Sun, Feb 1, 2026
[Archive]
Volume 23, Issue 7 (July 2025)
IJRM 2025, 23(7): 533-544 |
Back to browse issues page
Ethics code: R.ARAKMU.REC.1403.019
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soltani M, Shojafar E, Ghafarizadeh A A, Moslemi A, Jalali Mashayekhi F, Baazm M. Comparison of the effects of platelet-rich plasma and nanocurcumin on the sperm quality parameters in frozen-thawed semen of men with asthenoteratozoospermia: A lab trial study. IJRM 2025; 23 (7) :533-544
URL: http://ijrm.ir/article-1-3613-en.html
URL: http://ijrm.ir/article-1-3613-en.html
Mahsa Soltani1 

 , Elham Shojafar2
, Elham Shojafar2 

 , Ali Asghar Ghafarizadeh2
, Ali Asghar Ghafarizadeh2 

 , Azam Moslemi3
, Azam Moslemi3 

 , Farideh Jalali Mashayekhi4
, Farideh Jalali Mashayekhi4 

 , Maryam Baazm *5
, Maryam Baazm *5 




 , Elham Shojafar2
, Elham Shojafar2 

 , Ali Asghar Ghafarizadeh2
, Ali Asghar Ghafarizadeh2 

 , Azam Moslemi3
, Azam Moslemi3 

 , Farideh Jalali Mashayekhi4
, Farideh Jalali Mashayekhi4 

 , Maryam Baazm *5
, Maryam Baazm *5 


1- Students Research Committee, Arak University of Medical Sciences, Arak, Iran.
2- Rastak Infertility Treatment Center, Sina Hospital, Arak, Iran.
3- Department of Biostatistics, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
4- Department of Genetics and Biochemistry, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
5- Department of Anatomy, School of Medicine, Arak University of Medical Sciences, Arak, Iran. & Molecular and Medicine Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran. ,dr.baazm@arakmu.ac.ir
2- Rastak Infertility Treatment Center, Sina Hospital, Arak, Iran.
3- Department of Biostatistics, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
4- Department of Genetics and Biochemistry, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
5- Department of Anatomy, School of Medicine, Arak University of Medical Sciences, Arak, Iran. & Molecular and Medicine Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran. ,
Keywords: Cryopreservation, Spermatozoa, Asthenoteratozoospermia, Platelet-rich plasma, Nanocurcumin.
Full-Text [PDF 1757 kb]
(561 Downloads)
| Abstract (HTML) (649 Views)
Full-Text: (89 Views)
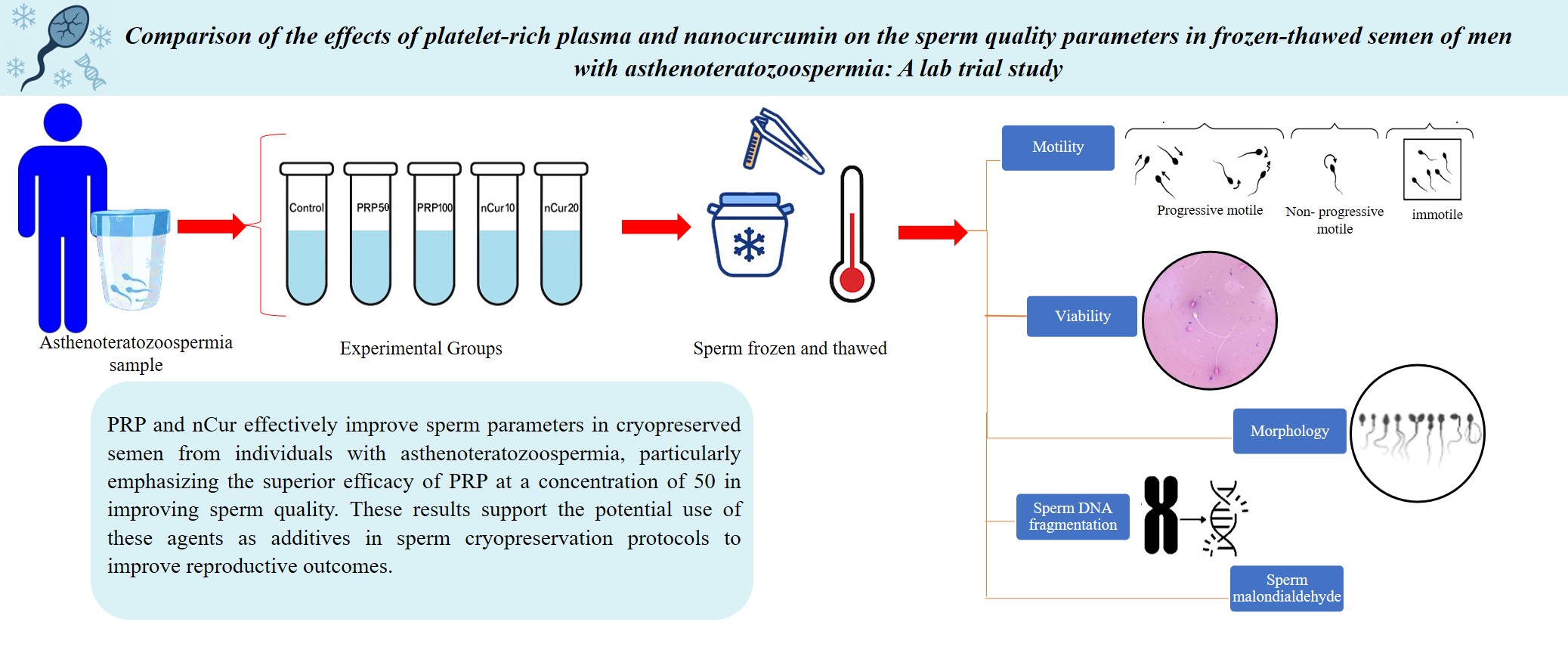
- Introduction
Sperm cryopreservation is a process that freezes sperm for long-term storage, enabling individuals to preserve their fertility for future use (1). This technique is beneficial in various diseases, such as cancers, autoimmune diseases, individuals with reproductive disorders, endangered animal protection, assisted reproduction, and research activities (2). Male infertility conditions such as oligozoospermia, azoospermia, teratozoospermia, asthenozoospermia, and asthenoteratozoospermia are also candidates for sperm cryopreservation (3). Despite the advantages of freezing, this method has some destructive effects on sperm motility, viability, and DNA integrity (2).
During spermatogenesis, mature sperm lose a significant amount of their cytoplasm, which reduces their antioxidant levels and makes them more susceptible to oxidative stress and damage (4). In contrast, cryopreservation disrupts the balance between reactive oxygen species (ROS) and antioxidant levels in sperm (5). Therefore, an increase in ROS production during the freezing process can negatively affect sperm parameters, induce DNA damage, and fragmentation. This underscores the importance of optimizing cryopreservation techniques to minimize oxidative stress, thereby preserving sperm quality and integrity for future use (6). To reduce the harmful effects of freezing on sperm, various strategies can be employed (2). These include incorporating enzymatic and non-enzymatic antioxidants into the cryopreservation medium (7), and using advanced cooling protocols that regulate the rate of temperature decline, thereby enhancing cell survival during thawing (8).
Platelet-rich plasma (PRP), a product of blood plasma with a higher platelet concentration, is currently used in various medical fields (9). The growth factors and cytokines found in PRP play a crucial role in stimulating cellular proliferation, angiogenesis, and collagen synthesis (10). This makes PRP a valuable tool in a variety of medical and cosmetic applications such as orthopedics, tissue regeneration, wound healing, scar regeneration, and skin rejuvenation (2). Some of these growth factors like platelet-derived growth factor, epidermal growth factor, insulin-like growth factor (IGF), and superoxide dismutase (SOD) are important factors for maintaining tissue and cell homeostasis, and have a positive impact on the quality of sperm cells (9).
Nanocurcumin (nCur), a novel form of curcumin (Cur), is recognized for its enhanced absorption and potential health benefits, including anti-inflammatory and antioxidant properties (11). Administration of nCur enhances sperm quality, particularly in models of oxidative stress induced by toxins like aluminium oxide (12), and varicocele (13). It can also improve total antioxidant capacity and reduce malondialdehyde (MDA) levels, indicating decreased oxidative damage (14). Cur has been effectively used in the cryopreservation of sperm from various species, including rams (15), fish (16), and rabbits (17). Studies have shown that it improves sperm motility and viability across these species.
Given the detrimental effects of cryo-injury on sperm quality during cryopreservation, and considering the beneficial properties of PRP and nCur, this study aimed to investigate the potential protective impacts of these 2 products on sperm parameters during the cryopreservation process of sperm in individuals with asthenoteratozoospermia.
2. Materials and Methods
During spermatogenesis, mature sperm lose a significant amount of their cytoplasm, which reduces their antioxidant levels and makes them more susceptible to oxidative stress and damage (4). In contrast, cryopreservation disrupts the balance between reactive oxygen species (ROS) and antioxidant levels in sperm (5). Therefore, an increase in ROS production during the freezing process can negatively affect sperm parameters, induce DNA damage, and fragmentation. This underscores the importance of optimizing cryopreservation techniques to minimize oxidative stress, thereby preserving sperm quality and integrity for future use (6). To reduce the harmful effects of freezing on sperm, various strategies can be employed (2). These include incorporating enzymatic and non-enzymatic antioxidants into the cryopreservation medium (7), and using advanced cooling protocols that regulate the rate of temperature decline, thereby enhancing cell survival during thawing (8).
Platelet-rich plasma (PRP), a product of blood plasma with a higher platelet concentration, is currently used in various medical fields (9). The growth factors and cytokines found in PRP play a crucial role in stimulating cellular proliferation, angiogenesis, and collagen synthesis (10). This makes PRP a valuable tool in a variety of medical and cosmetic applications such as orthopedics, tissue regeneration, wound healing, scar regeneration, and skin rejuvenation (2). Some of these growth factors like platelet-derived growth factor, epidermal growth factor, insulin-like growth factor (IGF), and superoxide dismutase (SOD) are important factors for maintaining tissue and cell homeostasis, and have a positive impact on the quality of sperm cells (9).
Nanocurcumin (nCur), a novel form of curcumin (Cur), is recognized for its enhanced absorption and potential health benefits, including anti-inflammatory and antioxidant properties (11). Administration of nCur enhances sperm quality, particularly in models of oxidative stress induced by toxins like aluminium oxide (12), and varicocele (13). It can also improve total antioxidant capacity and reduce malondialdehyde (MDA) levels, indicating decreased oxidative damage (14). Cur has been effectively used in the cryopreservation of sperm from various species, including rams (15), fish (16), and rabbits (17). Studies have shown that it improves sperm motility and viability across these species.
Given the detrimental effects of cryo-injury on sperm quality during cryopreservation, and considering the beneficial properties of PRP and nCur, this study aimed to investigate the potential protective impacts of these 2 products on sperm parameters during the cryopreservation process of sperm in individuals with asthenoteratozoospermia.
2. Materials and Methods
2.1. Study participants
In this lab trial study, semen samples of 20 men with asthenoteratospermia (17) who referred to Rastak and Ghavamzadeh Infertility Centers, Arak, Iran for infertility assessment from June-August 2024 were collected.
2.2. Sample size
In this lab trial study, semen samples of 20 men with asthenoteratospermia (17) who referred to Rastak and Ghavamzadeh Infertility Centers, Arak, Iran for infertility assessment from June-August 2024 were collected.
2.2. Sample size
The sample size was calculated by the following formula:
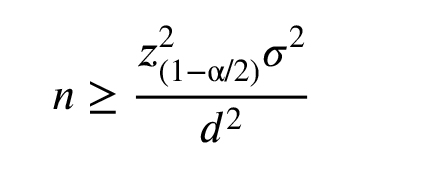
WhereZ
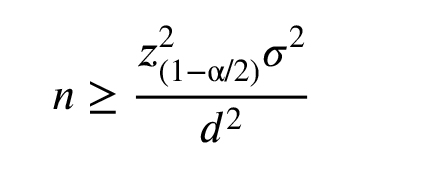
Where
Based on this formula, we needed at least 20 participants in each group.
2.3. Eligibility criteria
2.3. Eligibility criteria
Individuals with sperm motility ≤ 40% and sperm morphology ≤ 4% genitourinary were included in this study. They did not have a history of tract infections, azoospermia, severe oligospermia, leukocytospermia, cryptorchidism, varicocele, systemic diseases, hormonal disorders, obesity, alcohol consumption, diabetes, smoking, or substance abuse. The samples with higher motility and morphology were excluded from this study.
Semen samples were collected 3-5 days after the last ejaculation or sexual intercourse using the masturbation method, utilizing special non-toxic containers (2).
2.4. Semen sample preparation
Semen samples were collected 3-5 days after the last ejaculation or sexual intercourse using the masturbation method, utilizing special non-toxic containers (2).
2.4. Semen sample preparation
In this study, at first, the samples were incubated at 37°C for 30 min for liquification. Then, an equal volume of Ham's/F10 medium (HF10; BioIdea, Iran) was mixed with the semen and centrifuged at 3000 g for 6 min. After discarding the supernatant, 500 µl of HF10 was added to the pellet. The samples were then divided into 5 groups: control (no treatment), PRP50, PRP100, nCur10, and nCur20. Semen was gradually mixed with an equal volume of PRP or nCur, followed by the addition of sperm freezing medium (Cooper Surgical, Denmark) to the mixture. nCur (Exir Nano Sina, Iran) was added to each sample at concentrations of 10 µM (18) and 20 µM (12).
Cryovials containing the samples were positioned above the vapor of liquid nitrogen for 30 min at a height of 3-5 cm above the liquid nitrogen surface, after which they were immediately immersed in a liquid nitrogen tank. After 20 days, the cryovials were thawed in a water bath at 37°C. HF10, containing 10% human serum albumin (Kedrion SpA, Italy), was added to each sample and centrifuged at 300×g for 5 min. Following the removal of the supernatant, the resulting pellet was used to assess sperm parameters (2).
2.5. PRP preparation
Cryovials containing the samples were positioned above the vapor of liquid nitrogen for 30 min at a height of 3-5 cm above the liquid nitrogen surface, after which they were immediately immersed in a liquid nitrogen tank. After 20 days, the cryovials were thawed in a water bath at 37°C. HF10, containing 10% human serum albumin (Kedrion SpA, Italy), was added to each sample and centrifuged at 300×g for 5 min. Following the removal of the supernatant, the resulting pellet was used to assess sperm parameters (2).
2.5. PRP preparation
To prepare hemologous PRP, blood sample was taken from a volunteer, and the PRP kit (Rooyagen, Iran) was utilized following the manufacturer's instructions. Briefly, 1.5 ml of anticoagulant solution was added to 8.5 ml of blood and centrifuged at 1200 g for 10 min. The resulting supernatant was then centrifuged again at 3300×g for 6 min. The upper two-thirds of the platelet-free plasma were discarded, leaving the lower one-third, which contained the PRP. A cell analyzer was used to count platelets (in our case, it was 600,000 platelets/µl). By varying the volume of HF10 used to resuspend the pellet and adjusting centrifugation parameters, different PRP concentrations (ratios) were obtained (for PRP50, it was diluted 1:11 and for PRP100, it was diluted 1:5) to activate the PRP and release growth factors, the samples underwent freezing at -80°C followed by thawing at room temperature for 30 min before use (2).
2.6. Sperm parameters analysis: motility, count, morphology, and viability
2.6. Sperm parameters analysis: motility, count, morphology, and viability
After thawing each sample, the percentage of total motility, progressive and non-progressive sperm motility, and immotile sperm was evaluated based on WHO criteria and using a computer-assisted semen analysis device. For this analysis, 10 μl of the prepared sample was placed on a glass slide maintained at 37°C. Approximately 200 sperm from each sample was analyzed across 3 different microscopic fields, with results reported as a percentage. Sperm motility was classified into 3 categories: progressive motility, non-progressive motility, and immobile sperm (19). Eosin-Nigrosin staining was used for evaluating sperm viability. A smear was prepared on a glass slide, and after staining, the sperm were examined using light microscopy at x100 magnification (5).
The Diff-Quick staining kit (Ideh Varzan Farda, Iran) was used to evaluate the morphology of sperm. A smear was prepared from each sample on a glass slide and allowed to air-dry at room temperature. The staining procedure was then carried out according to the kit's instructions. Using a light microscope at x100 magnification, 200 sperm were examined per sample. The percentage of morphologically normal spermatozoa was reported (18).
2.7. Sperm DNA fragmentation index (DFI)
The Diff-Quick staining kit (Ideh Varzan Farda, Iran) was used to evaluate the morphology of sperm. A smear was prepared from each sample on a glass slide and allowed to air-dry at room temperature. The staining procedure was then carried out according to the kit's instructions. Using a light microscope at x100 magnification, 200 sperm were examined per sample. The percentage of morphologically normal spermatozoa was reported (18).
2.7. Sperm DNA fragmentation index (DFI)
Sperm DNA fragmentation was assessed using the sperm chromatin dispersion test with DFI kit (Ideh Varzan Farda, Iran). For this purpose, 50 μl of the semen sample was mixed with preheated low-melting agarose. Then, 25 μl of this mixture was carefully placed on a pre-coated slide provided in the kit. A coverslip was applied to evenly spread the sample. The slides were incubated at 4°C for 5 min. After incubation, the coverslip was then gently removed, and staining was performed according to the protocol. Using light microscopy at x100 magnification, 200 spermatozoa were examined per sample, and the size of the halo surrounding each sperm nucleus was assessed (10).
2.8. Sperm MDA levels analysis
2.8. Sperm MDA levels analysis
The levels of MDA were measured to assess the rate of lipid peroxidation in the frozen-thawed semen samples. MDA measurement was conducted using an MDA kit (Kushan Zist, Iran) according to the manufacture’s instruction. After thawing, the semen samples were lysed using sonication for 10 min, followed by centrifugation at 4000 g for 10 min, and the supernatant being used for the assay. The MDA level in each sample was determined by its color reaction with thiobarbituric acid reagent at 100oC for 1 hr, which its maximum absorbance is at 530 nm (20).
2.9. Ethical Considerations
2.9. Ethical Considerations
This study was approved by the Ethics Committee of Arak University of Medical Sciences, Arak, Iran (Code: IR.ARAKMU.REC.1403.019). Written informed consent was obtained from all participants. All data collected and analyzed in this study were handled in strict accordance with established confidentiality protocols. Access to personal and proprietary information was restricted to authorized research personnel only. Data were stored securely, with appropriate technical and organizational measures in place to prevent unauthorized access, disclosure, or misuse. Any identifying information was anonymized or de-identified prior to analysis to protect participant privacy. The study adhered to all relevant institutional and legal requirements for the protection of personal and sensitive data.
2.10. Statistical Analysis
All data were numerical and are expressed as mean ± SD. Prior to performing statistical comparisons, the normality of the data distribution was assessed using the Shapiro-Wilk test. Comparisons between groups were conducted using one-way analysis of variance (ANOVA). When the ANOVA indicated statistically significant differences, Tukey’s post-hoc test was applied for multiple pairwise comparisons. A p-value (p < 0.05) was considered statistically significant. All analyses were performed using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA).
3. Results
2.10. Statistical Analysis
All data were numerical and are expressed as mean ± SD. Prior to performing statistical comparisons, the normality of the data distribution was assessed using the Shapiro-Wilk test. Comparisons between groups were conducted using one-way analysis of variance (ANOVA). When the ANOVA indicated statistically significant differences, Tukey’s post-hoc test was applied for multiple pairwise comparisons. A p-value (p < 0.05) was considered statistically significant. All analyses were performed using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA).
3. Results
The participants were aged between 20 and 40 yr. The average semen volume was 3.1 ± 1.5 mL (mean ± SD), and the pH ranged from 7.2-7.8.
3.1. Effects of PRP and nCur on sperm parameters
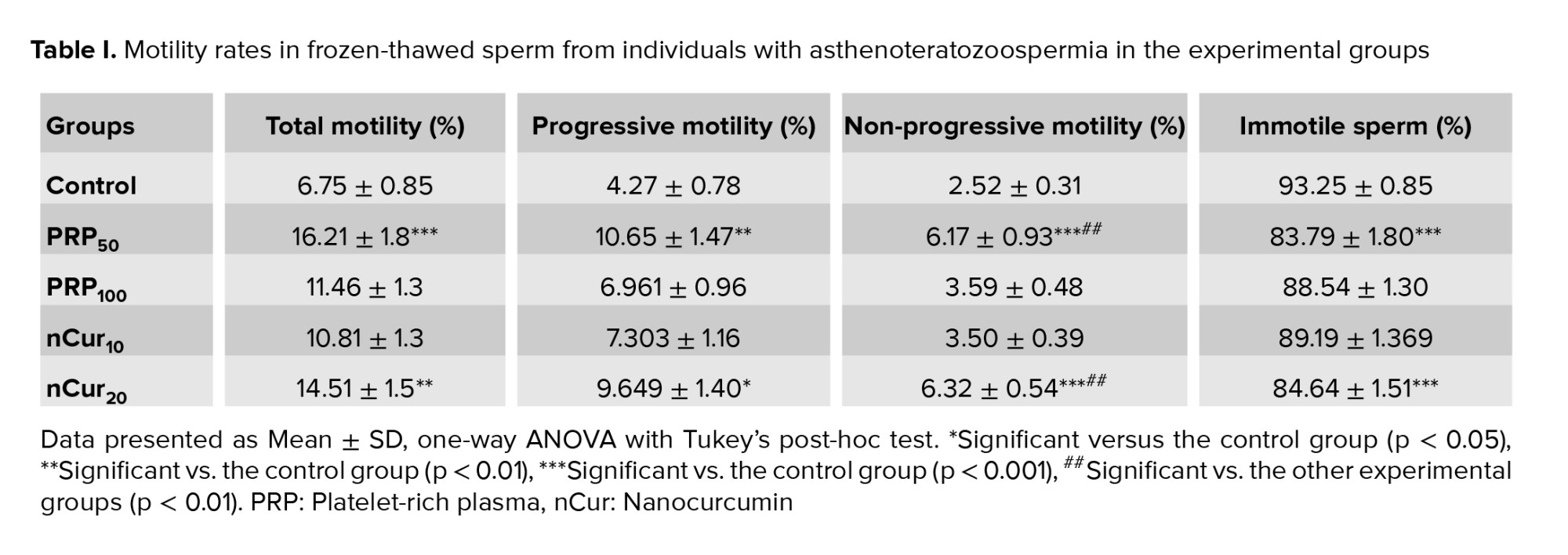
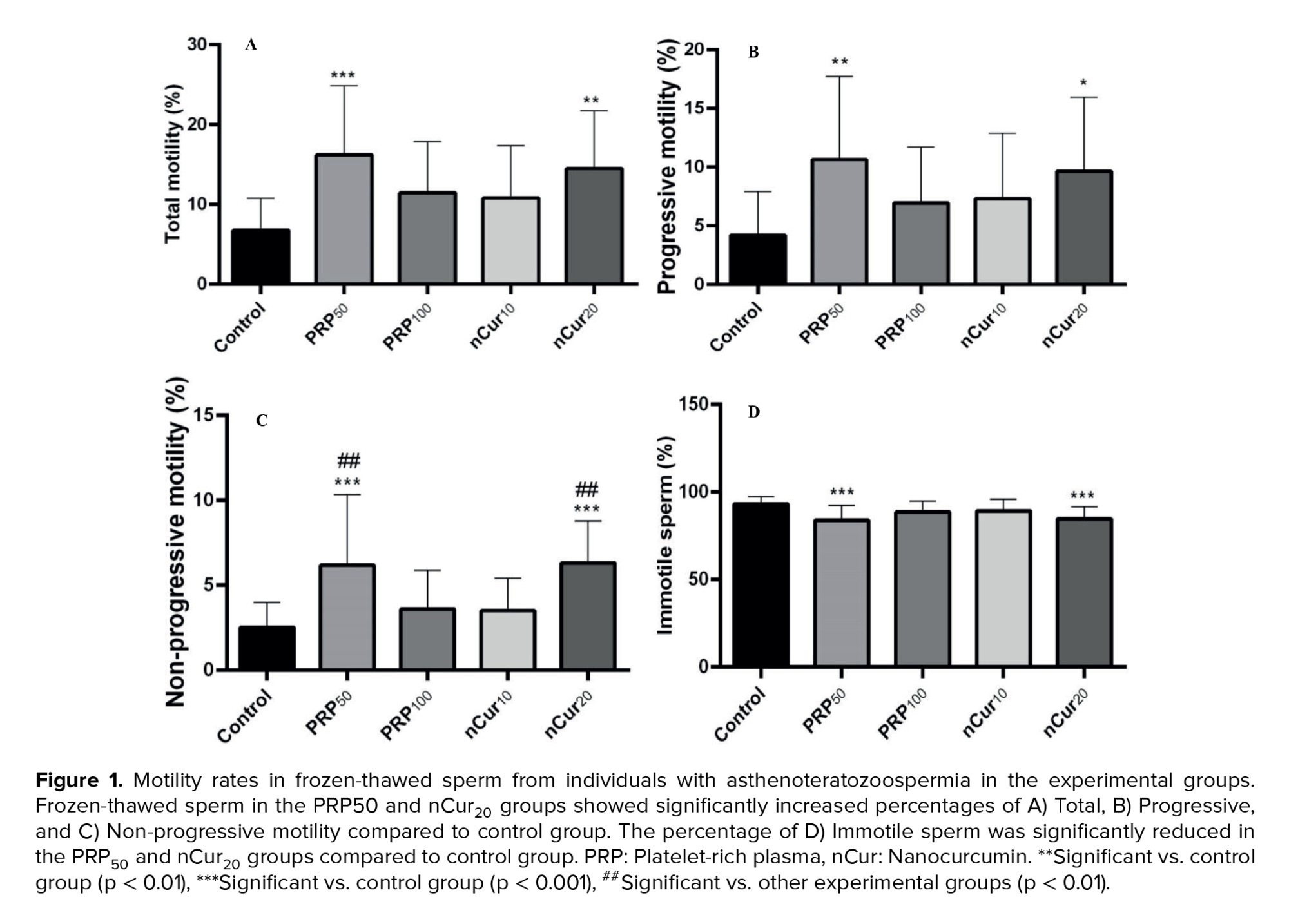
Our results indicated that the percentage of total sperm motility (all sperm that are moving, regardless of the direction or efficiency of their movement) (21), as well as progressive (swim in a forward direction, either in straight lines or large circles) (21) and non-progressive motility (sperm that exhibit movement but do not progress in a forward direction) (22), increased significantly in the PRP50 (p ˂ 0.001), and nCur20 (p = 0.001) groups compared to the control group. Additionally, the percentage of immotile sperm was significantly reduced in these experimental groups compared to the control group (p ˂ 0.001) (Figure 1A-D) (Table I).
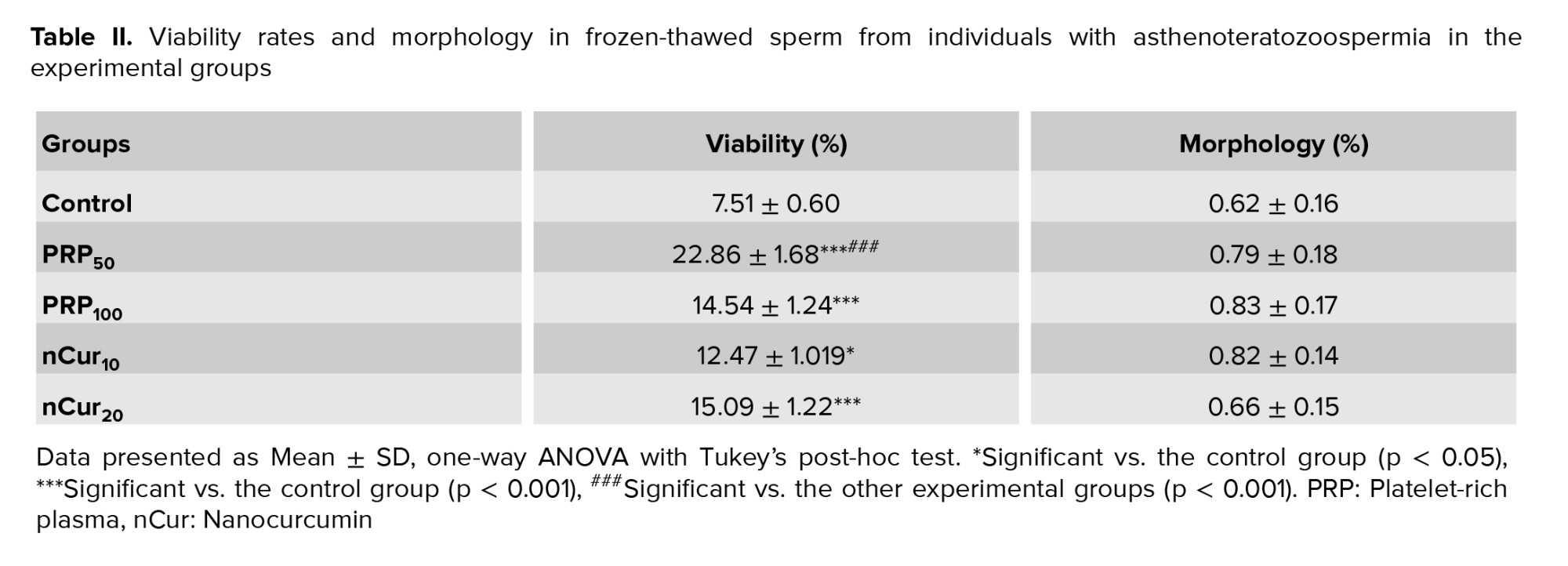
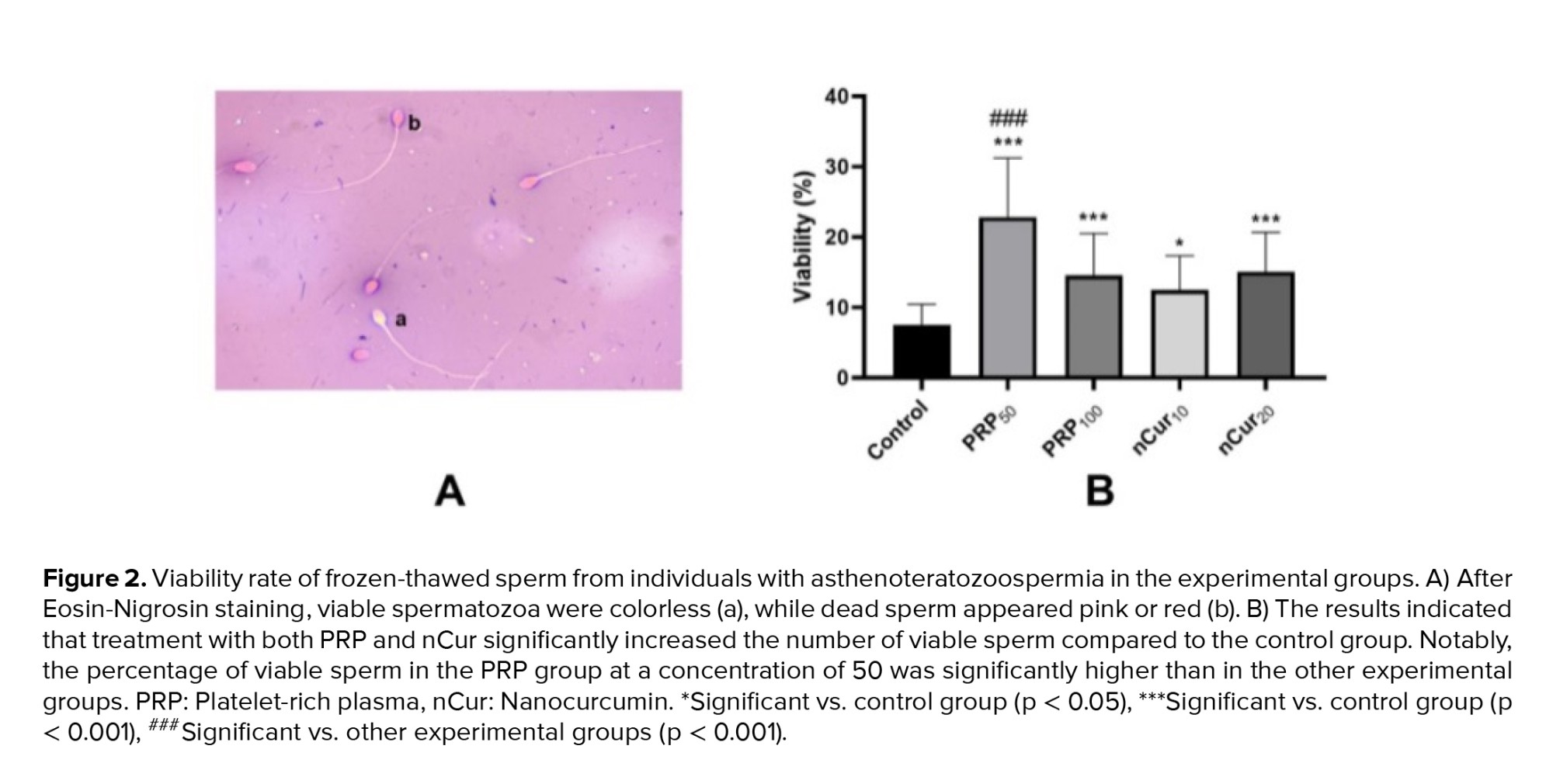
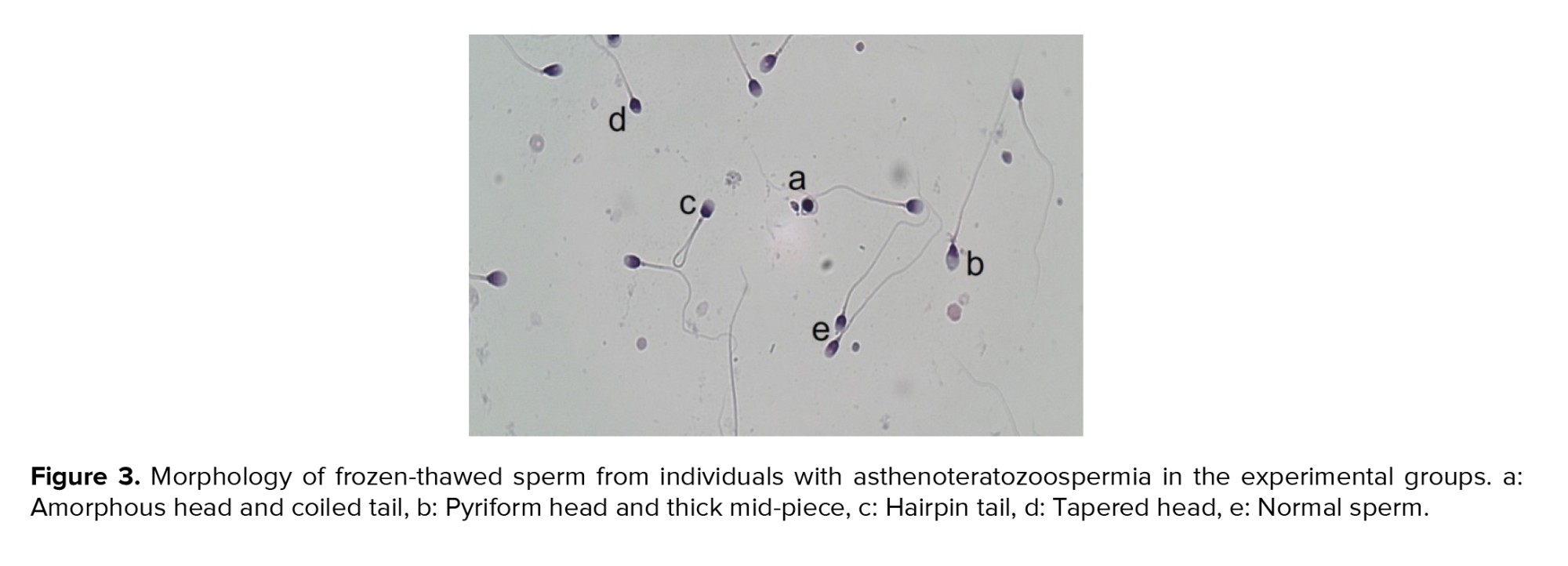
According to the kit, colorless spermatozoa were considered as viable, while pink or red color were counted as dead (Figure 2A). Compared to the control group, all treatment groups exhibited a significant increase in the number of viable sperm (p = 0.001). Notably, treatment with PRP at a concentration of 50 showed more pronounced effects compared to the other experimental groups (p < 0.001) (Figure 2B) (Table II). Our data revealed that PRP at concentrations of 50 (0.79 ± 0.18) and 100 (0.83 ± 0.17), as well as nCur at the concentrations of 10 (0.82 ± 0.14) and 20 (0.66 ± 0.15) did not improve sperm morphology following the freezing procedure (Figure 3) (Table II).
3.2. Effects of PRP and nCur on sperm DFI
3.1. Effects of PRP and nCur on sperm parameters
Our results indicated that the percentage of total sperm motility (all sperm that are moving, regardless of the direction or efficiency of their movement) (21), as well as progressive (swim in a forward direction, either in straight lines or large circles) (21) and non-progressive motility (sperm that exhibit movement but do not progress in a forward direction) (22), increased significantly in the PRP50 (p ˂ 0.001), and nCur20 (p = 0.001) groups compared to the control group. Additionally, the percentage of immotile sperm was significantly reduced in these experimental groups compared to the control group (p ˂ 0.001) (Figure 1A-D) (Table I).
According to the kit, colorless spermatozoa were considered as viable, while pink or red color were counted as dead (Figure 2A). Compared to the control group, all treatment groups exhibited a significant increase in the number of viable sperm (p = 0.001). Notably, treatment with PRP at a concentration of 50 showed more pronounced effects compared to the other experimental groups (p < 0.001) (Figure 2B) (Table II). Our data revealed that PRP at concentrations of 50 (0.79 ± 0.18) and 100 (0.83 ± 0.17), as well as nCur at the concentrations of 10 (0.82 ± 0.14) and 20 (0.66 ± 0.15) did not improve sperm morphology following the freezing procedure (Figure 3) (Table II).
3.2. Effects of PRP and nCur on sperm DFI
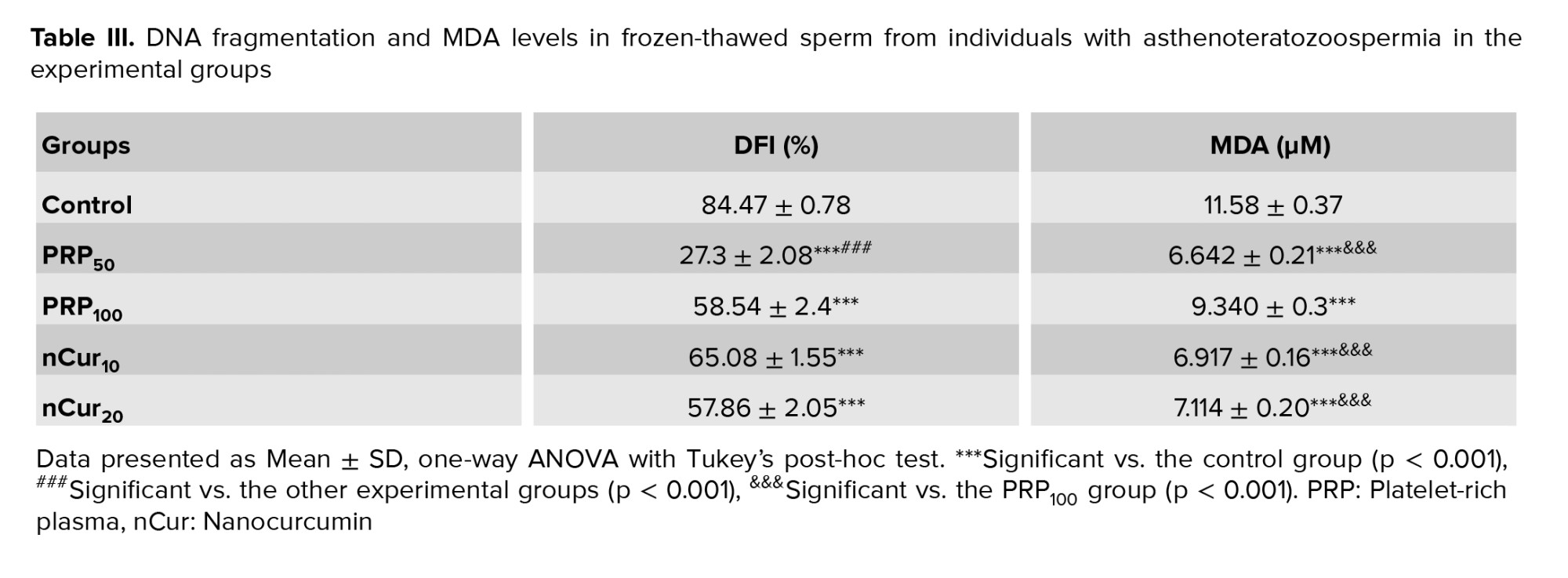
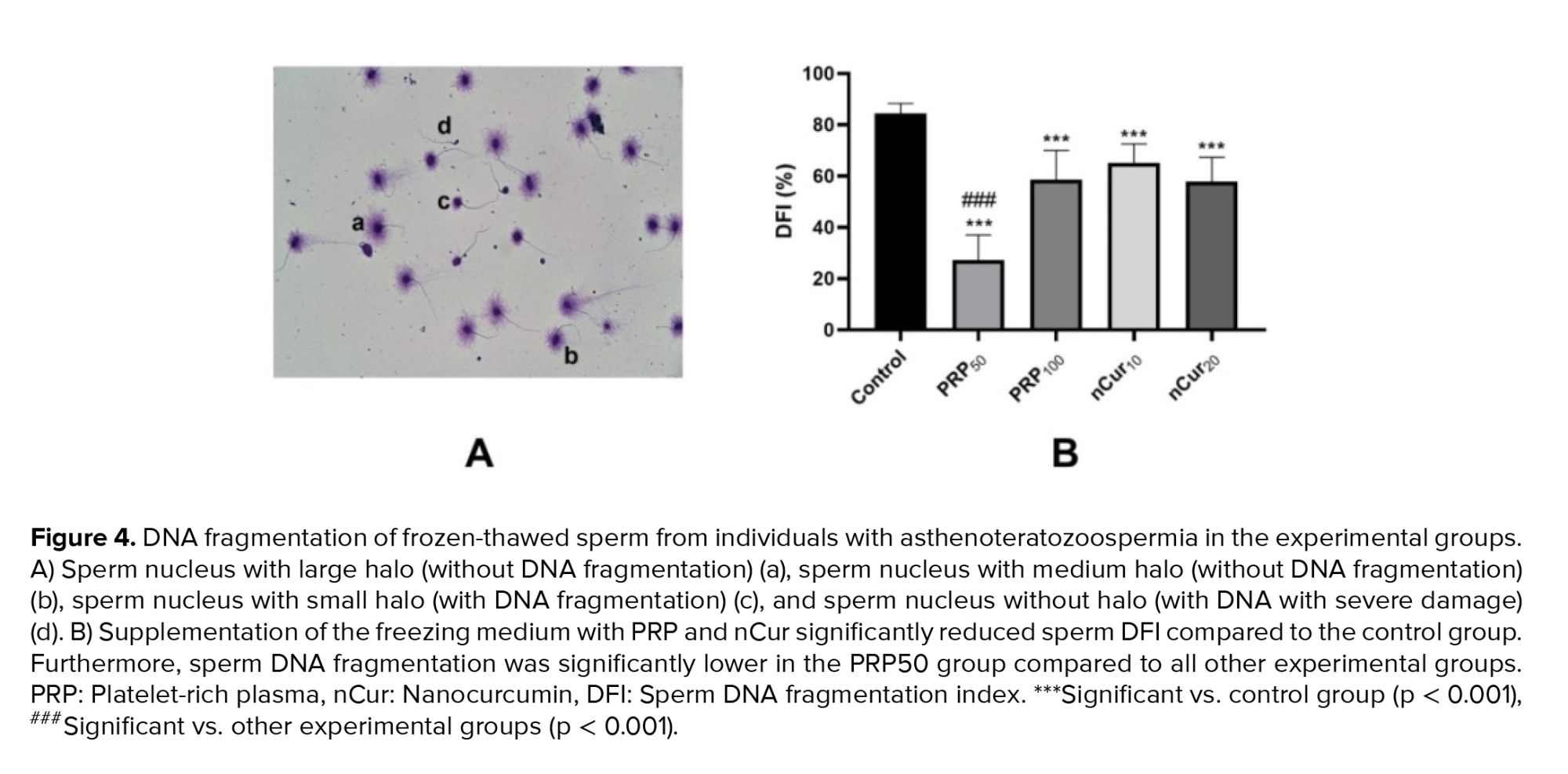
According to the kit, large or medium halos indicated intact DNA while small or absent halos signified DNA fragmentation (Figure 4A). Our data showed that supplementation of the freezing medium with PRP and nCur (all doses) significantly reduced DFI compared to the control group (p = 0.001). Among the treatment groups, PRP at a concentration of 50 was more effective in decreasing DFI than the other treatment groups (p < 0.001) (Figure 4B) (Table III).
3.3. Effects of PRP and nCur on sperm MDA level
3.3. Effects of PRP and nCur on sperm MDA level
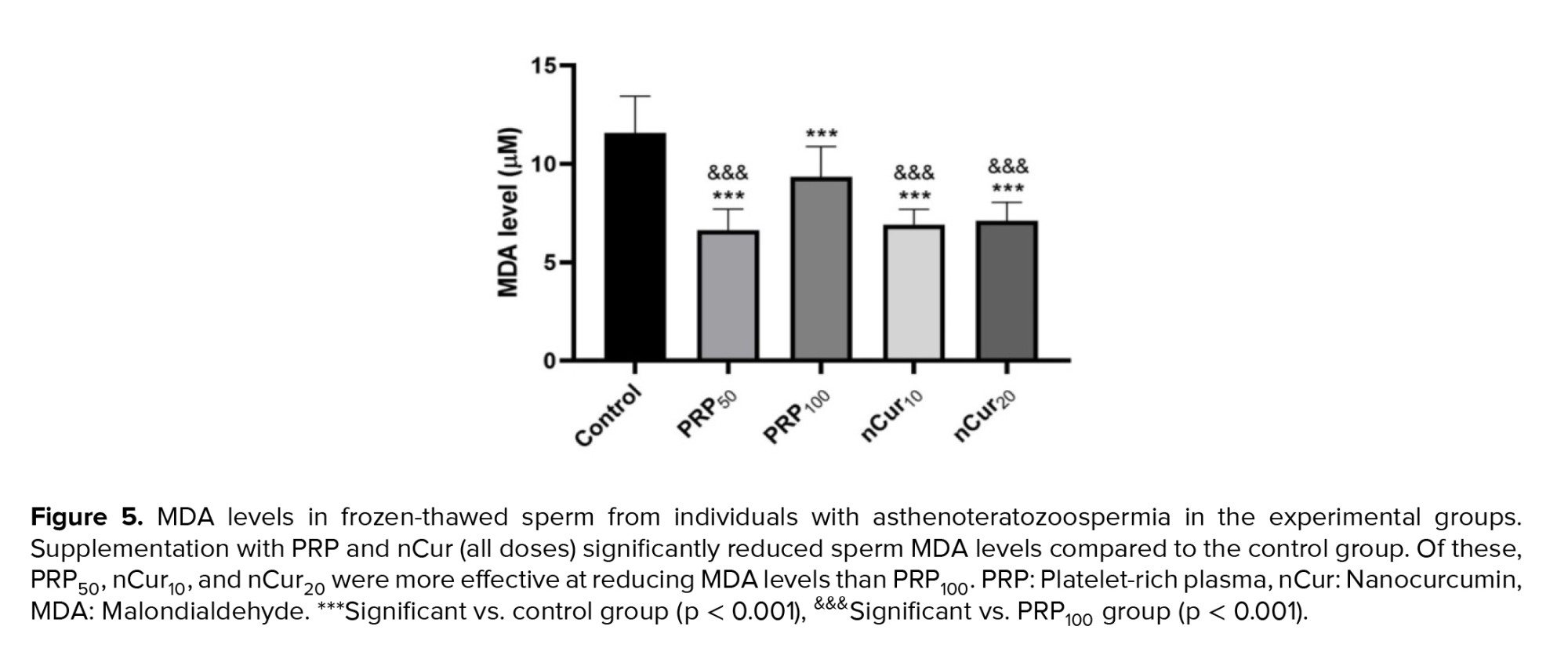
Our data showed that the use of PRP and nCur resulted in a significant decrease in MDA levels compared to the control group (p < 0.001). Among the treatment groups, PRP at the concentration of 50, along with nCur at 10 and 20, demonstrated a greater reduction in MDA levels than the PRP100 group (p < 0.001) (Figure 5) (Table III).
4. Discussion








4. Discussion
In the present study, we demonstrated that both PRP and nCur improved sperm parameters in cryopreserved sperm from individuals with asthenoteratozoospermia in a dose-dependent manner. Furthermore, PRP at lower concentrations produced better results than nCur.
Asthenoteratozoospermia is one of the causes of male infertility, and cryopreservation can be employed as a technique to manage and preserve fertility in these individuals. Despite significant advancements in cryopreservation techniques, this process inevitably results in varying degrees of damage. This includes plasma membrane disruption, DNA fragmentation, mitochondrial dysfunction, reduced motility and morphology, and increased apoptosis-all of which significantly affect sperm fertilization capacity (7).
Our data revealed that following freezing, the control (no treatment) group showed a more pronounced decline in sperm quality compared to treatment groups, while both DNA fragmentation and MDA levels increased.
Freezing and thawing processes elevate the levels of free radicals. Among the most significant ROS generated are superoxide anions, which are quickly converted into hydrogen peroxide and oxygen by the SOD enzyme (23). Hydrogen peroxide is toxic to cells because it can cross cell membranes and generate hydroxyl radicals. These hydroxyl radicals, with their potent oxidizing properties, can cause substantial damage to biological systems and surrounding molecules (5). Elevated levels of hydrogen peroxide can inactivate the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, resulting in decreased ATP production and, consequently, reduced sperm motility (1). In this study, we aimed to mitigate the destructive effects of freezing on sperm quality by adding heterologous PRP, which is rich in platelets and growth factors, or nCur as an antioxidant to the freezing media. We then compared the effects of these products on sperm parameters, MDA levels, and DNA fragmentation. The concentrations of PRP used in this study were based on prior research demonstrating beneficial effects on sperm quality. Specifically, mixing sperm with autologous PRP at a concentration of 100×10³/µl improved sperm parameters in oligoasthenoteratozoospermic samples (23). Similarly, heterologous PRP at the same concentration enhanced sperm quality in healthy men (2). Therefore, we selected PRP doses around this concentration to evaluate their effects in our experimental setup. Regarding nCur, we supplemented the freezing medium with 10 µM and 20 µM concentrations. These doses were chosen based on previous studies investigating the antioxidant and protective effects of curcumin derivatives on sperm and reproductive cells, which reported efficacy within this concentration range (11, 18). By selecting these doses, we aimed to explore the potential cryoprotective effects of nCur while maintaining consistency with established literature.
Our data revealed that adding heterologous PRP at a concentration of 50 and nCur at a concentration of 20 μM to the freezing medium produced similar effects in increasing total, progressive, and non-progressive sperm motility, while decreasing the percentage of immotile sperm. Additionally, all treatment groups demonstrated similar effects in increasing viability and decreasing DNA fragmentation; however, PRP at a concentration of 50 exhibited the best results. All treatment groups also showed comparable outcomes in reducing MDA levels compared to the control group, but the nCur groups and the PRP50 group outperformed the PRP100 group.
PRP contains a high concentration of growth factors and possesses antioxidant properties, allowing it to protect sperm from the harmful effects of cryopreservation. These characteristics play a direct role in enhancing sperm quality (10). The antioxidant capacity of PRP is related to zinc ions and SOD, which exhibit potent antioxidant effects and the ability to scavenge ROS (24). In addition, PRP contains various growth factors, including fibroblast growth factor, nerve growth factor, vascular endothelial growth factor, insulin-like growth factor-1 (IGF-I), and brain-derived neurotrophic factor. Fibroblast growth factor can activate specific receptors on sperm flagella, triggering an intracellular signaling cascade. This cascade enhances the activity of enzymes such as extracellular signal-regulated kinases and protein kinase B, ultimately leading to increased progressive sperm motility (4). Nerve growth factor, by activating specific receptors in the midpiece of sperm, has a significant impact on sperm viability and motility (24). Vascular endothelial growth factor is significantly present in spermatids, seminal plasma, and in Sertoli and Leydig cells (10). It helps in maintaining sperm motility by reducing oxidative stress through the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (2). IGF-I significantly enhances the integrity of the sperm plasma membrane, acrosomal membrane, and human sperm DNA stability, and by increasing intracellular calcium ion concentrations and improving calcium flux, IGF-I also enhances progressive sperm motility (9). Brain-derived neurotrophic factor, by activating cellular signaling pathways such as phosphatidylinositol 3-kinase/protein Kinase B signaling pathway, plays a crucial role in maintaining sperm integrity and function. This factor enhances the expression of anti-apoptotic proteins and reduces caspase activity, thereby preventing sperm cell death and improving sperm quality (25).
In this study, we added either 10 µM or 20 µM of nCur to the freezing medium. Our results indicated that the higher concentration had a more positive effect on sperm quality. Studies have shown that Cur can improve sperm quality by reducing oxidative stress and increasing the activity of antioxidant enzymes (12). Cur neutralizes harmful free radicals through its phenolic, beta-diketone, and methoxy functional groups (16). Moreover, the primary mechanism by which Cur acts on sperm is through the activation of the Nrf2 pathway. Nrf2 is a transcription factor that plays a crucial role in the cellular response to oxidative stress (12). We demonstrated that Cur can mitigate the harmful effects of cryptorchidism on testicular tissue by activating the Nrf2 pathway. This activation is essential for enhancing the cellular response to oxidative stress, thereby protecting testicular integrity and function (26). As a soluble form of Cur, nCur maintains its biological properties, which include anti-inflammatory, antioxidant, and anticancer effects (27). In our previous work, we utilized nCur to decrease inflammasome activity in a testicular torsion model (28). Sadraei et al. showed that the use of nCur in a varicocele rat model demonstrated better results in improving sperm quality compared to Cur (13).
Our data showed no significant improvement in sperm morphology following treatment with PRP and nCur. This lack of improvement may be related to the low quality of sperm in cases of asthenoteratospermia (1). The current study demonstrated that the low concentration of PRP is more effective in protecting sperm against freezing compared to higher concentrations of PRP. This may be due to elevated levels of amino acids in the high-concentration PRP (24), which can increase osmotic pressure and hypertonicity, thereby negatively affecting sperm membrane stability and motility during cryopreservation (29). While studies using homologous PRP report benefits at higher concentrations (e.g., 100) (1), our heterologous PRP showed optimal results at a concentration of 50. This difference likely stems from species-specific protein interactions: homologous PRP, benefiting from evolutionary compatibility, can be safely used at higher doses, whereas heterologous PRP carries dose-dependent risks such as immune-mediated membrane damage or growth factor toxicity. Therefore, both the source and concentration of PRP must be carefully tailored to ensure biological compatibility (30).
Our results showed similar effects of nCur at concentration of 20 μM compared to PRP; however, PRP was the best. Since higher concentration of nCur have shown better results compared to lower concentration, it is possible that using an even higher concentration could produce effects similar to those of PRP, especially considering the ease of use and affordability of nCur. However, further research is necessary in this area, particularly to evaluate its toxicity.
5. Conclusion
Asthenoteratozoospermia is one of the causes of male infertility, and cryopreservation can be employed as a technique to manage and preserve fertility in these individuals. Despite significant advancements in cryopreservation techniques, this process inevitably results in varying degrees of damage. This includes plasma membrane disruption, DNA fragmentation, mitochondrial dysfunction, reduced motility and morphology, and increased apoptosis-all of which significantly affect sperm fertilization capacity (7).
Our data revealed that following freezing, the control (no treatment) group showed a more pronounced decline in sperm quality compared to treatment groups, while both DNA fragmentation and MDA levels increased.
Freezing and thawing processes elevate the levels of free radicals. Among the most significant ROS generated are superoxide anions, which are quickly converted into hydrogen peroxide and oxygen by the SOD enzyme (23). Hydrogen peroxide is toxic to cells because it can cross cell membranes and generate hydroxyl radicals. These hydroxyl radicals, with their potent oxidizing properties, can cause substantial damage to biological systems and surrounding molecules (5). Elevated levels of hydrogen peroxide can inactivate the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, resulting in decreased ATP production and, consequently, reduced sperm motility (1). In this study, we aimed to mitigate the destructive effects of freezing on sperm quality by adding heterologous PRP, which is rich in platelets and growth factors, or nCur as an antioxidant to the freezing media. We then compared the effects of these products on sperm parameters, MDA levels, and DNA fragmentation. The concentrations of PRP used in this study were based on prior research demonstrating beneficial effects on sperm quality. Specifically, mixing sperm with autologous PRP at a concentration of 100×10³/µl improved sperm parameters in oligoasthenoteratozoospermic samples (23). Similarly, heterologous PRP at the same concentration enhanced sperm quality in healthy men (2). Therefore, we selected PRP doses around this concentration to evaluate their effects in our experimental setup. Regarding nCur, we supplemented the freezing medium with 10 µM and 20 µM concentrations. These doses were chosen based on previous studies investigating the antioxidant and protective effects of curcumin derivatives on sperm and reproductive cells, which reported efficacy within this concentration range (11, 18). By selecting these doses, we aimed to explore the potential cryoprotective effects of nCur while maintaining consistency with established literature.
Our data revealed that adding heterologous PRP at a concentration of 50 and nCur at a concentration of 20 μM to the freezing medium produced similar effects in increasing total, progressive, and non-progressive sperm motility, while decreasing the percentage of immotile sperm. Additionally, all treatment groups demonstrated similar effects in increasing viability and decreasing DNA fragmentation; however, PRP at a concentration of 50 exhibited the best results. All treatment groups also showed comparable outcomes in reducing MDA levels compared to the control group, but the nCur groups and the PRP50 group outperformed the PRP100 group.
PRP contains a high concentration of growth factors and possesses antioxidant properties, allowing it to protect sperm from the harmful effects of cryopreservation. These characteristics play a direct role in enhancing sperm quality (10). The antioxidant capacity of PRP is related to zinc ions and SOD, which exhibit potent antioxidant effects and the ability to scavenge ROS (24). In addition, PRP contains various growth factors, including fibroblast growth factor, nerve growth factor, vascular endothelial growth factor, insulin-like growth factor-1 (IGF-I), and brain-derived neurotrophic factor. Fibroblast growth factor can activate specific receptors on sperm flagella, triggering an intracellular signaling cascade. This cascade enhances the activity of enzymes such as extracellular signal-regulated kinases and protein kinase B, ultimately leading to increased progressive sperm motility (4). Nerve growth factor, by activating specific receptors in the midpiece of sperm, has a significant impact on sperm viability and motility (24). Vascular endothelial growth factor is significantly present in spermatids, seminal plasma, and in Sertoli and Leydig cells (10). It helps in maintaining sperm motility by reducing oxidative stress through the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (2). IGF-I significantly enhances the integrity of the sperm plasma membrane, acrosomal membrane, and human sperm DNA stability, and by increasing intracellular calcium ion concentrations and improving calcium flux, IGF-I also enhances progressive sperm motility (9). Brain-derived neurotrophic factor, by activating cellular signaling pathways such as phosphatidylinositol 3-kinase/protein Kinase B signaling pathway, plays a crucial role in maintaining sperm integrity and function. This factor enhances the expression of anti-apoptotic proteins and reduces caspase activity, thereby preventing sperm cell death and improving sperm quality (25).
In this study, we added either 10 µM or 20 µM of nCur to the freezing medium. Our results indicated that the higher concentration had a more positive effect on sperm quality. Studies have shown that Cur can improve sperm quality by reducing oxidative stress and increasing the activity of antioxidant enzymes (12). Cur neutralizes harmful free radicals through its phenolic, beta-diketone, and methoxy functional groups (16). Moreover, the primary mechanism by which Cur acts on sperm is through the activation of the Nrf2 pathway. Nrf2 is a transcription factor that plays a crucial role in the cellular response to oxidative stress (12). We demonstrated that Cur can mitigate the harmful effects of cryptorchidism on testicular tissue by activating the Nrf2 pathway. This activation is essential for enhancing the cellular response to oxidative stress, thereby protecting testicular integrity and function (26). As a soluble form of Cur, nCur maintains its biological properties, which include anti-inflammatory, antioxidant, and anticancer effects (27). In our previous work, we utilized nCur to decrease inflammasome activity in a testicular torsion model (28). Sadraei et al. showed that the use of nCur in a varicocele rat model demonstrated better results in improving sperm quality compared to Cur (13).
Our data showed no significant improvement in sperm morphology following treatment with PRP and nCur. This lack of improvement may be related to the low quality of sperm in cases of asthenoteratospermia (1). The current study demonstrated that the low concentration of PRP is more effective in protecting sperm against freezing compared to higher concentrations of PRP. This may be due to elevated levels of amino acids in the high-concentration PRP (24), which can increase osmotic pressure and hypertonicity, thereby negatively affecting sperm membrane stability and motility during cryopreservation (29). While studies using homologous PRP report benefits at higher concentrations (e.g., 100) (1), our heterologous PRP showed optimal results at a concentration of 50. This difference likely stems from species-specific protein interactions: homologous PRP, benefiting from evolutionary compatibility, can be safely used at higher doses, whereas heterologous PRP carries dose-dependent risks such as immune-mediated membrane damage or growth factor toxicity. Therefore, both the source and concentration of PRP must be carefully tailored to ensure biological compatibility (30).
Our results showed similar effects of nCur at concentration of 20 μM compared to PRP; however, PRP was the best. Since higher concentration of nCur have shown better results compared to lower concentration, it is possible that using an even higher concentration could produce effects similar to those of PRP, especially considering the ease of use and affordability of nCur. However, further research is necessary in this area, particularly to evaluate its toxicity.
5. Conclusion
This study demonstrated that both heterologous PRP and nCur can preserve sperm quality when added to the sperm freezing medium. Among the various concentrations tested, PRP at a concentration of 50 and nCur at 20 showed the most significant cryoprotective effects, with PRP yielding the best results overall. It is important to consider that the application of PRP and nCur as cryoprotectants in the freezing process can help to prevent further deterioration of sperm quality in asthenoteratozoospermia samples.
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author Contributions
E. Shojafar, AA. Ghafarizadeh, and M. Baazm had complete access to all data used in the study and took responsibility for the integrity of the data and accuracy of the data analysis, including the conceptualization and design of the study. M. Soltani, A. Moslemi, and F. Jalali Mashayekhi were responsible for the acquisition, analysis, and interpretation of the data. M. Soltani, A. Moslemi, and M. Baazm drafted the manuscript and conducted statistical analysis. All authors contributed to the critical revision of the manuscript for significant intellectual content and approved the final manuscript and took responsibility for the integrity of the data.
Acknowledgments
The authors thank the staff of Rastak and Ghavamzadeh Infertility Centers, Arak, Iran and participants of this study for their important contributions. This study was granted by the Council of Arak University of Medical Sciences, Arak, Iran (grant number: 7313 and grant number: 4615). Artificial intelligence was not used to prepare the manuscript in any way (translation, revision, grammar check, etc.).
Conflict of Interest
The authors declare that there is no conflict of interest.
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author Contributions
E. Shojafar, AA. Ghafarizadeh, and M. Baazm had complete access to all data used in the study and took responsibility for the integrity of the data and accuracy of the data analysis, including the conceptualization and design of the study. M. Soltani, A. Moslemi, and F. Jalali Mashayekhi were responsible for the acquisition, analysis, and interpretation of the data. M. Soltani, A. Moslemi, and M. Baazm drafted the manuscript and conducted statistical analysis. All authors contributed to the critical revision of the manuscript for significant intellectual content and approved the final manuscript and took responsibility for the integrity of the data.
Acknowledgments
The authors thank the staff of Rastak and Ghavamzadeh Infertility Centers, Arak, Iran and participants of this study for their important contributions. This study was granted by the Council of Arak University of Medical Sciences, Arak, Iran (grant number: 7313 and grant number: 4615). Artificial intelligence was not used to prepare the manuscript in any way (translation, revision, grammar check, etc.).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |