitamin E administration as an antioxidant leads to reverse this effect significantly (12). It is worth mentioning that our findings of deteriorated sperm parameters (sperm count, viability, and morphology) and injured seminiferous tubules caused by naproxen and diclofenac sodium were in parallel with the toxic effects of other reproductive toxicants such as sodium arsenite and manganese dioxide micro-and nanoparticles (12, 21).
With regards to the morphometric outcomes, the present study showed a decrease in the seminiferous tubules’ diameters and their germinal epithelium thickness in both naproxen and diclofenac sodium-administered groups. These findings were in accordance with the toxic effects of zinc oxide nanoparticles in mouse testis and di-butyl phthalate-induced oxidative stress in the testis of rats (22, 23). The reduction in seminiferous diameter may indicate increased loss of the germ cells through apoptosis (22). Also, the spermatogenesis process's rise results in an expansion of the seminiferous tubules’ diameter and thickness (24). Thus, the histomorphological alterations in our study might be attributed to the reduction in the number of spermatogonial cells and spermatogenesis. Furthermore, the significantly increased diameters of tubule lumens were manifested in both drug-administered groups of the present study. The larger lumen and thinner epithelium of the seminiferous tubules were suggested to be an evidence of increased intra-tubular pressure-mediated spermatogenic damage (25).
It is noteworthy that the body weights of rats administered the non-steroidal drugs significantly declined compared to the control rats. Researchers (14) mentioned that the diclofenac sodium treatment can reduce feed intake and body weight. As for naproxen, it is considered an inhibitor of glycogen synthase kinase-3β, and this pharmacological inhibitor caused a significant reduction in the weights of obese mice (26). Glycogen is the main origin for glucose and plays a crucial role in growing gonads during development (27). Also, inhibition of androgen biosynthesis may be indirectly responsible for significantly reducing the weights of the testis, epididymis, ventral prostate, and seminal vesicle caused by aspirin (nonsteroidal anti-inflammatory drug) treatment; as well as androgen hormones closely regulate the weight, size, and function of testes and other reproductive organs (28). These facts might be involved in the significant drop of testis weight; which was considerable in the naproxen-administered group. Otherwise, diclofenac sodium administration produced a nonsignificant decline in the testis weights. This insignificant decline did not agree with the results of a previous study (16).
5. Conclusion
It can be concluded from the current study that both naproxen and diclofenac sodium exert harms on the sperm parameters (sperm numbers, viability, and morphology), the testicular histology and morphometry in the albino rats when administered before their sexual maturity. These pathological effects are very deleterious to male fertility and can lead to potential infertility.
Acknowledgments
This study has not been supported financially from any organization or agency.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Griswold MD. Spermatogenesis: The commitment to meiosis. Physiol Rev 2016; 96: 1-17. [DOI:10.1152/physrev.00013.2015] [PMID] [PMCID]
2. Ding J, Shang X, Zhang Zh, Jing H, Shao J, Fei Q, et al. FDA-approved medications that impair human spermatogenesis. Oncotarget 2017; 8: 10714-10725. [DOI:10.18632/oncotarget.12956] [PMID] [PMCID]
3. Wallace JL. Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J Gastroenterol 2013; 19: 1861-1876. [DOI:10.3748/wjg.v19.i12.1861] [PMID] [PMCID]
4. Cooper C, Chapurlat R, Al-Daghri N, Herrero-Beaumont G, Bruyère O, Rannou F, et al. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs Aging 2019; 36: 15-24. [DOI:10.1007/s40266-019-00660-1] [PMID] [PMCID]
5. Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol 2013; 115: 909-919. [DOI:10.1152/japplphysiol.00061.2013] [PMID] [PMCID]
6. Pier B, Edmonds JW, Wilson L, Arabshahi A, Moore R, Bates GW, et al. Comprehensive profiling of prostaglandins in human ovarian follicular fluid using mass spectrometry. Prostaglandins Other Lipid Mediat 2018; 134: 7-15. [DOI:10.1016/j.prostaglandins.2017.11.001] [PMID] [PMCID]
7. Niringiyumukiza JD, Cai H, Xiang W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod Biol Endocrinol 2018; 16: 43. [DOI:10.1186/s12958-018-0359-5] [PMID] [PMCID]
8. Duffy DM. Novel contraceptive targets to inhibit ovulation: The prostaglandin E2 pathway. Hum Reprod Update 2015; 21: 652-670. [DOI:10.1093/humupd/dmv026] [PMID] [PMCID]
9. Frungieri MB, Calandra RS, Mayerhofer A, Matzkin ME. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction 2015; 149: R169-180. [DOI:10.1530/REP-14-0392] [PMID]
10. Kaewmala K, Uddin MJ, Cinar MU, Große-Brinkhaus C, Jonas E, Tesfaye D, et al. Investigation into association and expression of PLCz and COX-2 as candidate genes for boar sperm quality and fertility. Reprod Domest Anim 2012; 47: 213-223. [DOI:10.1111/j.1439-0531.2011.01831.x] [PMID]
11. Campos Silva R, Coelho Britto DM, de Fátima Pereira W, Brito-Melo GEA, Machado CT, Pedreira MM. Effect of short- and medium-term toxicity of doxorubicin on spermatogenesis in adult Wistar rats. Reprod Biol 2018; 18: 169-176. [DOI:10.1016/j.repbio.2018.03.002] [PMID]
12. Momeni HR, Eskandari N. Effect of vitamin E on sperm parameters and DNA integrity in sodium arsenite-treated rats. Iran J Reprod Med 2012; 10: 249-256.
13. Uzun B, Atli O, Perk BO, Burukoglu D, Ilgin S. Evaluation of the reproductive toxicity of naproxen sodium and meloxicam in male rats. Hum Exp Toxicol 2015; 34: 415-429. [DOI:10.1177/0960327114542886] [PMID]
14. Mousa AA, Elweza AE, Elbaz HT, Tahoun EAE-A, Shoghy KM, Elsayed I, et al. Eucalyptus Globulus protects against diclofenac sodium induced hepatorenal and testicular toxicity in male rats. J Tradit Complement Med 2020; 10: 521-528. [DOI:10.1016/j.jtcme.2019.11.002] [PMID] [PMCID]
15. Adeyemi WJ, Omoniyi JA, Olayiwola A, Ibrahim M, Ogunyemi O, Olayaki LA. Elevated reproductive toxicity effects of diclofenac after withdrawal: Investigation of the therapeutic role of melatonin. Toxicol Rep 2019; 6: 571-577. [DOI:10.1016/j.toxrep.2019.06.009] [PMID] [PMCID]
16. Vyas A, Purohit A, Ram H. Assessment of dose-dependent reproductive toxicity of diclofenac sodium in male rats. Drug Chem Toxicol 2019; 42: 478-486. [DOI:10.1080/01480545.2017.1421659] [PMID]
17. Oumaima A, Tesnim A, Zohra H, Amira S, Ines Z, Sana C, et al. Investigation on the origin of sperm morphological defects: Oxidative attacks, chromatin immaturity, and DNA fragmentation. Environ Sci Pollut Res Int 2018; 25: 13775-13786. [DOI:10.1007/s11356-018-1417-4] [PMID]
18. Jakubik-Uljasz J, Gill K, Rosiak-Gill A, Piasecka M. Relationship between sperm morphology and sperm DNA dispersion. Transl Androl Urol 2020; 9: 405-415. [DOI:10.21037/tau.2020.01.31] [PMID] [PMCID]
19. Ammar O, Mehdi M, Muratori M. Teratozoospermia: Its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology 2020; 8: 1095-1106. [DOI:10.1111/andr.12778] [PMID]
20. Shukla KK, Mahdi AA, Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci 2012; 4: 746-754. [DOI:10.2741/e415]
21. Yousefalizadegan N, Mousavi Z, Rastegar T, Razavi Y, Najafizadeh P. Reproductive toxicity of manganese dioxide in forms of micro- and nanoparticles in male rats. Int J Reprod BioMed 2019; 17: 361-370. [DOI:10.18502/ijrm.v17i5.4603] [PMID] [PMCID]
22. Moridian M, Khorsandi L, Talebi AR. Morphometric and stereological assessment of the effects of zinc oxide nanoparticles on the mouse testicular tissue. Bratisl Lek Listy 2015; 116: 321-325. [DOI:10.4149/BLL_2015_060] [PMID]
23. Abarikwu SO, Simple G, Onuoha CS. Morphometric evaluation of the seminiferous tubules and the antioxidant protective effects of gallic acid and quercetin in the testis and liver of Butyl Phthalate treated rats. Indian J Clin Biochem 2020; 35: 20-31. [DOI:10.1007/s12291-018-0788-0] [PMID] [PMCID]
24. Tripathi UK, Chhillar S, Kumaresan A, Aslam MKM, Rajak SK, Nayak S, et al. Morphometric evaluation of seminiferous tubule and proportionate numerical analysis of Sertoli and spermatogenic cells indicate differences between crossbred and purebred bulls. Vet World 2015; 8: 645-650. [DOI:10.14202/vetworld.2015.645-650] [PMID] [PMCID]
25. Ma L, Guo Y, Yuan Y, Li Y-G, Deng X-Z, Yang Z-W. Morphometric study of the testis and reproductive tract (including sperm granuloma) after vasectomy in mature rats. Asian J Androl 2016; 18: 66-73. [DOI:10.4103/1008-682X.150038] [PMID] [PMCID]
26. Motawi TMK, Bustanji Y, El-Maraghy SA, Taha MO, Al Ghussein MAS. Naproxen and cromolyn as new glycogen synthase kinase 3β inhibitors for amelioration of diabetes and obesity: An investigation by docking simulation and subsequent in vitro/in vivo biochemical evaluation. J Biochem Mol Toxicol 2013; 27: 425-436. [DOI:10.1002/jbt.21503] [PMID]
27. Villarroel-Espíndola F, Maldonado R, Mancilla H, vander Stelt K, Acuña AI, Covarrubias A, et al. Muscle glycogen synthase isoform is responsible for testicular glycogen synthesis: Glycogen overproduction induces apoptosis in male germ cells. J Cell Biochem 2013; 114: 1653-1664. [DOI:10.1002/jcb.24507] [PMID]
28. Vyas A, Ram H, Purohit A, Jatwa R. Adverse effects of subchronic dose of aspirin on reproductive profile of male rats. J Pharm 2016; 2016: 6585430. [DOI:10.1155/2016/6585430] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








