Sun, Feb 1, 2026
[Archive]
Volume 23, Issue 6 (June 2025)
IJRM 2025, 23(6): 475-484 |
Back to browse issues page
Ethics code: IR.SSU.RSI.REC.1402.016
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dehghani Firouzabadi R, Homayoon N, Arabian S, Dehghanpour F, Mangoli E, Barzegar H et al . Influence of assisted hatching on pregnancy outcomes in women with poor ovarian response: An RCT. IJRM 2025; 23 (6) :475-484
URL: http://ijrm.ir/article-1-3495-en.html
URL: http://ijrm.ir/article-1-3495-en.html
Razieh Dehghani Firouzabadi1 

 , Nahid Homayoon2
, Nahid Homayoon2 

 , Sahereh Arabian *3
, Sahereh Arabian *3 

 , Fatemeh Dehghanpour1
, Fatemeh Dehghanpour1 

 , Esmat Mangoli1
, Esmat Mangoli1 

 , Hamide Barzegar4
, Hamide Barzegar4 

 , Sajad Zare Garizi5
, Sajad Zare Garizi5 




 , Nahid Homayoon2
, Nahid Homayoon2 

 , Sahereh Arabian *3
, Sahereh Arabian *3 

 , Fatemeh Dehghanpour1
, Fatemeh Dehghanpour1 

 , Esmat Mangoli1
, Esmat Mangoli1 

 , Hamide Barzegar4
, Hamide Barzegar4 

 , Sajad Zare Garizi5
, Sajad Zare Garizi5 


1- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Department of Gynecology and Infertility, Shiraz Fertility Center, Shiraz, Iran.
3- Abnormal Uterine Bleeding Research Center, Semnan University of Medical Sciences, Semnan, Iran. ,sahereh_arabian@yahoo.com; s.arabian@semums.ac.ir
4- Neonatal Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Student Research Committee, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Department of Gynecology and Infertility, Shiraz Fertility Center, Shiraz, Iran.
3- Abnormal Uterine Bleeding Research Center, Semnan University of Medical Sciences, Semnan, Iran. ,
4- Neonatal Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Student Research Committee, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 437 kb]
(563 Downloads)
| Abstract (HTML) (495 Views)
Full-Text: (87 Views)
1. Introduction
Infertility, a significant global issue, affects 8-12% of couples (1). Since the first infant conceived through assisted reproductive technology was born in the United States in 1981, the use of advanced methods to address infertility has increased substantially (2). Advances in assisted reproductive technology have led to improved outcomes through various innovations. These include laboratory breakthroughs in fertilization, enhanced cryopreservation methods, and the implementation of blastocyst transfer. By employing these techniques, it is possible to choose embryos with a higher potential for successful implantation (3).
The embryo must hatch from its acellular glycoprotein-based outer layer, the zona pellucida (ZP), before it can implant in the uterus (4). Factors such as advanced maternal age, elevated follicle-stimulating hormone levels, and even embryo culture conditions have been associated with a thickening or hardening of the ZP, potentially hindering the embryo's ability to hatch and implant (5).
Assisted hatching (AH) was first introduced by Cohen et al. who reported the first successful pregnancy using this technique in 1988 (6). AH is a technique proposed to enhance embryo implantation. It involves artificially disrupting the ZP through various methods, such as zona thinning, zona drilling (creating a hole), complete zona removal, the use of chemicals, mechanical techniques, and lasers (7). The mechanism by which AH improves embryo implantation is not fully understood. Still, several theories suggest that AH facilitates earlier contact between the embryo and the endometrium during the critical implantation window. Early implantation is associated with successful pregnancies, while delayed implantation often leads to higher abortion rates (8). AH may enhance the exchange of essential nutrients and growth factors by creating artificial gaps in the ZP, thereby promoting embryo development and blastocyst formation. Experimental studies in mice support this theory, showing improved embryo development with AH (9).
Although some research has been conducted, the impact of AH remains uncertain. A randomized controlled trial showed no significant difference in live birth rates (LBR) between embryos with and without AH (10). A study conducted on the effect of AH revealed no statistically significant difference in implantation or pregnancy rates between the AH group and the control group (11). Another study demonstrated that AH significantly increased the clinical pregnancy rate in women aged 37 and older (12). In 2023, a large retrospective cohort study found that AH does not enhance pregnancy outcomes, even for participants with a poor prognosis (13). The variability in results from different studies highlights the need for further research to evaluate the effects of AH. A recent Cochrane review found no substantial difference in LBRs between women who underwent AH and those who did not. However, the review did indicate a slight improvement in clinical pregnancy rates with AH. A subgroup analysis suggested that AH might modestly increase clinical pregnancy rates in women with a poor prognosis (14).
This study aimed to compare the outcomes between the AH group and the control group (non-AH), as well as between fresh and frozen embryo transfers (ET), focusing on clinical pregnancy, chemical pregnancy, ongoing pregnancy, and miscarriage rates in women with poor ovarian response (POR).
2. Materials and Methods
2.1. Trial design and study population
In this randomized controlled clinical trial, 170 candidates for in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) who met the inclusion criteria at the Yazd Reproductive Sciences Institute, Yazd, Iran from December 2023-June 2024, enrolled using a convenience sampling method. Inclusion criteria were candidates for fresh or frozen-thawed ET with POR, classified as POSEIDON groups 3 and 4, aged between 18 and 42 yr. The POSEIDON criteria classify women based on factors such as age, ovarian reserve, and their response to stimulation. It differentiates between an "expected" low ovarian response (groups 3 and 4) and an "unexpected" low ovarian response (groups 1 and 2). Group 3 consists of women < 35 with either anti-Müllerian hormone (AMH) levels < 1.2 ng/ml, an antral follicle count < 5, or both. Group 4 includes women aged ≥ 35 who have AMH levels < 1.2 ng/ml, an antral follicle count < 5, or both (15). Exclusion criteria included structural anomalies of the uterus, poor-quality embryos, and severe male factor infertility. Women who fulfilled the inclusion criteria and were candidates for either a fresh or frozen ET cycle were randomly assigned to one of 2 equal groups. One group received AH, while the other group underwent standard ET without AH.
2.2. Sample size
Considering a 95% confidence level and 80% power, and assuming a 16% clinical pregnancy rate in the control group and a 32% rate in the intervention group, the minimum required sample size for each group was calculated to be 85, based on the following formula:

2.3. Randomization
The randomization list for the samples was generated using the Random Allocation1 software. Based on the order of participant referrals and inclusion criteria, the statistical consultant informed the researcher of each participant's assignment to either the intervention or control group, according to the participant's designated number on the randomization list. Participants who met the inclusion criteria, based on the randomization list order, were randomly allocated to either one of 2 hatching groups who underwent AH or a control group that underwent ET without intervention.
2.4. Controlled ovarian stimulation
After the initial examinations and evaluation of the gametes' condition, female gametes were retrieved through ovarian stimulation using standard methods (16). All women underwent controlled ovarian stimulation using the antagonist protocol. Stimulation began on the 2nd day of the menstrual cycle with 150-300 IU of recombinant follicle-stimulating hormone (Cinnal-F, CinnaGen, Tehran, Iran) and human menopausal gonadotropins (Humegnan, DarouPakhsh, Karaj, Iran), adjusted based on each woman's age, antral follicle count, and AMH levels. When at least one follicle had grown to a diameter of ≥ 14 mm, a gonadotropin-releasing hormone antagonist was introduced, with 0.25 mg of Cetrorelix Acetate (Cetroronax, Ronak, Tehran, Iran) administered. Dual triggering was carried out with 1 mg of Buserelin Acetate (CinnaFact, CinnaGen, Tehran, Iran) and 5000 IU of human chorionic gonadotropin (Folignan, DarouPakhsh, Karaj, Iran) when 2 or more follicles reached ≥ 17 mm (16).
2.5. Assisted reproductive procedures
The oocytes were collected 34-36 hr after the trigger. Cumulus oocyte complexes were harvested using SynVitro Flush media (without heparin, Origio, CooperSurgical, Denmark) and incubated in Universal IVF Medium (Origio) at 37°C with 5% CO2 and 6% O2. After 2-3 hr, the oocytes were denuded through a combination of chemical (80 IU/mL pre-warmed hyaluronidase, Irvine Scientific®, CA, USA) and mechanical methods. Fertilization was assessed 16-18 hr post-ICSI. Embryo quality and cleavage stage were assessed 42-44 hr post-ICSI.
Embryo grading followed the criteria described by Hill et al. in 1989 (17): grade A for embryos with evenly sized blastomeres and no fragmentation, grade B for embryos with slightly uneven blastomere sizes and up to 10% fragmentation, grade C for embryos with uneven blastomere sizes and 10-50% cytoplasmic fragmentation, and grade D for embryos with significantly uneven blastomere sizes, large black granules, and more than 50% fragmentation. Grades A and B were classified as high-quality embryos, while grade C was considered poor quality, and grade D embryos were discarded (18).
2.6. AH procedure
For AH, following the method we previously used in our center (19), the embryos in the experimental group underwent laser-AH on the morning of day 3. This was performed using a Nikon TE300 inverted microscope (Nikon, Tokyo, Japan) equipped with a Saturn system (Research Instruments LTD, UK). A 1480 nm wavelength infrared diode laser was applied for 605 µs to create a 10-12 μm hole in the ZP without any physical contact, with the process monitored via video. Following AH, the embryos underwent multiple washes and were subsequently stored until transfer. Control group embryos were maintained intact without any AH intervention.
2.7. Endometrial preparation and ET
For women undergoing frozen embryo transfer (FET), oral estradiol valerate (Estradiol Valerate, Aburaihan Co., Iran) at a dose of 6 mg daily was started on day 2 of the menstrual cycle. When the endometrial thickness reached 7 mm, progesterone (Actogest, Actover CO., Iran) 400 mg twice daily (administered as vaginal or rectal suppositories) and dydrogesterone (Dofamed, Actover CO., Iran) 10 mg twice daily were initiated. One or 2 best quality day 3 fresh or vitrified-warmed embryos were transferred using an RADA ET catheter from Behrad Rooyesh Rooyan Co., Iran. The administration of estradiol and progesterone continued until 12th wk of pregnancy (20).
2.8. Measurement variables and outcomes
The demographic characteristics of the women, including age, duration, and type of infertility, body mass index, and AMH level, were recorded. Additionally, treatment cycle characteristics, such as the use of frozen or fresh ET cycles and the number of embryos transferred, were documented. Pregnancy outcomes, including chemical pregnancy, clinical pregnancy, ongoing pregnancy, implantation rate, multiple pregnancy rate, and abortion rate, were assessed.
The primary outcome was clinical pregnancy, while the secondary outcomes included chemical pregnancy, ongoing pregnancy, implantation rate, multiple pregnancy rate, and early abortion rate. A chemical pregnancy was identified by a serum beta-human chorionic gonadotropin level of at least 50 IU/L, measured 14 days after ET. The implantation rate was defined as the number of intrauterine gestational sacs divided by the number of transferred embryos. Clinical pregnancy was confirmed through ultrasound detection of a fetal heartbeat 4 wk after the transfer. An ongoing pregnancy was determined by the continued presence of fetal cardiac activity on ultrasound at 12 wk post-ET. In cases where this activity was not observed, an early miscarriage was diagnosed (21). Luteal phase support continued until 12th wk of pregnancy.
2.9. Blinding
This study was open-label, and participants were aware of the procedures.
2.10. Ethical Considerations
The Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved the study (Code: IR.SSU.RSI.REC.1402.016) and registered with the Iranian Clinical Trial Registry (IRCT20091012002575N5, updated on 2025-03-17). All participants provided written informed consent, and potential complications and adverse effects of both treatments were explained to the participants. Data confidentiality was fully respected throughout the study. All participant information was securely stored, accessible only to the authorized research team.
2.11. Statistical Analysis
All data were entered and analyzed using the statistical package for the social sciences version 26 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive analysis included calculating the mean and standard deviation (SD) for continuous variables, while frequencies and percentages were used for categorical variables. Group comparisons of categorical variables were conducted using the Chi-square test, with Fisher's exact test applied when necessary. Independent t tests were employed to compare continuous variables. The common odds ratio was estimated using the Mantel-Haenszel method. A p < 0.05 was considered statistically significant.
3. Results
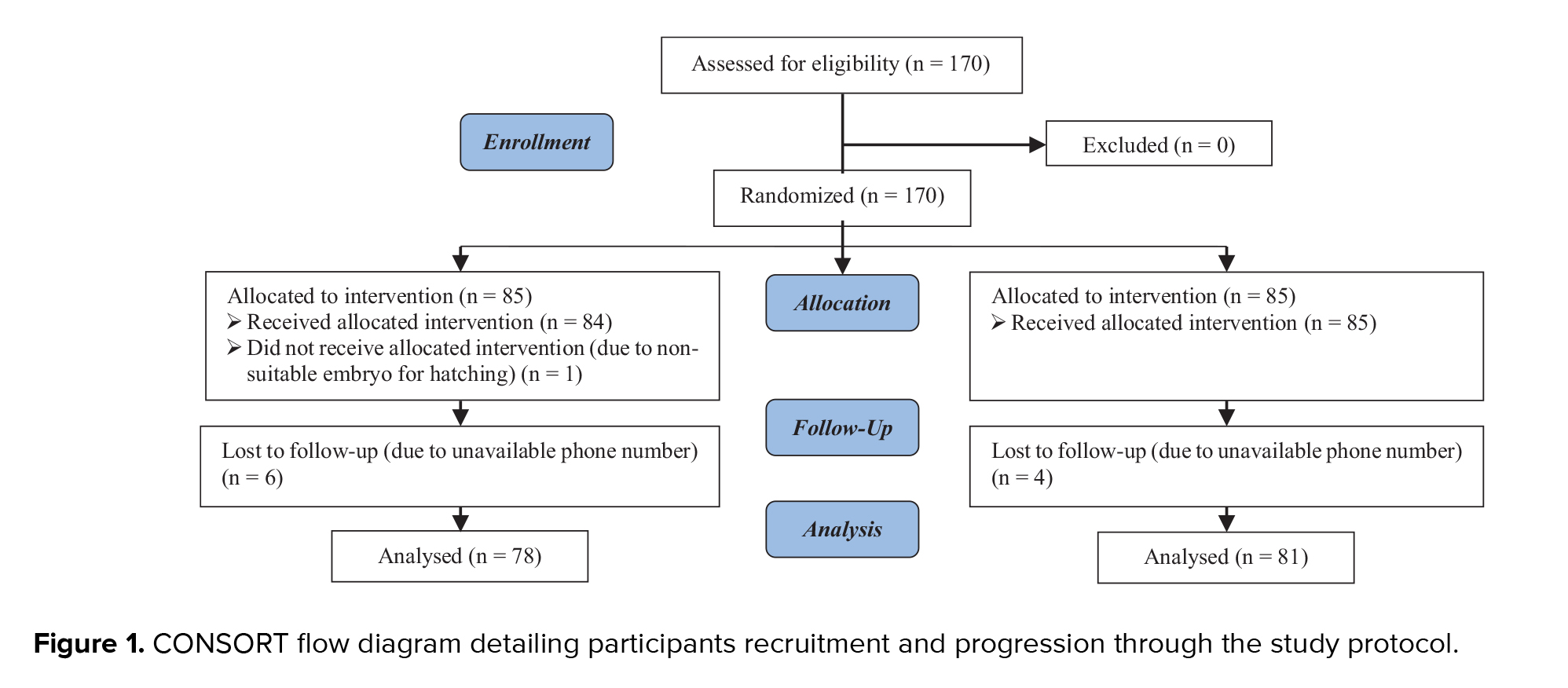
A total of 170 women candidates for IVF enrolled in the study, 85 in the hatching group and 85 in the control group. One woman in the hatching group was excluded from the study because the embryo had a thin ZP, making it inappropriate for AH. Furthermore, 6 participants from the hatching group and 4 from the control group were lost to follow-up due to unreachable phone numbers. A CONSORT flow diagram outlining the trial design is presented in figure 1.
3.1. Demographic data
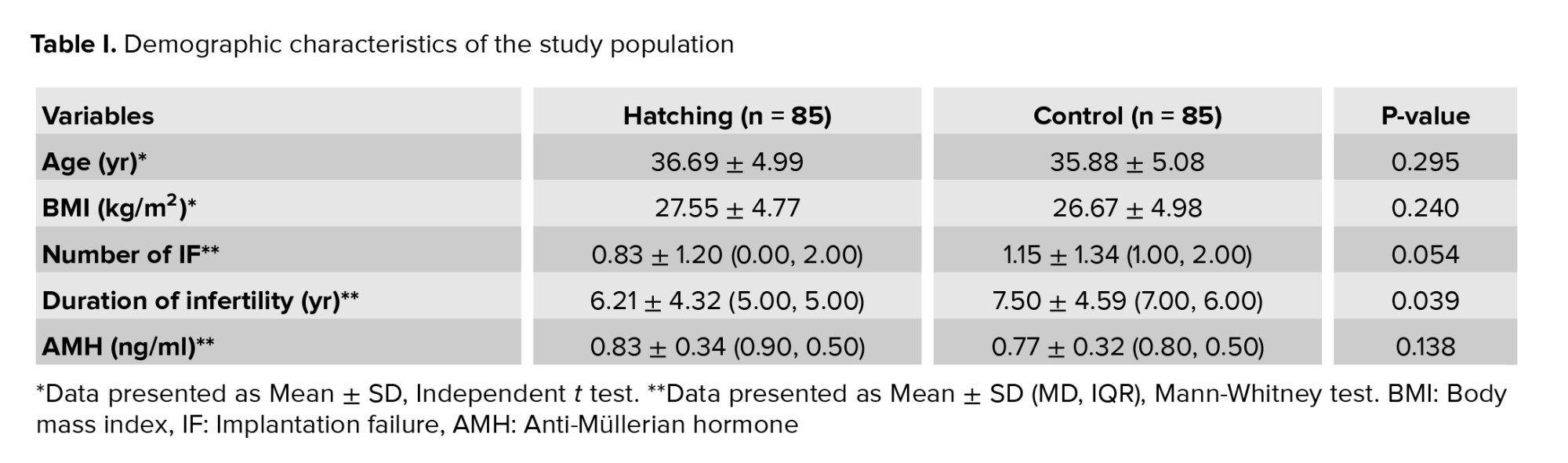
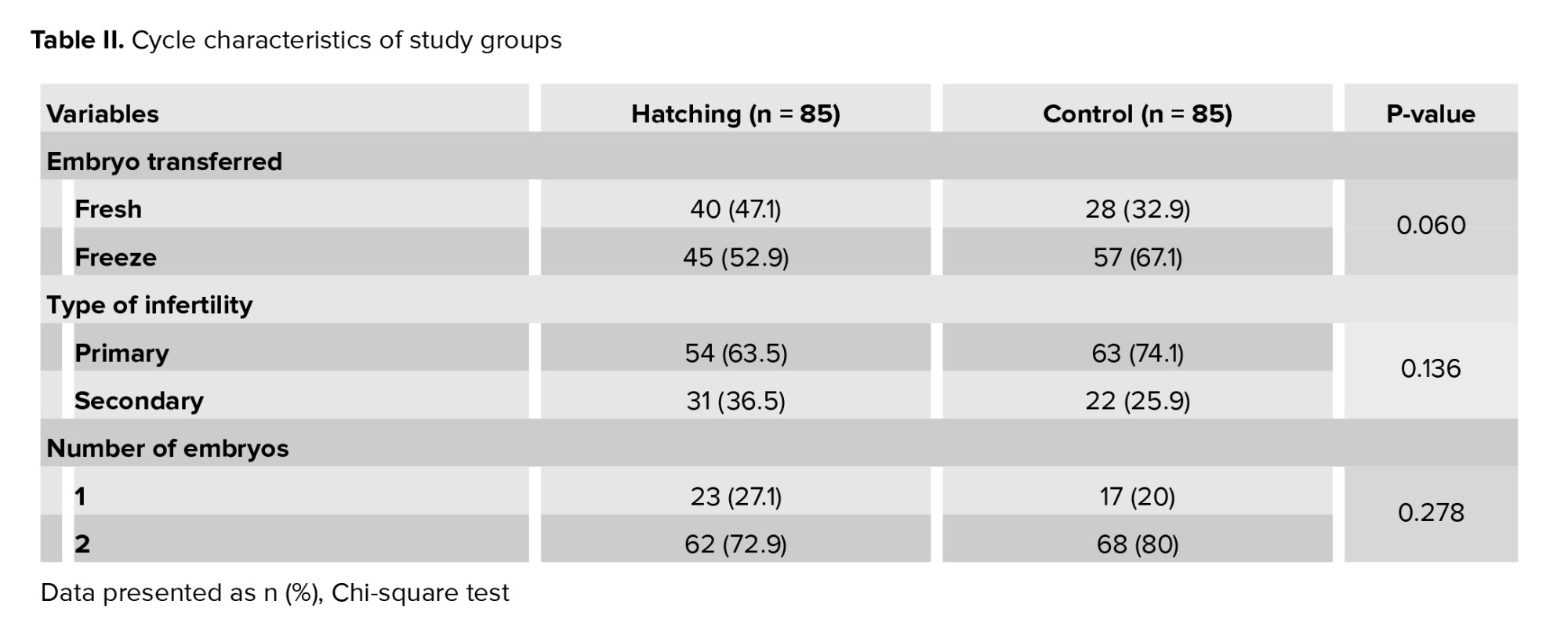
No significant difference was observed between the AH and control group for maternal age, body mass index, and AMH level. The number of previous implantation failures and the duration of infertility were higher in the control group (p = 0.05 and p = 0.039, respectively). The demographic data are illustrated in table I. Table II presents the cycle characteristics of the study groups. The hatching group underwent fresh ET in 47.1% of cycles and FET in 52.9% of cycles. In contrast, the control group had FET rates of 32.9% and 67.1%, respectively. No significant differences were observed in infertility type or number of embryos transferred between the 2 groups.
3.2. Reproductive outcomes
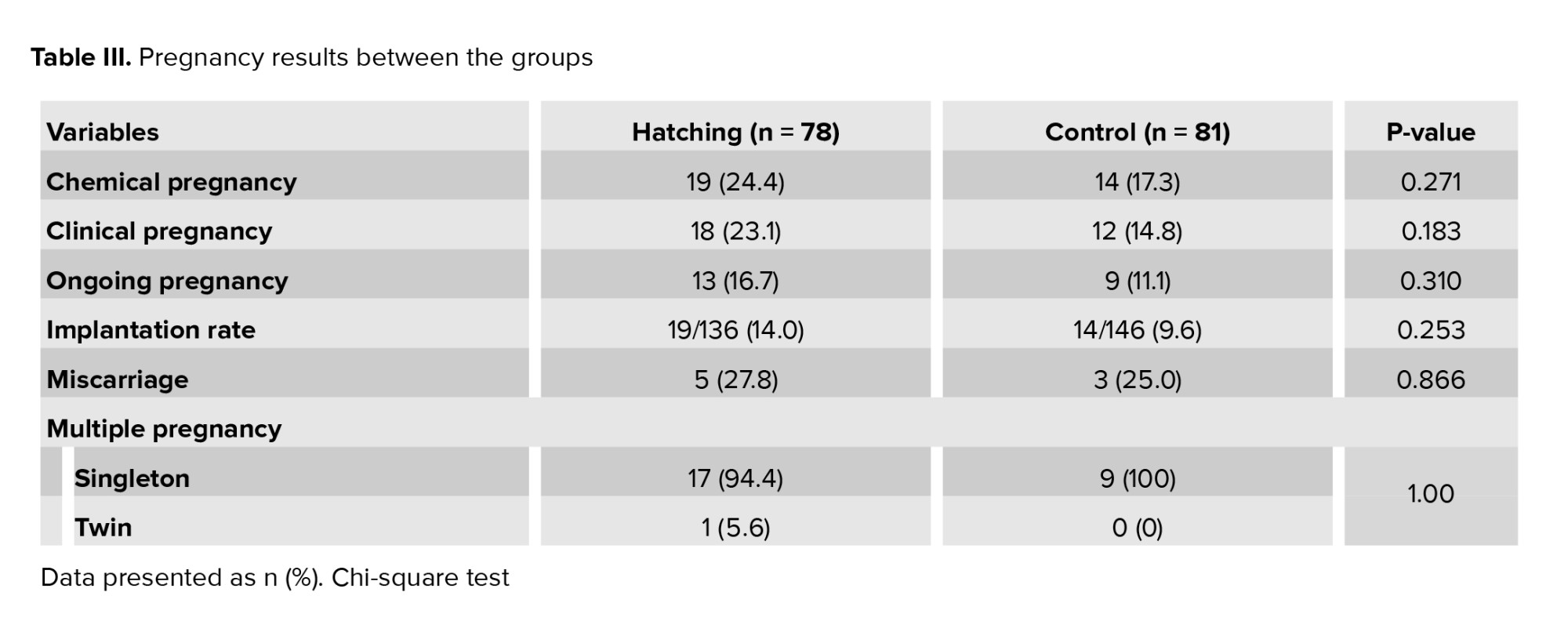
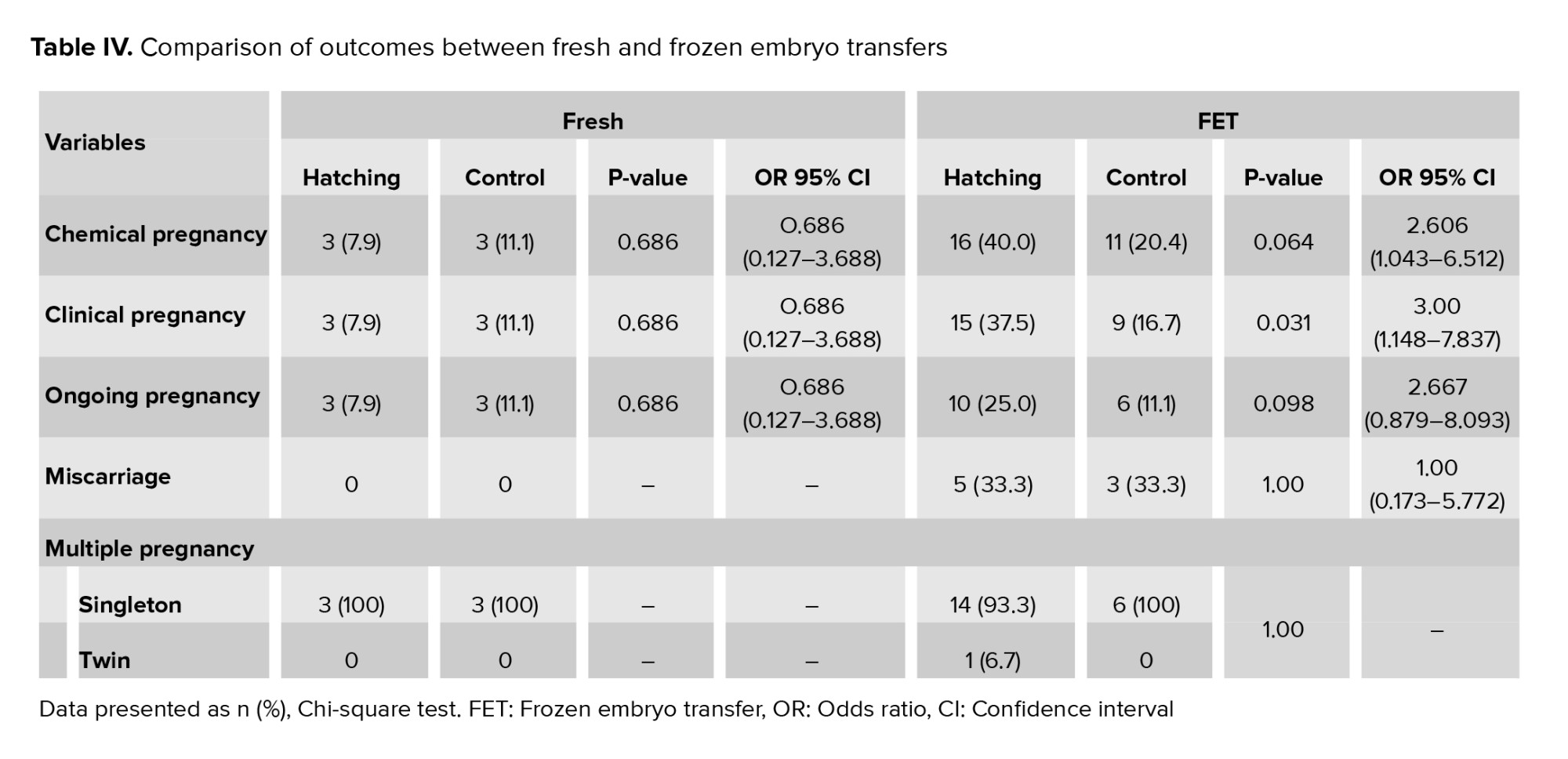
Table III summarizes IVF outcomes, including rates of implantation, chemical, clinical, and ongoing pregnancy, miscarriage, and multiple pregnancy. No statistically significant differences were observed between the groups in these outcomes. All twin pregnancies were dichorionic diamniotic, and no significant difference was observed in the rate of multiple pregnancies between the case and control groups. The results were assessed based on fresh and frozen ETs. No significant difference was observed in outcomes between the hatching and control women in the fresh transfer group. However, in the FET group, the number of positive chemical and clinical pregnancies was significantly higher in the hatching group compared to the control group (p = 0.038 and p = 0.022, respectively). The comparison of outcomes between fresh and frozen ETs in the hatching and control groups is shown in table IV. In pregnancies with FET, the odds of an ongoing pregnancy in the hatching group were 2.6 times higher than in the control group (95% CI: 0.87-8.09). Additionally, the chances of achieving a clinical pregnancy were 3 times higher in the hatching group compared to the control group (95% CI: 1.14-7.83)
4. Discussion
Our study found that AH did not significantly improve overall pregnancy outcomes in women with POR. However, a notable benefit was observed in frozen ETs cycles, where AH was associated with higher rates of clinical and ongoing pregnancies. A narrative review through a series of sensitivity analyses concluded that AH significantly improves pregnancy rates, implantation rates, and ongoing pregnancy rates in IVF/ICSI women with poor prognoses, especially in those with 2 or more previous implantation failures (22). However, the Cochrane systematic review and meta-analysis, which included 31 trials with a total of 5728 participants, did not find a significant difference in LBRs between the AH group and the control group. The clinical pregnancy rate for women who underwent AH demonstrated a slight improvement, reaching borderline statistical significance (OR = 1.13, 95% CI: 1.01-1.27) (14).
Our study on FET revealed that the likelihood of an ongoing pregnancy in the hatching group was 2.6 times higher than in the control group, with the odds of achieving a clinical pregnancy being 3 times greater in the hatching group. Different studies have reported varying results on this topic. For instance, a systematic review on randomized clinical trials evaluating the effect of AH found significant clinical pregnancy outcomes in women who underwent the transfer of frozen/thawed embryos without a prior history of implantation failure (23). A meta-analysis of 11 randomized controlled trials assessing the effect of laser-AH in frozen cycles revealed a significant increase in clinical pregnancy rates for the AH group. However, this increase did not result in a higher LBR (24). In their study involving 416 participants, authors demonstrated that AH improves pregnancy rates in frozen-thawed ICSI-ET cycles (12). This result aligns with the findings of other studies conducted to assess the impact of AH in FET cycles (25). In a study examining the effects of laser-AH on clinical outcomes in frozen-thawed cleavage-ETs among women with a history of repeated implantation failures, the AH group demonstrated significantly higher clinical pregnancy and implantation rates compared to the control group. While the live birth and multiple pregnancy rates were also higher in the AH group, these differences were not statistically significant (26). Another study analyzed 3535 FET cycles, finding a significantly higher LBR in the AH group (34.9%) compared to the non-AH group (31.4%, p = 0.024). Additionally, the AH group exhibited an insignificant reduction in pregnancy loss (p = 0.078). In contrast, the Cochrane systematic review found no significant increase in clinical pregnancy rates for FET (14). A recent study found that there is not enough evidence to say for sure whether AH helps with implantation or pregnancy rates in frozen ET (22).
Our study found no significant difference in miscarriage rates among the groups, aligning with a meta-analysis of 5 RCTs, which also reported no significant difference in miscarriage rates between AH and control groups (24). Likewise, a retrospective study by Pan et al. involving 924 women who conceived through frozen-thawed cleavage-stage ETs, found no significant difference in miscarriage rates between the AH and non-AH groups (27).
In our study, no difference was observed between groups regarding multiple pregnancies, and all twin pregnancies were dichorionic diamniotic. However, a systematic review found that multiple pregnancies were significantly increased in the AH group (23). In contrast to our results, other reports suggest that AH of cleavage-stage embryos is associated with an increased risk of monozygosity (13, 28, 29). ESHRE's 2023 good practice recommendations on add-ons in reproductive medicine indicate a potential correlation between AH and monozygotic twinning rates, but the evidence is currently inconclusive owing to a small sample size (30).
Since our center's protocol involves transferring cleavage-stage embryos, we performed hatching at the cleavage stage, focusing on assessing pregnancy rates. A recent study found that AH on low-grade cleavage-stage embryos on days 4 and 6 enhances both the blastocyst formation rate and the rate of usable blastocysts by day 7, and suggested that AH is a simple and effective technique for maximizing the value of low-grade cleavage-stage embryos (31).
The strength of our study lies in its focus on women with POR. Additionally, the case-control design allowed us to compare the results between the AH and control groups effectively.
One of the limitations of our study was that we did not consider LBRs. Although AH is considered safe and no increase in anomalies has been reported so far, it is essential to consider the potential for anomalies (32, 33).
5. Conclusion
Although our results demonstrate no significant difference between the hatching and control groups for pregnancy outcomes in women with POR, the evidence remains mixed regarding its effectiveness. This highlights the need for further research to clarify the role of AH in improving pregnancy outcomes.
Data Availability
Data supporting findings of this research are available from the corresponding author upon request.
Author Contributions
R. Dehghani Firouzabadi: Study design. R. Dehghani Firouzabadi, S. Arabian, N. Homayoon: Conducted the IVF procedures. F. Dehghanpour and E. Mangoli: Conducted embryonic procedures, N. Homayoon, S. Arabian, H. Barzegar, and S. Zare Garizi: Conducted data collection and analysis. All authors contributed to the literature review, assisted in drafting the manuscript, approved the final version, and took responsibility for the integrity of the data.
Acknowledgments
This research was supported by the Research and Clinical Center for Infertility at the Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (project no. 16601). The authors express sincere gratitude to the Yazd Reproductive Sciences Institute, Yazd, Iran and its staff for their support. We would also like to thank Dr. Farimah Shamsi for her invaluable statistical and methodological expertise. Artificial intelligence tools, including Google Bard (Gemini) 1.5 Pro and ChatGPT 4, were employed for grammar checking in specific sections.
Conflict of Interest
The authors declare that there is no conflict of interest.
Infertility, a significant global issue, affects 8-12% of couples (1). Since the first infant conceived through assisted reproductive technology was born in the United States in 1981, the use of advanced methods to address infertility has increased substantially (2). Advances in assisted reproductive technology have led to improved outcomes through various innovations. These include laboratory breakthroughs in fertilization, enhanced cryopreservation methods, and the implementation of blastocyst transfer. By employing these techniques, it is possible to choose embryos with a higher potential for successful implantation (3).
The embryo must hatch from its acellular glycoprotein-based outer layer, the zona pellucida (ZP), before it can implant in the uterus (4). Factors such as advanced maternal age, elevated follicle-stimulating hormone levels, and even embryo culture conditions have been associated with a thickening or hardening of the ZP, potentially hindering the embryo's ability to hatch and implant (5).
Assisted hatching (AH) was first introduced by Cohen et al. who reported the first successful pregnancy using this technique in 1988 (6). AH is a technique proposed to enhance embryo implantation. It involves artificially disrupting the ZP through various methods, such as zona thinning, zona drilling (creating a hole), complete zona removal, the use of chemicals, mechanical techniques, and lasers (7). The mechanism by which AH improves embryo implantation is not fully understood. Still, several theories suggest that AH facilitates earlier contact between the embryo and the endometrium during the critical implantation window. Early implantation is associated with successful pregnancies, while delayed implantation often leads to higher abortion rates (8). AH may enhance the exchange of essential nutrients and growth factors by creating artificial gaps in the ZP, thereby promoting embryo development and blastocyst formation. Experimental studies in mice support this theory, showing improved embryo development with AH (9).
Although some research has been conducted, the impact of AH remains uncertain. A randomized controlled trial showed no significant difference in live birth rates (LBR) between embryos with and without AH (10). A study conducted on the effect of AH revealed no statistically significant difference in implantation or pregnancy rates between the AH group and the control group (11). Another study demonstrated that AH significantly increased the clinical pregnancy rate in women aged 37 and older (12). In 2023, a large retrospective cohort study found that AH does not enhance pregnancy outcomes, even for participants with a poor prognosis (13). The variability in results from different studies highlights the need for further research to evaluate the effects of AH. A recent Cochrane review found no substantial difference in LBRs between women who underwent AH and those who did not. However, the review did indicate a slight improvement in clinical pregnancy rates with AH. A subgroup analysis suggested that AH might modestly increase clinical pregnancy rates in women with a poor prognosis (14).
This study aimed to compare the outcomes between the AH group and the control group (non-AH), as well as between fresh and frozen embryo transfers (ET), focusing on clinical pregnancy, chemical pregnancy, ongoing pregnancy, and miscarriage rates in women with poor ovarian response (POR).
2. Materials and Methods
2.1. Trial design and study population
In this randomized controlled clinical trial, 170 candidates for in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) who met the inclusion criteria at the Yazd Reproductive Sciences Institute, Yazd, Iran from December 2023-June 2024, enrolled using a convenience sampling method. Inclusion criteria were candidates for fresh or frozen-thawed ET with POR, classified as POSEIDON groups 3 and 4, aged between 18 and 42 yr. The POSEIDON criteria classify women based on factors such as age, ovarian reserve, and their response to stimulation. It differentiates between an "expected" low ovarian response (groups 3 and 4) and an "unexpected" low ovarian response (groups 1 and 2). Group 3 consists of women < 35 with either anti-Müllerian hormone (AMH) levels < 1.2 ng/ml, an antral follicle count < 5, or both. Group 4 includes women aged ≥ 35 who have AMH levels < 1.2 ng/ml, an antral follicle count < 5, or both (15). Exclusion criteria included structural anomalies of the uterus, poor-quality embryos, and severe male factor infertility. Women who fulfilled the inclusion criteria and were candidates for either a fresh or frozen ET cycle were randomly assigned to one of 2 equal groups. One group received AH, while the other group underwent standard ET without AH.
2.2. Sample size
Considering a 95% confidence level and 80% power, and assuming a 16% clinical pregnancy rate in the control group and a 32% rate in the intervention group, the minimum required sample size for each group was calculated to be 85, based on the following formula:

2.3. Randomization
The randomization list for the samples was generated using the Random Allocation1 software. Based on the order of participant referrals and inclusion criteria, the statistical consultant informed the researcher of each participant's assignment to either the intervention or control group, according to the participant's designated number on the randomization list. Participants who met the inclusion criteria, based on the randomization list order, were randomly allocated to either one of 2 hatching groups who underwent AH or a control group that underwent ET without intervention.
2.4. Controlled ovarian stimulation
After the initial examinations and evaluation of the gametes' condition, female gametes were retrieved through ovarian stimulation using standard methods (16). All women underwent controlled ovarian stimulation using the antagonist protocol. Stimulation began on the 2nd day of the menstrual cycle with 150-300 IU of recombinant follicle-stimulating hormone (Cinnal-F, CinnaGen, Tehran, Iran) and human menopausal gonadotropins (Humegnan, DarouPakhsh, Karaj, Iran), adjusted based on each woman's age, antral follicle count, and AMH levels. When at least one follicle had grown to a diameter of ≥ 14 mm, a gonadotropin-releasing hormone antagonist was introduced, with 0.25 mg of Cetrorelix Acetate (Cetroronax, Ronak, Tehran, Iran) administered. Dual triggering was carried out with 1 mg of Buserelin Acetate (CinnaFact, CinnaGen, Tehran, Iran) and 5000 IU of human chorionic gonadotropin (Folignan, DarouPakhsh, Karaj, Iran) when 2 or more follicles reached ≥ 17 mm (16).
2.5. Assisted reproductive procedures
The oocytes were collected 34-36 hr after the trigger. Cumulus oocyte complexes were harvested using SynVitro Flush media (without heparin, Origio, CooperSurgical, Denmark) and incubated in Universal IVF Medium (Origio) at 37°C with 5% CO2 and 6% O2. After 2-3 hr, the oocytes were denuded through a combination of chemical (80 IU/mL pre-warmed hyaluronidase, Irvine Scientific®, CA, USA) and mechanical methods. Fertilization was assessed 16-18 hr post-ICSI. Embryo quality and cleavage stage were assessed 42-44 hr post-ICSI.
Embryo grading followed the criteria described by Hill et al. in 1989 (17): grade A for embryos with evenly sized blastomeres and no fragmentation, grade B for embryos with slightly uneven blastomere sizes and up to 10% fragmentation, grade C for embryos with uneven blastomere sizes and 10-50% cytoplasmic fragmentation, and grade D for embryos with significantly uneven blastomere sizes, large black granules, and more than 50% fragmentation. Grades A and B were classified as high-quality embryos, while grade C was considered poor quality, and grade D embryos were discarded (18).
2.6. AH procedure
For AH, following the method we previously used in our center (19), the embryos in the experimental group underwent laser-AH on the morning of day 3. This was performed using a Nikon TE300 inverted microscope (Nikon, Tokyo, Japan) equipped with a Saturn system (Research Instruments LTD, UK). A 1480 nm wavelength infrared diode laser was applied for 605 µs to create a 10-12 μm hole in the ZP without any physical contact, with the process monitored via video. Following AH, the embryos underwent multiple washes and were subsequently stored until transfer. Control group embryos were maintained intact without any AH intervention.
2.7. Endometrial preparation and ET
For women undergoing frozen embryo transfer (FET), oral estradiol valerate (Estradiol Valerate, Aburaihan Co., Iran) at a dose of 6 mg daily was started on day 2 of the menstrual cycle. When the endometrial thickness reached 7 mm, progesterone (Actogest, Actover CO., Iran) 400 mg twice daily (administered as vaginal or rectal suppositories) and dydrogesterone (Dofamed, Actover CO., Iran) 10 mg twice daily were initiated. One or 2 best quality day 3 fresh or vitrified-warmed embryos were transferred using an RADA ET catheter from Behrad Rooyesh Rooyan Co., Iran. The administration of estradiol and progesterone continued until 12th wk of pregnancy (20).
2.8. Measurement variables and outcomes
The demographic characteristics of the women, including age, duration, and type of infertility, body mass index, and AMH level, were recorded. Additionally, treatment cycle characteristics, such as the use of frozen or fresh ET cycles and the number of embryos transferred, were documented. Pregnancy outcomes, including chemical pregnancy, clinical pregnancy, ongoing pregnancy, implantation rate, multiple pregnancy rate, and abortion rate, were assessed.
The primary outcome was clinical pregnancy, while the secondary outcomes included chemical pregnancy, ongoing pregnancy, implantation rate, multiple pregnancy rate, and early abortion rate. A chemical pregnancy was identified by a serum beta-human chorionic gonadotropin level of at least 50 IU/L, measured 14 days after ET. The implantation rate was defined as the number of intrauterine gestational sacs divided by the number of transferred embryos. Clinical pregnancy was confirmed through ultrasound detection of a fetal heartbeat 4 wk after the transfer. An ongoing pregnancy was determined by the continued presence of fetal cardiac activity on ultrasound at 12 wk post-ET. In cases where this activity was not observed, an early miscarriage was diagnosed (21). Luteal phase support continued until 12th wk of pregnancy.
2.9. Blinding
This study was open-label, and participants were aware of the procedures.
2.10. Ethical Considerations
The Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved the study (Code: IR.SSU.RSI.REC.1402.016) and registered with the Iranian Clinical Trial Registry (IRCT20091012002575N5, updated on 2025-03-17). All participants provided written informed consent, and potential complications and adverse effects of both treatments were explained to the participants. Data confidentiality was fully respected throughout the study. All participant information was securely stored, accessible only to the authorized research team.
2.11. Statistical Analysis
All data were entered and analyzed using the statistical package for the social sciences version 26 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive analysis included calculating the mean and standard deviation (SD) for continuous variables, while frequencies and percentages were used for categorical variables. Group comparisons of categorical variables were conducted using the Chi-square test, with Fisher's exact test applied when necessary. Independent t tests were employed to compare continuous variables. The common odds ratio was estimated using the Mantel-Haenszel method. A p < 0.05 was considered statistically significant.
3. Results
A total of 170 women candidates for IVF enrolled in the study, 85 in the hatching group and 85 in the control group. One woman in the hatching group was excluded from the study because the embryo had a thin ZP, making it inappropriate for AH. Furthermore, 6 participants from the hatching group and 4 from the control group were lost to follow-up due to unreachable phone numbers. A CONSORT flow diagram outlining the trial design is presented in figure 1.
3.1. Demographic data
No significant difference was observed between the AH and control group for maternal age, body mass index, and AMH level. The number of previous implantation failures and the duration of infertility were higher in the control group (p = 0.05 and p = 0.039, respectively). The demographic data are illustrated in table I. Table II presents the cycle characteristics of the study groups. The hatching group underwent fresh ET in 47.1% of cycles and FET in 52.9% of cycles. In contrast, the control group had FET rates of 32.9% and 67.1%, respectively. No significant differences were observed in infertility type or number of embryos transferred between the 2 groups.
3.2. Reproductive outcomes
Table III summarizes IVF outcomes, including rates of implantation, chemical, clinical, and ongoing pregnancy, miscarriage, and multiple pregnancy. No statistically significant differences were observed between the groups in these outcomes. All twin pregnancies were dichorionic diamniotic, and no significant difference was observed in the rate of multiple pregnancies between the case and control groups. The results were assessed based on fresh and frozen ETs. No significant difference was observed in outcomes between the hatching and control women in the fresh transfer group. However, in the FET group, the number of positive chemical and clinical pregnancies was significantly higher in the hatching group compared to the control group (p = 0.038 and p = 0.022, respectively). The comparison of outcomes between fresh and frozen ETs in the hatching and control groups is shown in table IV. In pregnancies with FET, the odds of an ongoing pregnancy in the hatching group were 2.6 times higher than in the control group (95% CI: 0.87-8.09). Additionally, the chances of achieving a clinical pregnancy were 3 times higher in the hatching group compared to the control group (95% CI: 1.14-7.83)





4. Discussion
Our study found that AH did not significantly improve overall pregnancy outcomes in women with POR. However, a notable benefit was observed in frozen ETs cycles, where AH was associated with higher rates of clinical and ongoing pregnancies. A narrative review through a series of sensitivity analyses concluded that AH significantly improves pregnancy rates, implantation rates, and ongoing pregnancy rates in IVF/ICSI women with poor prognoses, especially in those with 2 or more previous implantation failures (22). However, the Cochrane systematic review and meta-analysis, which included 31 trials with a total of 5728 participants, did not find a significant difference in LBRs between the AH group and the control group. The clinical pregnancy rate for women who underwent AH demonstrated a slight improvement, reaching borderline statistical significance (OR = 1.13, 95% CI: 1.01-1.27) (14).
Our study on FET revealed that the likelihood of an ongoing pregnancy in the hatching group was 2.6 times higher than in the control group, with the odds of achieving a clinical pregnancy being 3 times greater in the hatching group. Different studies have reported varying results on this topic. For instance, a systematic review on randomized clinical trials evaluating the effect of AH found significant clinical pregnancy outcomes in women who underwent the transfer of frozen/thawed embryos without a prior history of implantation failure (23). A meta-analysis of 11 randomized controlled trials assessing the effect of laser-AH in frozen cycles revealed a significant increase in clinical pregnancy rates for the AH group. However, this increase did not result in a higher LBR (24). In their study involving 416 participants, authors demonstrated that AH improves pregnancy rates in frozen-thawed ICSI-ET cycles (12). This result aligns with the findings of other studies conducted to assess the impact of AH in FET cycles (25). In a study examining the effects of laser-AH on clinical outcomes in frozen-thawed cleavage-ETs among women with a history of repeated implantation failures, the AH group demonstrated significantly higher clinical pregnancy and implantation rates compared to the control group. While the live birth and multiple pregnancy rates were also higher in the AH group, these differences were not statistically significant (26). Another study analyzed 3535 FET cycles, finding a significantly higher LBR in the AH group (34.9%) compared to the non-AH group (31.4%, p = 0.024). Additionally, the AH group exhibited an insignificant reduction in pregnancy loss (p = 0.078). In contrast, the Cochrane systematic review found no significant increase in clinical pregnancy rates for FET (14). A recent study found that there is not enough evidence to say for sure whether AH helps with implantation or pregnancy rates in frozen ET (22).
Our study found no significant difference in miscarriage rates among the groups, aligning with a meta-analysis of 5 RCTs, which also reported no significant difference in miscarriage rates between AH and control groups (24). Likewise, a retrospective study by Pan et al. involving 924 women who conceived through frozen-thawed cleavage-stage ETs, found no significant difference in miscarriage rates between the AH and non-AH groups (27).
In our study, no difference was observed between groups regarding multiple pregnancies, and all twin pregnancies were dichorionic diamniotic. However, a systematic review found that multiple pregnancies were significantly increased in the AH group (23). In contrast to our results, other reports suggest that AH of cleavage-stage embryos is associated with an increased risk of monozygosity (13, 28, 29). ESHRE's 2023 good practice recommendations on add-ons in reproductive medicine indicate a potential correlation between AH and monozygotic twinning rates, but the evidence is currently inconclusive owing to a small sample size (30).
Since our center's protocol involves transferring cleavage-stage embryos, we performed hatching at the cleavage stage, focusing on assessing pregnancy rates. A recent study found that AH on low-grade cleavage-stage embryos on days 4 and 6 enhances both the blastocyst formation rate and the rate of usable blastocysts by day 7, and suggested that AH is a simple and effective technique for maximizing the value of low-grade cleavage-stage embryos (31).
The strength of our study lies in its focus on women with POR. Additionally, the case-control design allowed us to compare the results between the AH and control groups effectively.
One of the limitations of our study was that we did not consider LBRs. Although AH is considered safe and no increase in anomalies has been reported so far, it is essential to consider the potential for anomalies (32, 33).
5. Conclusion
Although our results demonstrate no significant difference between the hatching and control groups for pregnancy outcomes in women with POR, the evidence remains mixed regarding its effectiveness. This highlights the need for further research to clarify the role of AH in improving pregnancy outcomes.
Data Availability
Data supporting findings of this research are available from the corresponding author upon request.
Author Contributions
R. Dehghani Firouzabadi: Study design. R. Dehghani Firouzabadi, S. Arabian, N. Homayoon: Conducted the IVF procedures. F. Dehghanpour and E. Mangoli: Conducted embryonic procedures, N. Homayoon, S. Arabian, H. Barzegar, and S. Zare Garizi: Conducted data collection and analysis. All authors contributed to the literature review, assisted in drafting the manuscript, approved the final version, and took responsibility for the integrity of the data.
Acknowledgments
This research was supported by the Research and Clinical Center for Infertility at the Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (project no. 16601). The authors express sincere gratitude to the Yazd Reproductive Sciences Institute, Yazd, Iran and its staff for their support. We would also like to thank Dr. Farimah Shamsi for her invaluable statistical and methodological expertise. Artificial intelligence tools, including Google Bard (Gemini) 1.5 Pro and ChatGPT 4, were employed for grammar checking in specific sections.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




