Sun, Feb 1, 2026
[Archive]
Volume 5, Issue 4 (7-2007)
IJRM 2007, 5(4): 89-93 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadzadeh A, Heidari M, Soltan Ghoraiee H, Ghaffari Novin M, Jeddi-Tehrani M, Akhondi M M, et al . The effect of pentoxifylline on the growth of endometrial implants and leukocytes in rats.. IJRM 2007; 5 (4) :89-93
URL: http://ijrm.ir/article-1-82-en.html
URL: http://ijrm.ir/article-1-82-en.html
Afsaneh Mohammadzadeh *1 
 , Mahnaz Heidari2
, Mahnaz Heidari2 
 , Haleh Soltan Ghoraiee3
, Haleh Soltan Ghoraiee3 
 , Marefat Ghaffari Novin4
, Marefat Ghaffari Novin4 
 , Mahmood Jeddi-Tehrani5
, Mahmood Jeddi-Tehrani5 
 , Mohammad Mahdi Akhondi5
, Mohammad Mahdi Akhondi5 
 , Hojjat Zeraati5
, Hojjat Zeraati5 
 , Farzaneh Mohammadzadeh6
, Farzaneh Mohammadzadeh6 
 , Pegah Ebadi4
, Pegah Ebadi4 


 , Mahnaz Heidari2
, Mahnaz Heidari2 
 , Haleh Soltan Ghoraiee3
, Haleh Soltan Ghoraiee3 
 , Marefat Ghaffari Novin4
, Marefat Ghaffari Novin4 
 , Mahmood Jeddi-Tehrani5
, Mahmood Jeddi-Tehrani5 
 , Mohammad Mahdi Akhondi5
, Mohammad Mahdi Akhondi5 
 , Hojjat Zeraati5
, Hojjat Zeraati5 
 , Farzaneh Mohammadzadeh6
, Farzaneh Mohammadzadeh6 
 , Pegah Ebadi4
, Pegah Ebadi4 

1- Department of Reproductive Endocrinology and Embryology, Reproductive Biotechnology Research Center, Avesina Research Institute, ACECR, Tehran, Iran , af23mohammadzadeh@yahoo.com
2- Departmet of Nanotechnology , Nanobiotechnology Research Center , Avesina Research Institute, ACECR, Tehran, Iran
3- Department of Reproductive and Biotechnology Genetic, Reproductive Biotechnology Research Center, Avesina Research Institute ,ACECR, Tehran, Iran
4- Department of Reproductive Endocrinology and Embryology, Reproductive Biotechnology Research Center, Avesina Research Institute, ACECR, Tehran, Iran
5- Department of Monoclonal Antibody, Monoclonal Antibody Research Center , Avesina Research Institute ,ACERCR, Tehran, Iran
6- Anatomy Department, Iran University of Medical Science, Tehran, Iran
2- Departmet of Nanotechnology , Nanobiotechnology Research Center , Avesina Research Institute, ACECR, Tehran, Iran
3- Department of Reproductive and Biotechnology Genetic, Reproductive Biotechnology Research Center, Avesina Research Institute ,ACECR, Tehran, Iran
4- Department of Reproductive Endocrinology and Embryology, Reproductive Biotechnology Research Center, Avesina Research Institute, ACECR, Tehran, Iran
5- Department of Monoclonal Antibody, Monoclonal Antibody Research Center , Avesina Research Institute ,ACERCR, Tehran, Iran
6- Anatomy Department, Iran University of Medical Science, Tehran, Iran
Full-Text [PDF 92 kb]
(653 Downloads)
| Abstract (HTML) (3922 Views)
Full-Text: (406 Views)
Introduction
Endometriosis is defined as ectopic growth of endometrial stroma and glandular tissues (1,2). Although nearly three quarters of century have been passed since the initial description of endometriosis (3), our current understanding of the etiology and pathphysiology of the disease has still been remained unclear (1-4). After the first description of the disease by Sampson in 1927 several different hypotheses have been suggested to explain the mechanisms for the development of endometriosis (4,5). However, Sampson’s theory of retrograde menstruation has gained most supportive evidence (4, 5). Retrograde menstruation is the reflux of menses through fallopian tubes toward the ectopic sites especially in peritoneal cavity. There are some reports about endometriosis in surgical incision in women who have tolerated operation especially cesarean section. It seems it is due to direct implantation of viable endometrial cells into incision (4,5). These findings support Sampson's theory as well (4, 5). In addition,changes in cell-mediated immunity related with endometriosis have been reported in women, but the relevant data are inconsistent (2-5). A reduction in proliferation of peripheral blood lymphocytes in response to recognition of endometrial antigens and cells has also been reported (2-6).
It was shown that the ratio of T-helper to T-suppressor cells is increased in the peripheral blood of the affected women; on the contrary other studies have indicated no remarkable differences in peripheral lymphocytes profiles (4-7).On the other hand, peritoneal macrophages have revealed an increase in total number, concentration, and activation status (8).
Tumor necrosis factor-alpha (TNF-a) is regarded as one of the cytokines that has been gained recent attention in the pathphysiology of autoimmune diseases (9). TNF-a is a major product of activated macrophages. It can activate the inflammatory leukocytes which can lead to the production of other pro inflammatory cytokines, including interleukin-1 (IL-1), IL-6, and additional TNF-a (9). TNF-a can stimulate endometrial cell adhesion, as well as, induce matrix metalloproteinases (MMPs) expression, both of which are necessary events in the initial development of endometriosis (10, 11).
Considering above mention facts, TNF-a has an important role in endometriosis, therefore anti TNF-a drugs may be proper drug for this disease. PX reduces both the production and action of cytokines such as TNF-a through the elevation of intracellular cyclic AMP levels and subsequent down regulation of cytokine production and action (4, 5, 12).
PX is a member of Methylgezantine family which inhibits Phosphodiesterase in platlets and leads to the elevation of CAMP levels in them and decreases the aggregation of them in circulation. Therefore, it is useful in cerebrovascular diseases (4, 5,13). PX can inhibit phosphodiesterase in monocytes and macrophages and suppress cytokineproduction specially TNF-a in macrophages (4,5,12,13).
In other immunological disease like sever refractory rheumatoid arthritis (14), inflammatory bowel disease (15), and recurrent aphthous stomatitis (16), PX had a suppressive effect on these diseases .Therefore , it seems that anti TNF-a effect of this drug can be useful in treatment of endometriosis. Based on several studies, rats are good model for the study on endometriosis. They don’t have menstrual cycles and endometriosis isn't induced in them spontaneously (17,18).
Steinleitner et al (1991) induced endometriosis in female rats surgically and mentioned PX could increase fertility rates in comparison with normal saline (90% vs 2.3 %). They suggested that PX could raise fertility rates in endometriosis in rats (19). Nothnick et al (1994) reported that PX could modulate rat endometriotic implant growth and produce implant- specific proteins (ENDO-1& ENDO-2) (20).
Balach et al (1997) reported that PX versus placebo could improve fertility rates in women but not significantly (21). Although in animal studies (19, 20), PX could have a good effect on endometriosis, but the human studies couldn’t be able to show the significant changes in treated group with PX (21, 22).
In order to determine the effect of PX on endometriosis, we decided to do our study in female rats. In this study, we induced endometriosis in female rats surgically and evaluated the effect of PX versus placebo on the endometriosis.
The aims of this study were investigating : 1) The effect of PX on the growth of endometrial implants in female rats especially in different places. 2)The effect of PX on leukocyte count in serum, in order to determine the immune response with this drug.
Materials and methods
Our randomized clinical trial study was done on 20 female rats’ sparauge- dwley (2 months old). This study was performed in Avesina Research Institute under support of Iranian Academic Center for Education, Culture and Research (ACECR). The rats were housed individually in hanging cages. The facility was maintained on a 14-h light, 10-h darkness schedule. Female rats have 4- 5 days estrous cycles (24). For evaluation of phases in female rats we did vaginal Pap smear daily (25). Only those rats exhibited normal 4-5 day estrous cycle were subjected to surgical induction of endometriosis. All of the rats had a file that the processes were written in them step by step.
In proestrous phase or the phase of follicular growth and peak estrogen secretion, rats were anesthetized intra peritoneally (i.p) with 20 mg/ kg ketamine hydrochloride. In first operation, the left uterine horn and associated fat tissue were removed. Left ovary was saved. The removed uterine horn was cut longitudely and divided to 6 pieces (2×2mm). Then, endometrium was separated from underling myometrium and 4 pieces were sutured with 4-0 round nylon to the different parts of peritoneal cavity; 1 piece near right kidney as right peritoneal implant, 1 piece near mesanteric artery as left peritoneal implant and 2 pieces in both ovaries (25). Then, 2 pieces of endometrial implants were sutured in subcutaneous of the rat’s skin as right and left subcutaneous implants (25).
One hour after surgery, the animals could move and after 4 hours, they fed regularly. Two days later, they were put near the male rat for starting their Estrous cycles (24,25). They were kept into close consideration for 2 months. During this period, 10-12 Esterous cycles were recorded for each rat. Vaginal cytology was evaluated daily as an indirect index of ovarian activity and fortunately all of them had this normal Estrous cycle.
Rats (n=20) were randomly assigned to two groups; in treated group (n=10), PX (manufactured by STADA, Ampoule, 100mg/5ml) in dose of 5mg/kg twice a day was administered subcutaneously (s.c) and in control group (n=10) normal saline as placebo was administered with the same dose (19, 20).
In second laparotomy, which was done in Proestrous phase too, at first we collected 5 milliliter of blood by heart puncture and sent to laboratory for measuring the number of Leukocyte count in serum. Then, the skin was cut and the endometrial tissues removed and the abdominal cavity was examined and all of our observations about adhesions, cystic formation, size and site of implantation were recorded carefully. The endometriotic implants in each animal were measured (length x width) and the average size (mm2) for each place was calculated along with the total implant mass (mm2; defined as the sum of the size of all implants in the same places) (20). The tissues were put in 10% formalin and sent to laboratory for microscopic evaluation. Endometriosis was confirmed by observation of gland and stroma of endometrium in implants. The surgeries were done by one surgeon and the slides were evaluated by one pathologist blindly.
Statistical analysis
These data were analyzed by SPSS program (t-test and Mann-Whitney for quantitive and Chi-Square and if needed Fisher exact test for qualitive variants). p<0.05 was considered as significant.
Results
Age, weight and size of implanted fragments were similar in both groups. Daily pap smear showed that the number of Esterous cycles was similar in both groups.
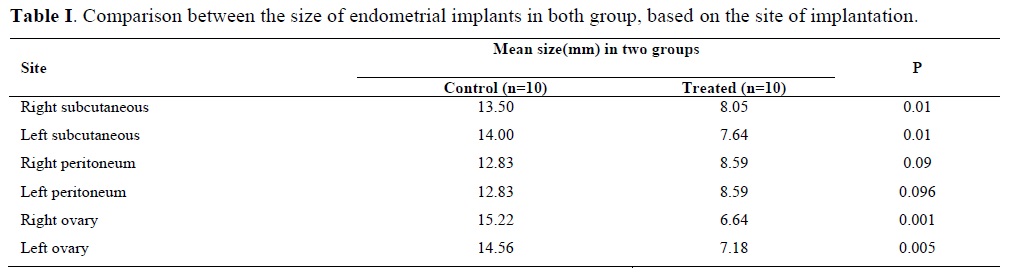
The mean size of the implants in both groups is shown in Table I. In treated group, the size of implants in right subcutaneous (8.05 vs. 13.50mm) p<0.01, left subcutaneous (7.64 vs. 14.00 mm) p<0.01, right ovary (6.64 vs. 15.22mm) p<0.001 and left ovary (7.18 vs. 14.56 mm)p<0.005 were significantly decreased. In right and left peritoneum (8.59 vs. 12.83 and 8.59 vs. 12.83 mm) p<0.09, the size of implants were decreased in treated group compare with control group but this was not significant.
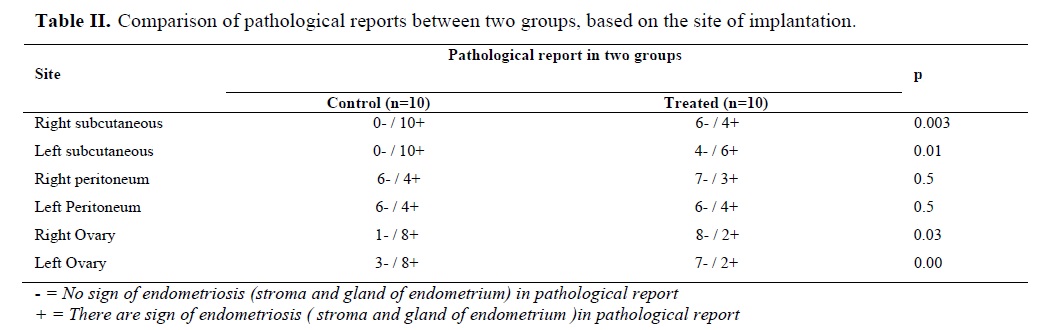
In treated group the results indicate, the total count of Leukocyte was less than control group (5259.54± 178.78 vs. 15833± 259.27) p<0.02 (Table II). The number of Estorous cycle was similar between two groups.
Discussion
Our findings showed that in treated group with PX, the size of endometrial implants were smaller than control group .This finding was similar to Nothnick et al study (1994) who reported that PX could modulate growth in endometriotic implant rat and also rise in production process of implant- specific proteins (ENDO-1& ENDO-2) (20).This is different from our study because we studied the size of endometrial fragments in different places and compared it with the same places in control group.
In treated group, the size of implants was decreased significantly as follows: in right subcutaneous, left subcutaneous, right ovary and left ovary. In right and left peritoneum, the size of implants was decreased in comparison with control group but not significantly. These findings suggest that the suppressive effect of PX was not similar in all places. This might be valuable when we are making decision for treatment of different types of endometriosis.
At the time of our study, there were a few studies on the effect of PX in female rats (19, 20) and in human (21, 22).
Although in animal studies (19, 20), Pentoxifylline could have a good effect on endometriosis, the human studies (21, 22) couldn’t be able to show the significant changes in treated group with PX.
In treated group, the total count of Leukocytes was decreased. These findings suggested that endometriosis in rats was accompanied with important changes in Leukocyte count in serum and PX could decrease the total count of Leukocyte. It seems that alternation in immune system in endometriosis which might be accompanied with alternation in Leukocyte count, can suppress endometrial growth in rats. This effect of PX was not evaluated in different studies on rats (19-21). Therefore this finding is nearly new and needs further studies for better evaluation. In order to better understanding of the suppressive effect of the PX, we suggest further studies on natural killer (NK) cells and TNF-a during treatment.
In our study, PX didn’t have any adverse effect on menstrual cycles in treated group. This is an important benefit in treatment with PX. Other drugs suppress ovarian function and induce hypoestrogenic state in women. Hot flash and osteoporesis with these drugs can hurt women (4,5). Based on our findings, under PX treatment, ovaries can secret hormones, these symptoms aren’t seen and fertility ability will be intact. These findings are similar to Steinleitner et al study (1991) findings. They induced endometriosis in female rats through surgical process and noticed that treatment with intra peritoneal PX in comparison with normal saline could increase fertility rates (90% vs. 2.3 %). They suggested that PX could increase fertility rates in endometriosis in rats (19).
In this study we didn’t allow the rats to get pregnant. This effect can be evaluated with future study.
Conclusion
PX can decrease the size of endometrial implants especially in ovaries and subcutaneous areas. In addition, significant reduction in Leukocyte count was noticed after PX therapy.
Endometriosis is defined as ectopic growth of endometrial stroma and glandular tissues (1,2). Although nearly three quarters of century have been passed since the initial description of endometriosis (3), our current understanding of the etiology and pathphysiology of the disease has still been remained unclear (1-4). After the first description of the disease by Sampson in 1927 several different hypotheses have been suggested to explain the mechanisms for the development of endometriosis (4,5). However, Sampson’s theory of retrograde menstruation has gained most supportive evidence (4, 5). Retrograde menstruation is the reflux of menses through fallopian tubes toward the ectopic sites especially in peritoneal cavity. There are some reports about endometriosis in surgical incision in women who have tolerated operation especially cesarean section. It seems it is due to direct implantation of viable endometrial cells into incision (4,5). These findings support Sampson's theory as well (4, 5). In addition,changes in cell-mediated immunity related with endometriosis have been reported in women, but the relevant data are inconsistent (2-5). A reduction in proliferation of peripheral blood lymphocytes in response to recognition of endometrial antigens and cells has also been reported (2-6).
It was shown that the ratio of T-helper to T-suppressor cells is increased in the peripheral blood of the affected women; on the contrary other studies have indicated no remarkable differences in peripheral lymphocytes profiles (4-7).On the other hand, peritoneal macrophages have revealed an increase in total number, concentration, and activation status (8).
Tumor necrosis factor-alpha (TNF-a) is regarded as one of the cytokines that has been gained recent attention in the pathphysiology of autoimmune diseases (9). TNF-a is a major product of activated macrophages. It can activate the inflammatory leukocytes which can lead to the production of other pro inflammatory cytokines, including interleukin-1 (IL-1), IL-6, and additional TNF-a (9). TNF-a can stimulate endometrial cell adhesion, as well as, induce matrix metalloproteinases (MMPs) expression, both of which are necessary events in the initial development of endometriosis (10, 11).
Considering above mention facts, TNF-a has an important role in endometriosis, therefore anti TNF-a drugs may be proper drug for this disease. PX reduces both the production and action of cytokines such as TNF-a through the elevation of intracellular cyclic AMP levels and subsequent down regulation of cytokine production and action (4, 5, 12).
PX is a member of Methylgezantine family which inhibits Phosphodiesterase in platlets and leads to the elevation of CAMP levels in them and decreases the aggregation of them in circulation. Therefore, it is useful in cerebrovascular diseases (4, 5,13). PX can inhibit phosphodiesterase in monocytes and macrophages and suppress cytokineproduction specially TNF-a in macrophages (4,5,12,13).
In other immunological disease like sever refractory rheumatoid arthritis (14), inflammatory bowel disease (15), and recurrent aphthous stomatitis (16), PX had a suppressive effect on these diseases .Therefore , it seems that anti TNF-a effect of this drug can be useful in treatment of endometriosis. Based on several studies, rats are good model for the study on endometriosis. They don’t have menstrual cycles and endometriosis isn't induced in them spontaneously (17,18).
Steinleitner et al (1991) induced endometriosis in female rats surgically and mentioned PX could increase fertility rates in comparison with normal saline (90% vs 2.3 %). They suggested that PX could raise fertility rates in endometriosis in rats (19). Nothnick et al (1994) reported that PX could modulate rat endometriotic implant growth and produce implant- specific proteins (ENDO-1& ENDO-2) (20).
Balach et al (1997) reported that PX versus placebo could improve fertility rates in women but not significantly (21). Although in animal studies (19, 20), PX could have a good effect on endometriosis, but the human studies couldn’t be able to show the significant changes in treated group with PX (21, 22).
In order to determine the effect of PX on endometriosis, we decided to do our study in female rats. In this study, we induced endometriosis in female rats surgically and evaluated the effect of PX versus placebo on the endometriosis.
The aims of this study were investigating : 1) The effect of PX on the growth of endometrial implants in female rats especially in different places. 2)The effect of PX on leukocyte count in serum, in order to determine the immune response with this drug.
Materials and methods
Our randomized clinical trial study was done on 20 female rats’ sparauge- dwley (2 months old). This study was performed in Avesina Research Institute under support of Iranian Academic Center for Education, Culture and Research (ACECR). The rats were housed individually in hanging cages. The facility was maintained on a 14-h light, 10-h darkness schedule. Female rats have 4- 5 days estrous cycles (24). For evaluation of phases in female rats we did vaginal Pap smear daily (25). Only those rats exhibited normal 4-5 day estrous cycle were subjected to surgical induction of endometriosis. All of the rats had a file that the processes were written in them step by step.
In proestrous phase or the phase of follicular growth and peak estrogen secretion, rats were anesthetized intra peritoneally (i.p) with 20 mg/ kg ketamine hydrochloride. In first operation, the left uterine horn and associated fat tissue were removed. Left ovary was saved. The removed uterine horn was cut longitudely and divided to 6 pieces (2×2mm). Then, endometrium was separated from underling myometrium and 4 pieces were sutured with 4-0 round nylon to the different parts of peritoneal cavity; 1 piece near right kidney as right peritoneal implant, 1 piece near mesanteric artery as left peritoneal implant and 2 pieces in both ovaries (25). Then, 2 pieces of endometrial implants were sutured in subcutaneous of the rat’s skin as right and left subcutaneous implants (25).
One hour after surgery, the animals could move and after 4 hours, they fed regularly. Two days later, they were put near the male rat for starting their Estrous cycles (24,25). They were kept into close consideration for 2 months. During this period, 10-12 Esterous cycles were recorded for each rat. Vaginal cytology was evaluated daily as an indirect index of ovarian activity and fortunately all of them had this normal Estrous cycle.
Rats (n=20) were randomly assigned to two groups; in treated group (n=10), PX (manufactured by STADA, Ampoule, 100mg/5ml) in dose of 5mg/kg twice a day was administered subcutaneously (s.c) and in control group (n=10) normal saline as placebo was administered with the same dose (19, 20).
In second laparotomy, which was done in Proestrous phase too, at first we collected 5 milliliter of blood by heart puncture and sent to laboratory for measuring the number of Leukocyte count in serum. Then, the skin was cut and the endometrial tissues removed and the abdominal cavity was examined and all of our observations about adhesions, cystic formation, size and site of implantation were recorded carefully. The endometriotic implants in each animal were measured (length x width) and the average size (mm2) for each place was calculated along with the total implant mass (mm2; defined as the sum of the size of all implants in the same places) (20). The tissues were put in 10% formalin and sent to laboratory for microscopic evaluation. Endometriosis was confirmed by observation of gland and stroma of endometrium in implants. The surgeries were done by one surgeon and the slides were evaluated by one pathologist blindly.
Statistical analysis
These data were analyzed by SPSS program (t-test and Mann-Whitney for quantitive and Chi-Square and if needed Fisher exact test for qualitive variants). p<0.05 was considered as significant.
Results
Age, weight and size of implanted fragments were similar in both groups. Daily pap smear showed that the number of Esterous cycles was similar in both groups.
The mean size of the implants in both groups is shown in Table I. In treated group, the size of implants in right subcutaneous (8.05 vs. 13.50mm) p<0.01, left subcutaneous (7.64 vs. 14.00 mm) p<0.01, right ovary (6.64 vs. 15.22mm) p<0.001 and left ovary (7.18 vs. 14.56 mm)p<0.005 were significantly decreased. In right and left peritoneum (8.59 vs. 12.83 and 8.59 vs. 12.83 mm) p<0.09, the size of implants were decreased in treated group compare with control group but this was not significant.
In treated group the results indicate, the total count of Leukocyte was less than control group (5259.54± 178.78 vs. 15833± 259.27) p<0.02 (Table II). The number of Estorous cycle was similar between two groups.


Discussion
Our findings showed that in treated group with PX, the size of endometrial implants were smaller than control group .This finding was similar to Nothnick et al study (1994) who reported that PX could modulate growth in endometriotic implant rat and also rise in production process of implant- specific proteins (ENDO-1& ENDO-2) (20).This is different from our study because we studied the size of endometrial fragments in different places and compared it with the same places in control group.
In treated group, the size of implants was decreased significantly as follows: in right subcutaneous, left subcutaneous, right ovary and left ovary. In right and left peritoneum, the size of implants was decreased in comparison with control group but not significantly. These findings suggest that the suppressive effect of PX was not similar in all places. This might be valuable when we are making decision for treatment of different types of endometriosis.
At the time of our study, there were a few studies on the effect of PX in female rats (19, 20) and in human (21, 22).
Although in animal studies (19, 20), Pentoxifylline could have a good effect on endometriosis, the human studies (21, 22) couldn’t be able to show the significant changes in treated group with PX.
In treated group, the total count of Leukocytes was decreased. These findings suggested that endometriosis in rats was accompanied with important changes in Leukocyte count in serum and PX could decrease the total count of Leukocyte. It seems that alternation in immune system in endometriosis which might be accompanied with alternation in Leukocyte count, can suppress endometrial growth in rats. This effect of PX was not evaluated in different studies on rats (19-21). Therefore this finding is nearly new and needs further studies for better evaluation. In order to better understanding of the suppressive effect of the PX, we suggest further studies on natural killer (NK) cells and TNF-a during treatment.
In our study, PX didn’t have any adverse effect on menstrual cycles in treated group. This is an important benefit in treatment with PX. Other drugs suppress ovarian function and induce hypoestrogenic state in women. Hot flash and osteoporesis with these drugs can hurt women (4,5). Based on our findings, under PX treatment, ovaries can secret hormones, these symptoms aren’t seen and fertility ability will be intact. These findings are similar to Steinleitner et al study (1991) findings. They induced endometriosis in female rats through surgical process and noticed that treatment with intra peritoneal PX in comparison with normal saline could increase fertility rates (90% vs. 2.3 %). They suggested that PX could increase fertility rates in endometriosis in rats (19).
In this study we didn’t allow the rats to get pregnant. This effect can be evaluated with future study.
Conclusion
PX can decrease the size of endometrial implants especially in ovaries and subcutaneous areas. In addition, significant reduction in Leukocyte count was noticed after PX therapy.
Type of Study: Original Article |
References
1. Akanda VA, Hunt LP, Cahill DJ, Jenkins JM. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum Reprod 2004 ;19:96-103. [DOI:10.1093/humrep/deh045] [PMID]
2. Denny E, Khan KS. Systematic reviews of qualitative evidence. What are the experiences of women with endometriosis. J Obstet Gynaecol 2006;26:501-506. [DOI:10.1080/01443610600797301] [PMID]
3. Tomassetti C, Meuleman C, Pexster SA, Mihalyi A, Kyama C, Sima P, D'Hooghe TM. Endometriosis, recurrent miscarriage and implantation failure : is there an immunological link? Reprod Biomed Online 2006;13:58-64. [DOI:10.1016/S1472-6483(10)62016-0]
4. Berkkangolu M, Arici A. Immunology and endometriosis . Am J Reprod.Immunol 2003;50:48-59. [DOI:10.1034/j.1600-0897.2003.00042.x] [PMID]
5. Nothnick WB. Treating endometriosis as an autoimmune disease. Fertil Steril 2001;76 :223-231. [DOI:10.1016/S0015-0282(01)01878-7]
6. Kyama.CM, Debrock S, Mwenda JM, D'Hooghe TM. Potential involvement of the immune system in development of endometriosis .Reprod Biol Endocrinol 2003;1:123-139. [DOI:10.1186/1477-7827-1-123] [PMID] [PMCID]
7. Maeda N, Lzumiya C, Yamamoto Y, Yomamato Y, Oguri H, Kusume T, Fukaya T. Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis .Fertil Steril 2002 ;77:297-302. [DOI:10.1016/S0015-0282(01)02964-8]
8. Gomez-Tores MJ, Acien P, Campos A, Velasco I. Embriotoxicity of peritoneal fluid in women with endometriosis: Its relation with cytokins and lymphocyte population.Hum Reprod 2002 ;17: 777-781. [DOI:10.1093/humrep/17.3.777] [PMID]
9. Richter O, Mallman P, Vanderven H, Krebs D. TNF-α Secretion by Peritoneal macrophages in endometriosis. Zentralbl Gynakol 1998;120:332-336.
10. Jerzak M,Baranowski W,Rechberger T, Gorski A. Enhanced Tcell interactions with extracellular matrix proteins in infertile women with endometriosis. Immunol Lett 2002; 81:65-70. [DOI:10.1016/S0165-2478(01)00337-6]
11. Bruner KL, Matrstan IM, Rodgers WI, Gorstein F, Osteen K. Suppression of matrix metalloproteinase inhibits establishment of ectopic lesions by human tissue in nude mice. J Clin Invest 1997; 99: 2851-2857. [DOI:10.1172/JCI119478] [PMID] [PMCID]
12. Sullivan GW, Caper HT, Novick WJ Jr, Mandell GL. Induction of the inflammatory action of IL1, TNF-α, on neutrofil function by Pentoxifylline. Infect Immun. 1988 ;56:1722-1729.
13. Ward A, Clissold SP. Pentoxifylline .A review of it's pharmacodynamic and pharmacokinetic properties and it's therapeutic efficacy. Drugs 1987;34:50-97. [DOI:10.2165/00003495-198734010-00003] [PMID]
14. Maksymowych WP, Avina-Zubeta A, Luong MH, Kussel AS. An open study of pentoxifylline in the treatment of sever refractory rheumatoid arthritis. J Rheumatol 1995; 22:625-629.
15. Reimund JM, Bumont S, Muller CD, Kenny JS, kedinger M, Bauman R, et al . In vitro effects of pentoxifylline on inflammatory cytokine release in patients with inflammatory bowel disease. Gut 1997 ;40: 475-480. [DOI:10.1136/gut.40.4.475] [PMID] [PMCID]
16. Thornhill MH, Baccaglini L, Theaker E, Pemberton MN. A randomized Double-blind Placcebo-controlled of Pentoxifylline for the treatment of Recurrent Aphthous Stomatitis. Arch Dermatol 2007;143:463-470. [DOI:10.1001/archderm.143.4.463] [PMID]
17. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil steril 1985 ;44 :684-699.
18. Sharpe- Timms KL. Using rats as a research model for the study of endometriosis. Ann NY Acad Sci 2002 ;955:318-327. [DOI:10.1111/j.1749-6632.2002.tb02792.x] [PMID]
19. Steinleitner A, Lambert H, Suarez M, Serpa N, Roy S. Immunomodulation in the treatment of endometriosis - associated subfertility : use of Pentoxifylline to reverse the inhibition of fertilization by surgically induced endometriosis in a rodent model. Fertil Steril 1991;56:975-979. [DOI:10.1016/S0015-0282(16)54674-3]
20. Nothnick WB, Curry TE, Vernon MW. Immunomodulation of rat endometriotic implant growth and protein production. AJRI 1994;31:151-162. [DOI:10.1111/j.1600-0897.1994.tb00860.x] [PMID]
21. Balasch J, Creus M, Fabregues F, Carmona F, Martinez-Roman S, Manau D, Vanrell JA. Pentoxifylline versus placebo in the treatment of infertility associated with minimal or mild endometriosis: a pilot randomized clinical trial. Hum Reprod 1997;12:2046-2050. [DOI:10.1093/humrep/12.9.2046] [PMID]
22. Alborzi S, Ghotbi S, Parsanezhad ME, Dehbashi S, Alborzi S, Alborzi M. Pentoxifylline therapy after laparoscopic surgery for different stages of endometriosis: a prospective, double blind,randomized placebo-controled study. J Minim Invasive Gynecol 2007;14:54-58. [DOI:10.1016/j.jmig.2006.06.024] [PMID]
23. Smith MG, Hau J, Hoosier GL. Handbook of laboratory animal science, 2nd ed , U.S.A, CRC press . 2003.p. 51-62.
24. Waggie K. Manual of microbiologic monitoring of laboratory animals, 2nd ed , U.S.A, DIAN publishing. 1996:59-165.
25. Mohammadzadeh A, Heidari M, Soltan Ghoraii H, Zarnani AH, Ghaffari Novin M, Akhondi MM Mossavie Jarahi A, Mohammadzadeh F. Induction of endometriosis by implantation of endometrial fragments in female rats. IJRM 2006; 4:63-67.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


